Occurrence of Aflatoxin M1 (AFM1) in Donkey Milk Collected in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Nutrition
2.2. Sampling
2.3. Solvents and Reagents
2.4. Chromatographic Apparatus and Conditions
2.5. Sample Preparation and Immunoaffinity Clean-Up Procedures
2.6. Quantification
3. Results and Discussion
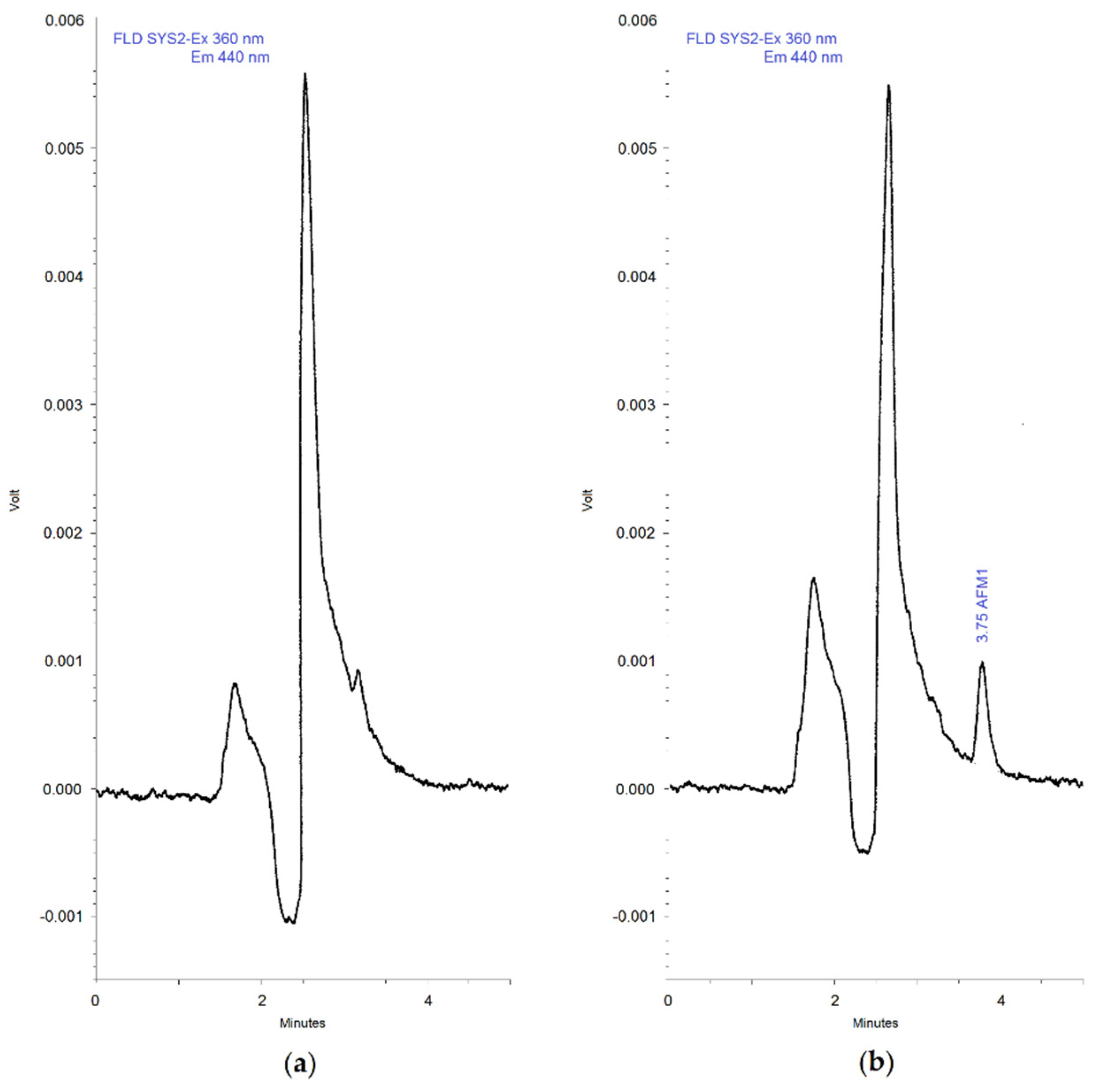
3.1. Method Development
3.2. Assay Validation
3.3. Occurrence of AFM1 in Milk Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef]
- Souroullas, K.; Aspri, M.; Papademas, P. Donkey milk as a supplement in infant formula: Benefits and technological challenges. Food Res. Int. 2018, 109, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Aspri, M.; Economou, N.; Papademas, P. Donkey milk: An overview on functionality, technology, and future prospects. Food Rev. Int. 2017, 33, 316–333. [Google Scholar] [CrossRef]
- Polidori, P.; Ariani, A.; Vincenzetti, S. Use of donkey milk in cases of cow’s milk protein allergies. Int. J. Child Health Nutr. 2015, 4, 174–179. [Google Scholar] [CrossRef]
- Iacono, G.; Scalici, C. Uso del latte d’asina come soluzione di problemi pratici. In Latte di Asina-Produzione, Caratteristiche e Gestione Dell’azienda Asinina; Milonis, E., Polidori, P., Eds.; Fondazione Iniziative Zooprofilattiche e Zootecniche: Brescia, Italy, 2011; pp. 259–266. [Google Scholar]
- Kocic, H.; Stankovic, M.; Tirant, M.; Lotti, T.; Arsic, I. Favorable effect of creams with skimmed donkey milk encapsulated in nanoliposomes on skin physiology. Dermatol. Ther. 2020, 33, e13511:1–e13511:7. [Google Scholar] [CrossRef]
- Colavita, G.; Amadoro, C.; Salimei, E. Latte d’asina: Aspetti igienico-sanitari e normativi. Argomenti SIVEMP 2011, 3, 61–70. [Google Scholar]
- ANSA. Coldiretti, +90% Asini in 10 Anni, Richiesta Latte e Cosmesi. Available online: https://www.mdpi.com/2306-7381/7/3/121 (accessed on 1 September 2020).
- Benkerroum, N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Kuilman, M.E.; Maas, R.F.; Fink-Gremmels, J. Cytochrome P450-mediated metabolism and cytotoxicity of aflatoxin B(1) in bovine hepatocytes. Toxicol. In Vitro 2000, 14, 321–327. [Google Scholar] [CrossRef]
- Wacoo, P.A.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Tozzi, B.; Liponi, G.B.; Meucci, V.; Casini, L.; Dall’Asta, C.; Intorre, L.; Gatta, D. Aflatoxins M1 and M2 in the milk of donkeys fed with naturally contaminated diet. Dairy Sci. Technol. 2016, 96, 513–523. [Google Scholar] [CrossRef]
