Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Chickens and Experimental Design
2.2. Body Weight of Chickens
2.3. RNA Extraction, Reverse Transcription, and Quantitative Polymerase Chain Reaction (PCR) Assays
2.4. Statistical Analysis
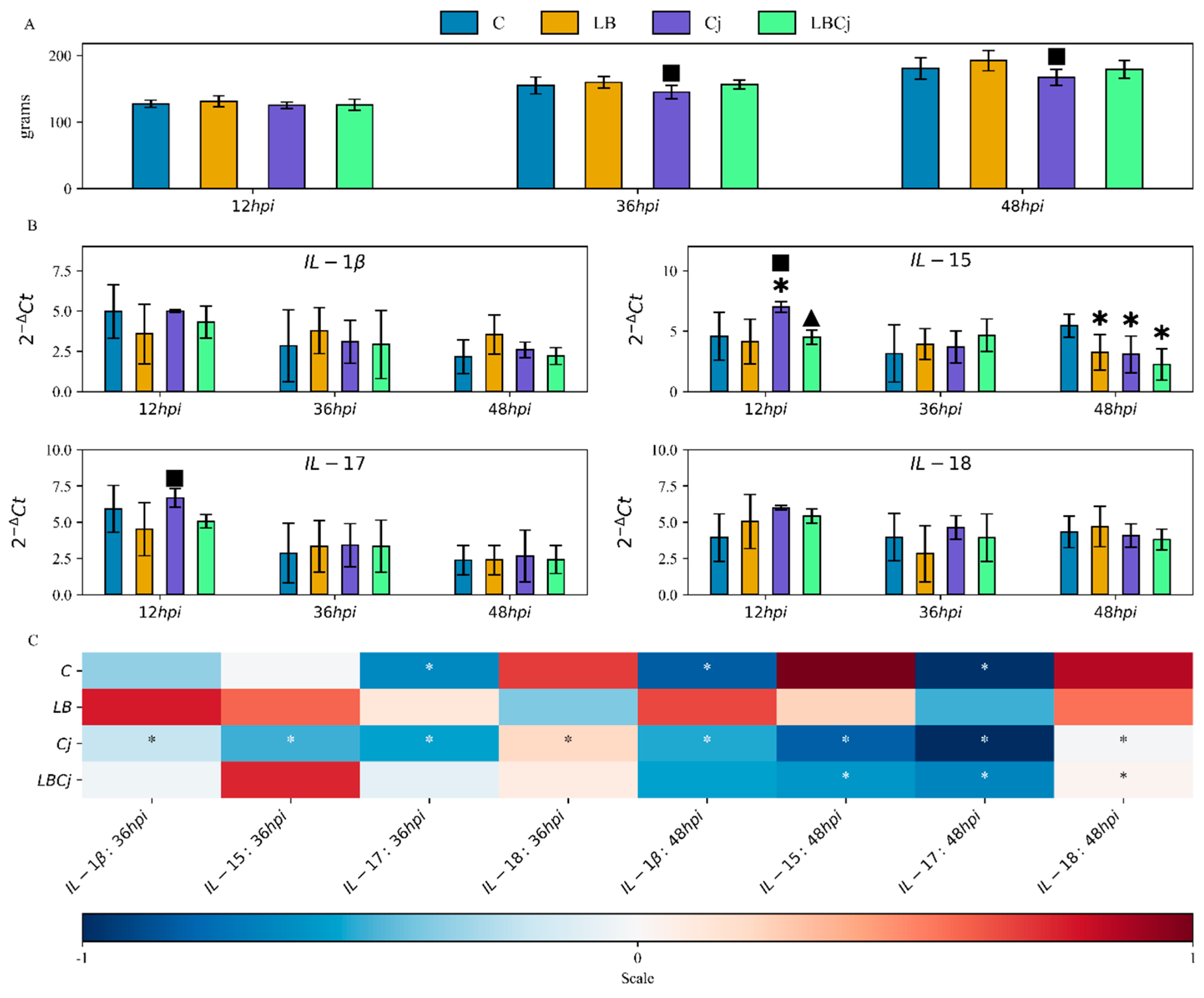
3. Results
3.1. Body Weight of Chickens
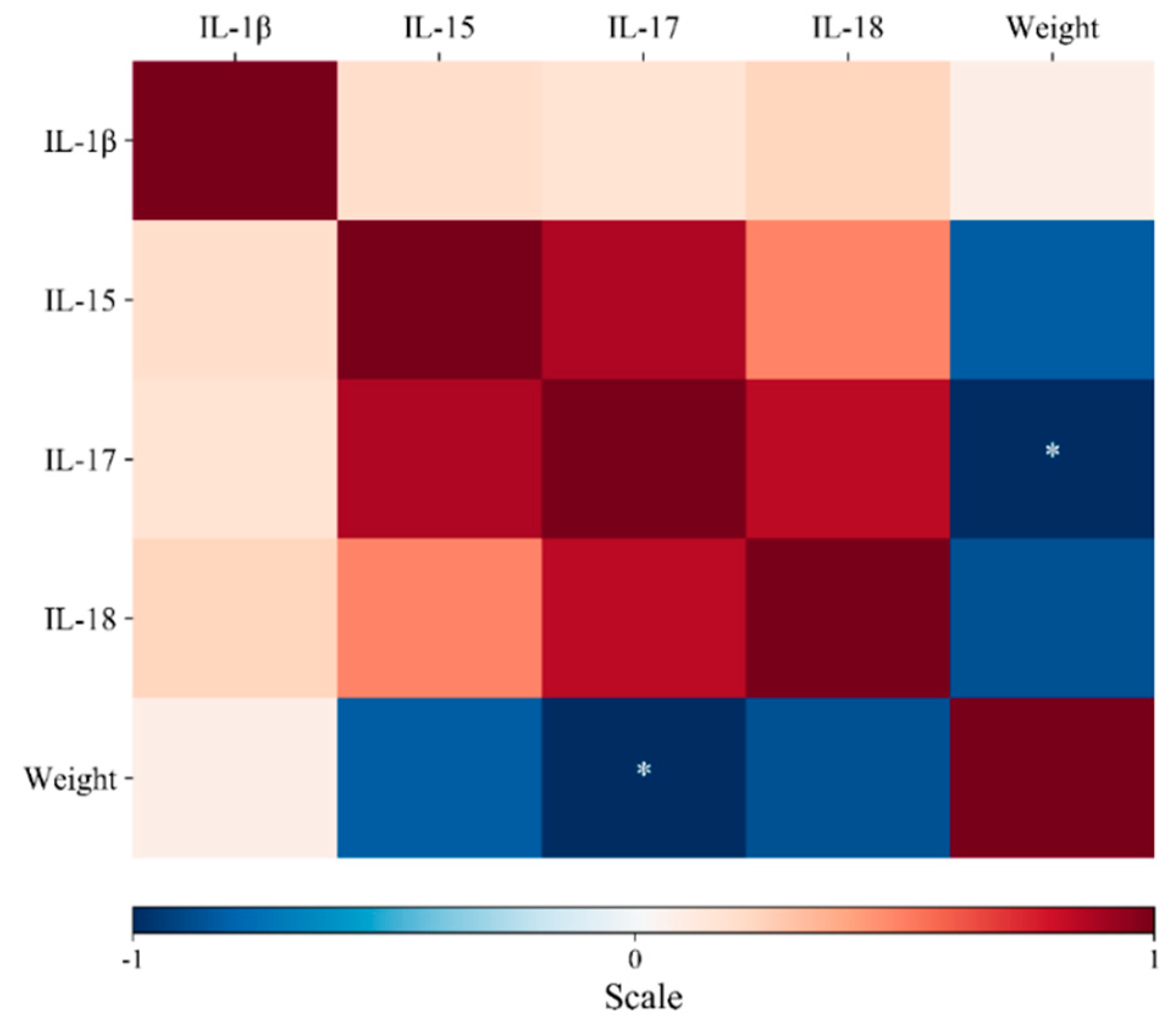
3.2. Cytokine Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roux, F.; Sproston, E.; Rotariu, O.; MacRae, M.; Sheppard, S.K.; Bessell, P.; Smith-Palmer, A.; Cowden, J.; Maiden, M.C.J.; Forbes, K.J.; et al. Elucidating the aetiology of human Campylobacter coli infections. PLoS ONE 2013, 8, e64504. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Re-Thinking the chicken–Campylobacter jejuni interaction: A review. Avian Pathol. 2018, 47, 352–363. [Google Scholar] [CrossRef]
- Connerton, P.L.; Richards, P.J.; Lafontaine, G.M.; O’kane, P.M.; Ghaffar, N.; Cummings, N.J.; Smith, D.L.; Fish, N.M.; Connerton, I.F. The effect of the timing of exposure to Campylobacter jejuni 6 days vs. 20. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Nyati, K.K.; Prasad, K.N.; Agrawal, V.; Husain, N. Matrix metalloproteinases-2 and -9 in Campylobacter jejuni-induced paralytic neuropathy resembling Guillain-Barré syndrome in chickens. Microb. Pathog. 2017, 111, 395–401. [Google Scholar] [CrossRef]
- Han, Z.; Willer, T.; Pielsticker, C.; Gerzova, L.; Rychlik, I.; Rautenschlein, S. Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken. Gut Pathog. 2016, 8, 1–14. [Google Scholar] [CrossRef]
- Li, X.; Swaggerty, C.L.; Kogut, M.H.; Chiang, H.I.; Wang, Y.; Genovese, K.J.; He, H.; Zhou, H. Gene expression profiling of the local cecal response of genetic chicken lines that differ in their susceptibility to Campylobacter jejuni colonization. PLoS ONE 2010, 5, e11827. [Google