- Hernández-Martínez, R.; Navarro-Blasco, I. Aflatoxin levels and exposure assessment of Spanish infant cereals. Food Addit. Contam. Part B Surveill. 2010, 3, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M. A review on aflatoxins reduction in food. Iran. J. Health Saf. Environ. 2015, 3, 445–459. [Google Scholar]
- Tam, J.; Mankotia, M.; Mably, M.; Pantazopoulos, P.; Neil, R.J.; Calway, P.; Scott, P.M. Survey of breakfast and infant cereals for aflatoxins B1, B2, G1 and G2. Food Addit. Contam. 2006, 23, 693–699. [Google Scholar] [CrossRef]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M 1 in milk and milk products: A worldwide review. J. Food Prot. 1996, 59, 1079–1090. [Google Scholar] [CrossRef]
- Hussain, I.; Anwar, J. A study on contamination of aflatoxin M1 in raw milk in the Punjab province of Pakistan. Food Control 2008, 19, 393–395. [Google Scholar] [CrossRef]
- López, C.E.; Ramos, L.L.; Ramadán, S.S.; Bulacio, L.C. Presence of aflatoxin M1 in milk for human consumption in Argentina. Food Control 2003, 14, 31–34. [Google Scholar] [CrossRef]
- Pierides, M.; El-Nezami, H.; Peltonen, K.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Prot. 2000, 63, 645–650. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Chi, F.; Broomhead, J. Mycotoxins and Dairy Cattle: A Review for Dairy Producers; Oil-Dri Corporation of America: Chicago, IL, USA, 2009. [Google Scholar]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carryover to dairy milk: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 172–180. [Google Scholar] [CrossRef]
- Sarica, D.Y.; Has, O.; Taşdelen, S.; Ezer, Ü. Occurrence of aflatoxin M1 in milk, white cheese and yoghurt from Ankara, Turkey markets. Biol. Chem. Res. 2015, 2015, 36–49. [Google Scholar]
- Torović, L. Aflatoxin M1 in processed milk and infant formulae and corresponding exposure of adult population in Serbia in 2013–2014. Food Addit. Contam. Part B Surveill. 2015, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Bognanno, M.; La Fauci, L.; Ritieni, A.; Tafuri, A.; De Lorenzo, A.; Micari, P.; Di Renzo, L.; Ciappellano, S.; Sarullo, V.; Galvano, F. Survey of the occurrence of Aflatoxin M1 in ovine milk by HPLC and its confirmation by MS. Mol. Nutr. Food Res. 2006, 50, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Mariotti, A.; Zizzadoro, C.; Zaghini, A.; Ormas, P.; Altafini, A.; Belloli, C. A clonal cell line (BME-UV1) as a possible model to study bovine mammary epithelial metabolism: Metabolism and cytotoxicity of aflatoxin B1. Toxicon 2009, 53, 400–408. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Diaz, G.J.; Murcia, H.W. Biotransformation of aflatoxin B1 and its relationship with the differential toxicological response to aflatoxin in commercial poultry species. In Aflatoxins: Biochemistry and Molecular Biology; Guevara-Gonzalez, R.G., Ed.; InTech: Rijeka, Croatia, 2011; pp. 3–20. ISBN 978-953-307-395-8. [Google Scholar]
- Pietri, A.; Piva, G. Aflatoxins in foods. Ital. J. Public Health 2007, 4, 32–38. [Google Scholar] [CrossRef]
- IARC. Chemical agents and related occupations. A review of human carcinogens. Monogr. Eval. Carcinog. Risks Hum. 2012, 100F, 225–248. [Google Scholar]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170:1–2170:10. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, M.A.; Ha, J.K. Mycotoxins and their biotransformation in the rumen: A review. Asian-Aust. J. Anim. Sci. 2010, 23, 1250–1260. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the biological and health effects of aflatoxins on body organs and body systems. In Aflatoxins-Recent Advances and Future Prospects; Razzaghi-Abyaneh, M., Ed.; InTech: Rijeka, Croatia, 2013; pp. 239–266. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- El-Desouky, T.A.; Mohamed, S.R.; Abou-Arab, A.A.K.; Salim, A.B. Occurrence of aflatoxin B1 and M1 in some Egyptian chicken organs and their affected by ozonated water. Open Sci. J. Mod. Phys. 2014, 1, 24–30. [Google Scholar]
- Gargees, M.T.; Shareef, A.M. Reducing liver aflatoxin M1 residues in chicks with mycofix plus 3.0® during aflatoxicosis. Iraqi J. Vet. Sci. 2009, 23, 37–44. [Google Scholar]
- Jurišić, N.; Schwartz-Zimmermann, H.E.; Kunz-Vekiru, E.; Moll, W.D.; Schweiger, W.; Fowler, J.; Berthiller, F. Determination of aflatoxin biomarkers in excreta and ileal content of chickens. Poult. Sci. 2019, 98, 5551–5561. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Grenier, B.; Applegate, T.J. Aflatoxins in Poultry. Purdue Extension, Purdue University. 2013. Available online: https://www.extension.purdue.edu/extmedia/AS/AS-615-W.pdf (accessed on 1 September 2020).