Scholar] [CrossRef]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karaffová, V.; Revajová, V.; Koščová, J.; Gancarčíková, S.; Nemcová, R.; Ševčíková, Z.; Herich, R.; Levkut, M., Sr. Local intestinal immune response including NLRP3 inflammasome in broiler chicken infected with Campylobacter jejuni after administration of Lactobacillus reuteri B1/1. Food Agric. Immunol. 2020, 31, 937–949. [Google Scholar] [CrossRef]
- Karaffová, V.; Marcinková, E.; Bobíková, K.; Herich, R.; Revajová, V.; Stašová, D.; Kavuľová, A.; Levkutová, M.; Levkut, M.; Lauková, A.; et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and SIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017, 62, 89–97. [Google Scholar]
- Laukova, A.; Pogany Simonova, M.; Kubasova, I.; Gancarcikova, S.; Placha, I.; Scerbova, J.; Revajova, V.; Herich, R.; Levkut, M.; Strompfova, V. Pilot experiment in chickens challenged with Campylobacter jejuni CCM6191 administered enterocin M-producing probiotic strain Enterococcus faecium CCM8558 to check its protective effect. Czech J. Anim. Sci. 2017, 62, 491–500. [Google Scholar] [CrossRef]
- Mortada, M.; Cosby, D.E.; Shanmugasundaram, R.; Selvaraj, R.K. In vivo and in vitro assessment of commercial probiotic and organic acid feed additives in broilers challenged with Campylobacter coli. J. Appl. Poult. Res. 2020, 29, 435–446. [Google Scholar] [CrossRef]
- Elhadidy, M.; Miller, W.G.; Arguello, H.; Álvarez-Ordóñez, A.; Duarte, A.; Dierick, K.; Botteldoorn, N. Genetic basis and clonal population structure of antibiotic resistance in Campylobacter jejuni isolated from broiler carcasses in Belgium. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mäesaar, M.; Meremäe, K.; Ivanova, M.; Roasto, M. Antimicrobial resistance and multilocus sequence types of Campylobacter jejuni isolated from Baltic broiler chicken meat and Estonian human patients. Poult. Sci. 2018, 97, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.; Verwoolde, M.B.; Zhang, J.; Smidt, H.; De Vries Reilingh, G.; Kemp, B.; Lammers, A. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult. Sci. 2016, 95, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genom. 2017, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef]
- Baldwin, S.; Hughes, R.J.; Van, T.T.H.; Moore, R.J.; Stanley, D. At-hatch administration of probiotic to chickens can introduce beneficial changes in gut microbiota. PLoS ONE 2018, 13, e0194825. [Google Scholar] [CrossRef]
- Huang, L.; Luo, L.; Zhang, Y.; Wang, Z.; Xia, Z. Effects of the dietary probiotic, Enterococcus Faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia Coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins 2019, 11, 946–956. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodème, M. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef]
- Šefcová, M.; Larrea-Álvarez, M.; Larrea-Álvarez, C.; Karaffová, V.; Revajová, V.; Gancarčíková, S.; Ševčíková, Z.; Herich, R. Lactobacillus fermentum Administration Modulates Cytokine Expression and Lymphocyte Subpopulation Levels in Broiler Chickens Challenged with Campylobacter coli. Foodborne Pathog. Dis. 2020, 17, 485–493. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Stašová, D.; Husáková, E.; Bobíková, K.; Karaffová, V.; Levkutová, M.; Levkut, M. Expression of cytokines in chicken peripheral blood mononuclear cells after stimulation by probiotic bacteria and Campylobacter jejuni in vitro. Food Agric. Immunol. 2015, 26, 813–820. [Google Scholar] [CrossRef]
- Lehri, B.; Seddon, A.M.; Karlyshev, A.V. Lactobacillus fermentum 3872 as a potential tool for combatting Campylobacter jejuni infections. Virulence 2017, 8, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Cobb-Vantress. Broiler Management Guide. Available online: https://cobb-vantress.com (accessed on 23 August 2020).