- European Commission. Commission Regulation (EC) 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L159, 7–24. [Google Scholar]
- European Commission. Commission Regulation (EC) 165/2010 of 26 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, L50, 8–11. [Google Scholar]
- Decastelli, L.; Lai, J.; Gramaglia, M.; Monaco, A.; Nachtmann, C.; Oldano, F.; Ruffier, M.; Sezian, A.; Bandirola, C. Aflatoxins occurrence in milk and feed in Northern Italy during 2004–2005. Food Control 2007, 18, 1263–1266. [Google Scholar] [CrossRef]
- Armorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Occurrence of aflatoxin M1 in conventional and organic milk offered for sale in Italy. Mycotoxin Res. 2016, 32, 237–246. [Google Scholar] [CrossRef]
- Fusi, F.; Scalvenzi, A.; Angelucci, A.; Bolzoni, G.; Bertocchi, L. Aflatossine/2: La fase di allerta non può dirsi conclusa. Inf. Zootec. 2013, 9, 54–60. Available online: http://www.izsler.it/izs_bs/allegati/415/aflatossineIZ_2013_09.pdf (accessed on 1 September 2020).
- Capei, R.; Neri, P. Occurrence of aflatoxin M1 in milk and yoghurt offered for sale in Florence (Italy). Ann. Ig. 2002, 14, 313–319. [Google Scholar]
- Danieli, P.P.; Giangolini, G.; Giontella, D.; Bernabucci, U.; Ronchi, B. Approccio epidemiologico allo studio delle contaminazioni da aflatossine B1 e M1 nel sistema di allevamento del bovino da latte, Rapporti ISTISAN 05/42. In Proceedings of the 1st National conference Mycotoxins in agri-food chain, Rome, Italy, 29–30 November 2004; Miraglia, M., Brera, C., Eds.; Istituto Superiore di Sanità: Rome, Italy, 2005; pp. 108–119. [Google Scholar]
- Galvano, F.; Galofaro, V.; Ritieni, A.; Bognanno, M.; De Angelis, A.; Galvano, G. Survey of the occurrence of aflatoxin M1 in dairy products marketed in Italy: Second year of observation. Food Addit. Contam. 2001, 18, 644–646. [Google Scholar] [CrossRef]
- Ghidini, S.; Zanardi, E.; Battaglia, A.; Varisco, G.; Ferretti, E.; Campanini, G.; Chizzolini, R. Comparison of contaminant and residue levels in organic and conventional milk and meat products from Northern Italy. Food Addit. Contam. 2005, 22, 9–14. [Google Scholar] [CrossRef]
- Nachtmann, C.; Gallina, S.; Rastelli, M.; Ferro, G.L.; Decastelli, L. Regional monitoring plan regarding the presence of aflatoxin M1 in pasteurized and UHT milk in Italy. Food Control 2007, 18, 623–629. [Google Scholar] [CrossRef]
- Santini, A.; Raiola, A.; Ferrantelli, V.; Giangrosso, G.; Macaluso, A.; Bognanno, M.; Galvano, F.; Ritieni, A. AflatoxinM1 in raw, UHT milk and dairy products in Sicily (Italy). Food Addit. Contam. Part B 2013, 6, 181–186. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Olivastri, A.M.A.; Tofalo, R.; Perpetuini, G.; Suzzi, G. A one-year survey on aflatoxin M1 in raw milk. Ital. J. Food Sci. 2015, 27, 271–276. [Google Scholar] [CrossRef]
- Malacarne, M.; Criscione, A.; Franceschi, P.; Bordonaro, S.; Formaggioni, P.; Marletta, D.; Summer, A. New insights into chemical and mineral composition of donkey milk throughout nine months of lactation. Animals 2019, 9, 1161. [Google Scholar] [CrossRef]