- Šefcová, M.; Levkut, M.; Bobíková, K.; Karaffová, V.; Revajová, V.; Cingeľová Maruščáková, I.; Levkutová, M.; Ševčíková, Z.; Herich, R.; Levkut, M. Cytokine response after stimulation of culture cells by zinc and probiotic strain. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef]
- Kolesarova, M.; Spisakova, V.; Matulova, M.; Crhanova, M.; Sisak, F.; Rychlik, I. Characterisation of basal expression of selected cytokines in the liver, spleen, and respiratory, reproductive and intestinal tract of hens. Vet. Med. 2011, 56, 325–332. [Google Scholar] [CrossRef]
- De Boever, S.; Vangestel, C.; De Backer, P.; Croubels, S.; Sys, S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008, 122, 312–317. [Google Scholar] [CrossRef]
- Bhat, I.A.; Naykoo, N.A.; Qasim, I.; Ganie, F.A.; Yousuf, Q.; Bhat, B.A.; Rasool, R.; Aziz, S.A.; Shah, Z.A. Association of interleukin 1 Beta (IL-1β) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene 2014, 2, 123–133. [Google Scholar] [CrossRef]
- Konjar, Š.; Ferreira, C.; Blankenhaus, B.; Veldhoen, M. Intestinal barrier interactions with specialized CD8 T cells. Front. Immunol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Mensikova, M.; Stepanova, H.; Faldyna, M. Interleukin-17 in veterinary animal species and its role in various diseases: A review. Cytokine 2013, 64, 11–17. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Kogut, M.H. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine 2011, 53, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lillehoj, H.S.; Lee, Y.; Bravo, D.; Lillehoj, E.P. Dietary antibiotic growth promoters down-regulate intestinal inflammatory cytokine expression in chickens challenged with LPS or co-infected with Eimeria maxima and Clostridium perfringens. Front. Vet. Sci. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, S.; Jung, S.K.; Kim, D.; Oh, K.B.; Yang, H.; Lee, H.C.; Jin, J.Y.; Sun, L.H.; Lee, S.; Byun, S.J. Oral administration of Lactobacillus reuteri expressing a 3D8 single-chain variable fragment (ScFv) enhances chicken growth and conserves immune homeostasis. 3 Biotech 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef]
- Barjesteh, N.; Hodgins, D.C.; St. Paul, M.; Quinteiro-Filho, W.M.; DePass, C.; Monteiro, M.A.; Sharif, S. Induction of chicken cytokine responses in vivo and in vitro by lipooligosaccharide of Campylobacter jejuni HS:10. Vet. Microbiol. 2013, 164, 122–130. [Google Scholar] [CrossRef]
- Sanad, Y.M.; Kwoni, J.; Kashoma, I.; Zhang, X.; Kassem, I.I.; Saif, Y.M.; Rajashekara, G. Insights into potential pathogenesis mechanisms associated with Campylobacter jejuni-induced abortion in ewes. BMC Vet. Res. 2014, 10, 1–13. [Google Scholar] [CrossRef]
- Shaughnessy, R.G.; Meade, K.G.; McGivney, A.B.; Allan, B.; O’Farrelly, C. Global gene expression analysis of chicken caecal response to Campylobacter jejuni. Vet. Immunol. Immunopathol. 2011, 142, 64–71. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens—In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Song, M.; Hu, Y.; Pan, B.; Cai, J.; Wang, M. Eimeria tenella: Interleukin 17 contributes to host immunopathology in the gut during experimental infection. Exp. Parasitol. 2013, 133, 121–130. [Google Scholar] [CrossRef]
- Fonseca, B.B.; Fernandez, H.; Rossi, D.A. (Eds.) Campylobacter spp. and Related Organisms in Poultry; Springer: Cham, Switzerland, 2016; pp. 1–206. [Google Scholar]
- Smith, C.K.; Abuoun, M.; Cawthraw, S.A.; Humphrey, T.J.; Rothwell, L.; Kaiser, P.; Barrow, P.A.; Jones, M.A. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol. Med. Microbiol. 2008, 54, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Pasmans, F.; Heyndrickx, M.; Van Immerseel, F.; Martel, A.; Van Deun, K.; Haesebrouck, F. A Tolerogenic Mucosal Immune Response Leads to Persistent Campylobacter Jejuni Colonization in the Chicken Gut. Crit. Rev. Microbiol. 2012, 38, 17–29. [Google Scholar] [CrossRef] [PubMed]
| Day of Experiment | Control (number of chickens) | LB Group (number of chickens) | Cj Group (number of chickens) | LBCj Group (number of chickens) |
|---|---|---|---|---|
| 0 d | 27 | 27 | 27 | 27 |
| 1 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 2 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 3 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 4 d | 27 | 27 L. fermentum dose 109 CFU/0.2 mL individually per os | 27 C. jejuni dose 108 CFU/0.2 mL individually per os | 27 L. fermentum dose 109 CFU/0.2 mL + C. jejuni dose 108 CFU/0.2 mL individually per os |
| 5 d (12 hpi) sample collection | 18 | 18 L. fermentum dose 109 CFU/0.2 mL individually per os | 18 | 18 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 6d (36 hpi) sample collection | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os |
| 7d (48 hpi) sample collection | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os | 9 | 9 L. fermentum dose 109 CFU/0.2 mL individually per os |
| Primer | Sequence 5′-3′ | Reference |
|---|---|---|
| IL-1β For IL-1β Rev | GAAGTGCTTCGTGCTGGAGT ACTGGCATCTGCCCAGTTC | [26] |
| IL-15 For IL-15 Rev | TGGAGCTGATCAAGACATCTG CATTACAGGTTCCTGGCATTC | [27] |
| IL-17 For IL-17 Rev | TATCAGCAAACGCTCACTGG AGTTCACGCACCTGGAATG | [26] |
| IL-18 For IL-18 Rev | ACGTGGCAGCTTTTGAAGAT GCGGTGGTTTTGTAACAGTG | [25] |
| GAPDH For GAPDH Rev | CCTGCATCTGCCCATTT GGCACGCCATCACTATC | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šefcová, M.; Larrea-Álvarez, M.; Larrea-Álvarez, C.; Revajová, V.; Karaffová, V.; Koščová, J.; Nemcová, R.; Ortega-Paredes, D.; Vinueza-Burgos, C.; Levkut, M.; et al. Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens. Vet. Sci. 2020, 7, 121. https://doi.org/10.3390/vetsci7030121
Šefcová M, Larrea-Álvarez M, Larrea-Álvarez C, Revajová V, Karaffová V, Koščová J, Nemcová R, Ortega-Paredes D, Vinueza-Burgos C, Levkut M, et al. Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens. Veterinary Sciences. 2020; 7(3):121. https://doi.org/10.3390/vetsci7030121
Chicago/Turabian StyleŠefcová, Miroslava, Marco Larrea-Álvarez, César Larrea-Álvarez, Viera Revajová, Viera Karaffová, Jana Koščová, Radomíra Nemcová, David Ortega-Paredes, Christian Vinueza-Burgos, Mikuláš Levkut, and et al. 2020. "Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens" Veterinary Sciences 7, no. 3: 121. https://doi.org/10.3390/vetsci7030121
APA StyleŠefcová, M., Larrea-Álvarez, M., Larrea-Álvarez, C., Revajová, V., Karaffová, V., Koščová, J., Nemcová, R., Ortega-Paredes, D., Vinueza-Burgos, C., Levkut, M., & Herich, R. (2020). Effects of Lactobacillus Fermentum Supplementation on Body Weight and Pro-Inflammatory Cytokine Expression in Campylobacter Jejuni-Challenged Chickens. Veterinary Sciences, 7(3), 121. https://doi.org/10.3390/vetsci7030121