- Marletta, D.; Tidona, F.; Bordonaro, S. Donkey milk proteins: Digestibility and nutritional significance. In Milk Protein—From Structure to Biological Properties and Health Aspects; Gigli, I., Ed.; InTech: Rijeka, Croatia, 2016; pp. 199–209. [Google Scholar] [CrossRef]
- Polidori, P.; Vincenzetti, S. Use of donkey milk in children with cow’s milk protein allergy. Foods 2013, 2, 151–159. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Foghini, L.; Pucciarelli, S.; Polzonetti, V.; Cammertoni, N.; Beghelli, D.; Polidori, P. Hypoallergenic properties of donkey’s milk: A preliminary study. Vet. Ital. 2014, 50, 99–107. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; Giraudi, G. Occurrence of aflatoxin M1 in dairy products. In Aflatoxins Detection, Measurement and Control; Torres-Pacheco, I., Ed.; InTech: Rijeka, Croatia, 2011; pp. 3–20. ISBN 978-953-307-711-6. [Google Scholar]
- Cammilleri, G.; Graci, S.; Collura, R.; Buscemi, M.D.; Vella, A.; Macaluso, A.; Giaccone, V.; Giangrosso, G.; Cicero, A.; Lo Dico, G.M.; et al. Aflatoxin M 1 in cow, sheep, and donkey milk produced in Sicily, Southern Italy. Mycotoxin Res. 2019, 35, 47–53. [Google Scholar] [CrossRef]
- Costamagna, D.; Gaggiotti, M.; Chiericatti, C.A.; Costabel, L.; Audero, G.M.L.; Taverna, M.; Signorini, M.L. Quantification of aflatoxin M1 carry-over rate from feed to soft cheese. Toxicol. Rep. 2019, 6, 782–787. [Google Scholar] [CrossRef]
- Walte, H.G.; Schwake-Anduschus, C.; Geisen, R.; Fritsche, J. Aflatoxin: Food chain transfer from feed to milk. J. Verbr. Lebensm. 2016, 11, 295–297. [Google Scholar] [CrossRef]
- Pettersson, H.; Bertilsson, J.; Wennberg, O. Carry-over of aflatoxin from dairy cattle feed to milk. In Proceedings of the International Symposium of the World Association of Veterinary Food Hygienists. Healthy Animals, Safe Foods, Healthy Man, Stockholm, Sweden, 2–7 July 1989; pp. 97–102. [Google Scholar]
- Masoero, F.; Gallo, A.; Moschini, M.; Piva, G.; Diaz, D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 2007, 1, 1344–1350. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Piva, G.; Binder, E.M.; Schatzmayr, D.; Rodrigues, I. Aflatoxin transfer from naturally contaminated feed to milk of dairy cows and the efficacy of a mycotoxin deactivating product. Int. J. Dairy Sci. 2009, 4, 34–42. [Google Scholar] [CrossRef]
- Sumantri, I.; Murti, T.W.; Van der Poel, A.F.B.; Boehm, J.; Agus, A. Carry-over of aflatoxin B1-feed into aflatoxin M1-milk in dairy cows treated with natural sources of aflatoxin and bentonite. J. Indones. Trop. Anim. Agric. 2012, 37, 271–277. [Google Scholar] [CrossRef]
- Britzi, M.; Friedman, S.; Miron, J.; Solomon, R.; Cuneah, O.; Shimshoni, J.A.; Soback, S.; Ashkenazi, R.; Armer, S.; Shlosberg, A. Carry-over of aflatoxin B1 to aflatoxin M1 in high yielding Israeli cows in mid-and late-lactation. Toxins 2013, 5, 173–183. [Google Scholar] [CrossRef]
- Gonçalves, B.L.; Gonçalves, J.L.; Rosim, R.E.; Cappato, L.P.; Cruz, A.; Oliveira, C.; Corassin, C.H. Effects of different sources of Saccharomyces cerevisiae biomass on milk production, composition, and aflatoxin M1 excretion in milk from dairy cows fed aflatoxin B1. J. Dairy Sci. 2017, 100, 5701–5708. [Google Scholar] [CrossRef]
- Völkel, I.; Schröer-Merker, E.; Czerny, C.P. The carry-over of mycotoxins in products of animal origin with special regard to its implications for the European food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 39, 1–27. [Google Scholar]
- Battacone, G.; Palomba, M.; Usai, M.G.; Pulina, G. Transfer of aflatoxin from feed to milk and curd in Sarda ewes with different milk production level. Ital. J. Anim. Sci. 2003, 2, 530–532. [Google Scholar] [CrossRef]
- Battacone, G.; Nudda, A.; Palomba, M.; Pascale, M.; Nicolussi, P.; Pulina, G. Transfer of aflatoxin B1 from feed to milk and from milk to curd and whey in dairy sheep fed artificially contaminated concentrates. J. Dairy Sci. 2005, 88, 3063–3069. [Google Scholar] [CrossRef]
- Mazzette, A.; Decandia, M.; Acciaro, M.; Fenu, A.; Dias Francesconi, A.H.; Battacone, G. Excretion of Aflatoxin M1 in milk of goats fed diet contaminated by Aflatoxin B1. Ital. J. Anim. Sci. 2009, 8, 631–633. [Google Scholar] [CrossRef][Green Version]
- Battacone, G.; Nudda, A.; Rassu, S.P.G.; Decandia, M.; Pulina, G. Excretion pattern of aflatoxin M1 in milk of goats fed a single dose of aflatoxin B1. J. Dairy Sci. 2012, 95, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Nageswara Rao, S.B.; Chopra, R.C. Influence of sodium bentonite and activated charcoal on aflatoxin M1 excretion in milk of goats. Small Rumin. Res. 2001, 41, 203–213. [Google Scholar] [CrossRef]
- Mugerwa, S.; Kabirizi, J.; Zziwa, E. Effect of supplementing lactating goats fed on aflatoxin contaminated feed with calcium bentonite and activated charcoal on aflatoxin M1 concentration, excretion and carryover in milk. Uganda J. Agric. Sci. 2015, 16, 83–92. [Google Scholar] [CrossRef]
- Kos, J.; Lević, J.; Đuragić, O.; Kokić, B.; Miladinović, I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Bilandžić, N.; Božić, Đ.; Đokić, M.; Sedak, M.; Kolanović, B.S.; Varenina, I.; Cvetnić, Ž. Assessment of aflatoxin M1 contamination in the milk of four dairy species in Croatia. Food Control 2014, 43, 18–21. [Google Scholar] [CrossRef]
- Malissiova, E.; Manouras, A. Monitoring aflatoxin M1 levels in donkey milk produced in Greece, intended for human consumption. World Mycotoxin J. 2017, 10, 203–206. [Google Scholar] [CrossRef]
- Gross, M.; Ploetz, C.P.; Gottschalk, C. Immunochemical detection of mycotoxins in donkey milk. Mycotoxin Res. 2019, 35, 83–87. [Google Scholar] [CrossRef]
| AFM1 Spiking Levels (µg/L) | Recovery (%) 1 | M (%) 2 |
|---|---|---|
| 0.0125 | 87.7 | 89.0 |
| 0.025 | 86.6 | |
| 0.075 | 89.7 | |
| 0.125 | 92.2 |
| Milk Sample | Animal | Sampling Period | AFM1 (µg/L) | Milk Sample | Animal | Sampling Period | AFM1 (µg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 41 | Jun–Aug | <LOD | 33 | 96 | Nov | <LOD |
| 2 | 33 | Jun–Aug | <LOD | 34 | 22 | Nov | <LOD |
| 3 | 64 | Jun–Aug | <LOD | 35 | 25 | Nov | <LOD |
| 4 | 41 | Jun–Aug | <LOD | 36 | 41 | Nov | <LOD |
| 5 | 13 | Jun–Aug | <LOD | 37 | 83 | Nov | <LOD |
| 6 | 83 | Jun–Aug | <LOD | 38 | 45 | Nov | <LOD |
| 7 | 6 | Jun–Aug | <LOD | 39 | 90 | Nov | <LOD |
| 8 | 23 | Jun–Aug | <LOD | 40 | 86 | Nov | <LOD |
| 9 | 92 | Jun–Aug | <LOD | 41 | 97 | Nov | <LOD |
| 10 | 6 | Jun–Aug | <LOD | 42 | 13 | Nov | <LOD |
| 11 | 23 | Jun–Aug | <LOD | 43 | 93 | Nov | <LOD |
| 12 | 92 | Nov | <LOD | 44 | 60 | Nov | <LOD |
| 13 | 94 | Nov | <LOD | 45 | 92 | Nov | <LOD |
| 14 | 89 | Nov | <LOD | 46 | 36 | Nov | 0.0044 |
| 15 | 22 | Nov | <LOD | 47 | 91 | Nov | <LOD |
| 16 | 25 | Nov | <LOD | 48 | 65 | Nov | <LOD |
| 17 | 41 | Nov | <LOD | 49 | 9 | Nov | <LOD |
| 18 | 83 | Nov | <LOD | 50 | 85 | Nov | <LOD |
| 19 | 45 | Nov | <LOD | 51 | 23 | Nov | <LOD |
| 20 | 90 | Nov | <LOD | 52 | 94 | Nov | <LOD |
| 21 | 13 | Nov | <LOD | 53 | 22 | Nov | <LOD |
| 22 | 29 | Nov | <LOD | 54 | 25 | Nov | <LOD |
| 23 | 64 | Nov | <LOD | 55 | 41 | Nov | <LOD |
| 24 | 58 | Nov | <LOD | 56 | 83 | Nov | <LOD |
| 25 | 42 | Nov | <LOD | 57 | 45 | Nov | <LOD |
| 26 | 23 | Nov | <LOD | 58 | 90 | Nov | <LOD |
| 27 | 66 | Nov | <LOD | 59 | 86 | Nov | <LOD |
| 28 | 94 | Nov | <LOD | 60 | 97 | Nov | <LOD |
| 29 | 21 | Nov | <LOD | 61 | 13 | Nov | <LOD |
| 30 | 54 | Nov | <LOD | 62 | 60 | Nov | <LOD |
| 31 | 89 | Nov | <LOD | 63 1 | - | Nov | <LOD |
| 32 | 1 | Nov | <LOD |
| Sampling Area | Sampling Period | Type of Milk | N° Samples Collected | % Positive | Concentration Range | References | |
|---|---|---|---|---|---|---|---|
| (µg/kg o ng/L) | |||||||
| Min | Max | ||||||
| Serbia | 2013 | Donkey milk | 5 | 60 | 0.005 1 | 0.035 1 | [74] |
| Cow milk | 150 | 98.7 | 0.01 1 | 1.2 1 | |||
| Goat milk | 10 | 80 | 0.008 1 | 0.24 1 | |||
| Breast milk | 10 | 60 | 0.006 1 | 0.022 1 | |||
| Infant formula | 1 | 0 | - | - | |||
| Croatia | 2013 | Donkey milk | 14 | − 3 | 3.43 2 | 10.4 2 | [75] |
| Cow milk | 337 | − 3 | 2.69 2 | 162.3 2 | |||
| Goat milk | 32 | − 3 | 2.78 2 | 40.8 2 | |||
| Sheep milk | 19 | − 3 | 2.11 2 | 5.87 2 | |||
| Sicily (Southern Italy) | 2013–2016 | Donkey milk | 84 | 0 | - | - | [57] |
| Cow milk | 170 | 11.1 | − 3 | 0.03 1 | |||
| Sheep milk | 133 | 3.6 | − 3 | 0.15 1 | |||
| Greece | 2015 | Donkey milk | 36 | 13.9 | 5.6 2 | 26.5 2 | [76] |
| Germany | − 3 | Donkey milk | 6 | 0 | - | - | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altafini, A.; Tassinari, M.; Guerrini, A.; Roncada, P. Occurrence of Aflatoxin M1 (AFM1) in Donkey Milk Collected in Northern Italy. Vet. Sci. 2020, 7, 176. https://doi.org/10.3390/vetsci7040176
Altafini A, Tassinari M, Guerrini A, Roncada P. Occurrence of Aflatoxin M1 (AFM1) in Donkey Milk Collected in Northern Italy. Veterinary Sciences. 2020; 7(4):176. https://doi.org/10.3390/vetsci7040176
Chicago/Turabian StyleAltafini, Alberto, Marco Tassinari, Alessandro Guerrini, and Paola Roncada. 2020. "Occurrence of Aflatoxin M1 (AFM1) in Donkey Milk Collected in Northern Italy" Veterinary Sciences 7, no. 4: 176. https://doi.org/10.3390/vetsci7040176
APA StyleAltafini, A., Tassinari, M., Guerrini, A., & Roncada, P. (2020). Occurrence of Aflatoxin M1 (AFM1) in Donkey Milk Collected in Northern Italy. Veterinary Sciences, 7(4), 176. https://doi.org/10.3390/vetsci7040176





