Serological Evidence of West Nile Virus in Wild Birds in Bangladesh
Abstract
1. Introduction
2. Materials and Methods
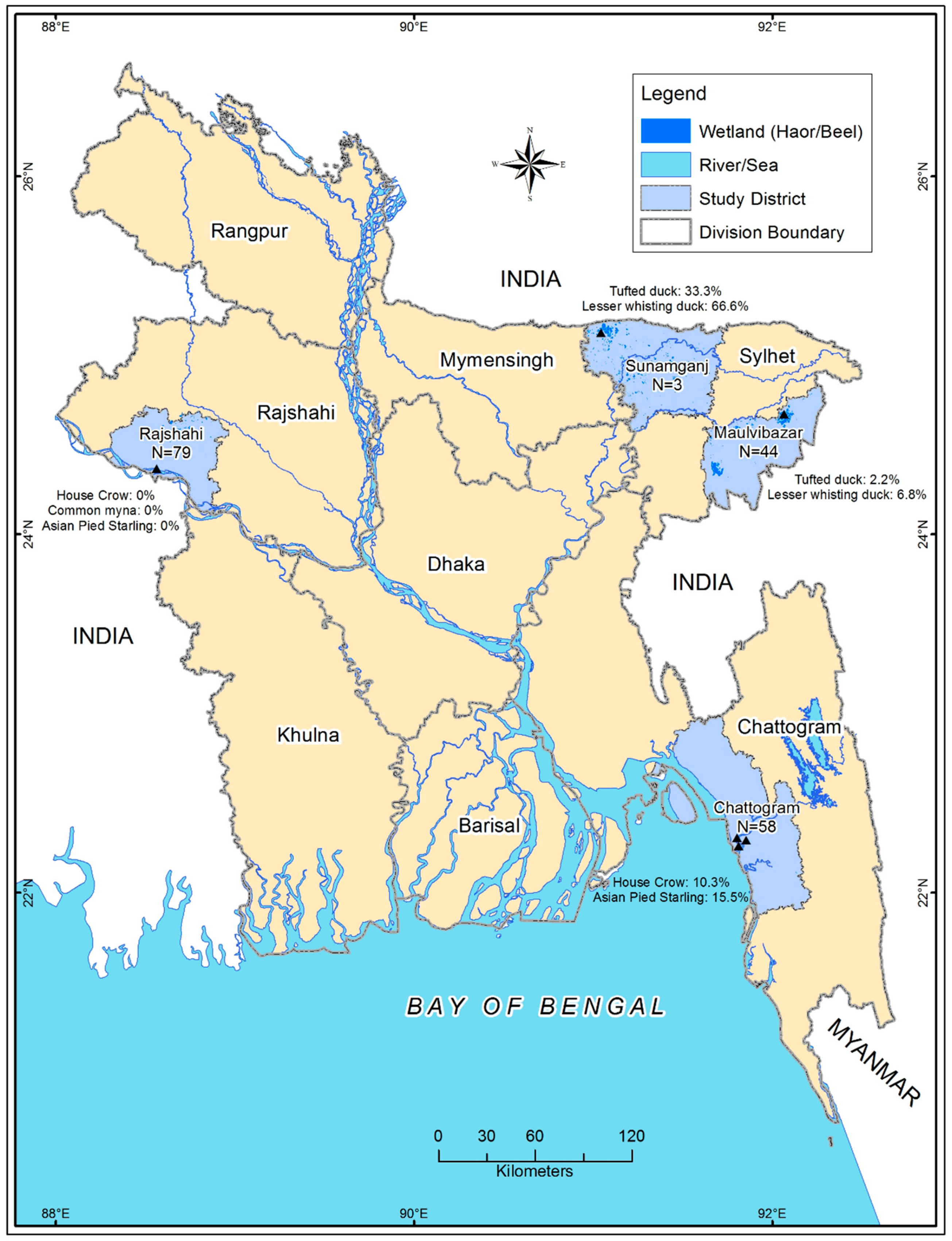
2.1. Study Time and Location
2.2. Sample Collection and Laboratory Analysis
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pradier, S.; Lecollinet, S.; Leblond, A. West Nile virus epidemiology and factors triggering change in its distribution in Europe. Rev. Sci. Tech. 2012, 31, 829–844. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Abraham, A.M. Epidemiological and clinical aspects on West Nile virus, a globally emerging pathogen. Infect. Dis. 2016, 48, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Barbachano-Guerrero, A.; Vásquez-Aguilar, A.A.; Aguirre, A.A.; Zavala-Norzagaray, A.A.; Gonzalez, E.C.; Terrazas, A.L.; Aguilar-Faisal, J.L. West nile virus prevalence in wild birds from Mexico. J. Wildl. Dis. 2019, 55, 425–431. [Google Scholar] [PubMed]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Martinez-De La Puente, J.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Soriguer, R.; Figuerola, J. Mosquito community influences West Nile virus seroprevalence in wild birds: Implications for the risk of spillover into human populations. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Michel, F.; Fischer, D.; Eiden, M.; Fast, C.; Reuschel, M.; Müller, K.; Rinder, M.; Urbaniak, S.; Brandes, F.; Schwehn, R. West Nile virus and Usutu virus monitoring of wild birds in Germany. Int. J. Environ. Res. Public Health 2018, 15, 171. [Google Scholar] [CrossRef]
- Khatun, T.; Chatterjee, S. Emergence of West Nile virus in West Bengal, India: A new report. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 178–184. [Google Scholar] [CrossRef]
- Somveille, M.; Manica, A.; Butchart, S.H.; Rodrigues, A.S. Mapping global diversity patterns for migratory birds. PLoS ONE 2013, 8, e70907. [Google Scholar] [CrossRef]
- Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Khan, S.A.; Rahman, M.K.; Järhult, J.D.; Hoque, M.A. Prevalence and Diversity of Avian Influenza Virus Hemagglutinin Sero-Subtypes in Poultry and Wild Birds in Bangladesh. Vet. Sci. 2020, 7, 73. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hoque, M.A.; Debnath, N.C.; Yamage, M.; Klaassen, M. Are poultry or wild birds the main reservoirs for avian influenza in Bangladesh? Ecohealth 2017, 14, 490–500. [Google Scholar] [CrossRef]
- Hassan, M.M. Who is the Culprit: Ecology and Epidemiology of Avian Influenza at the Wildlife-Poultry Interface in Bangladesh. Ph.D Thesis, Deakin Univeristy, Melbourne, Australia, 2017. [Google Scholar]
- Hassan, M.M.; El Zowalaty, M.E.; Islam, A.; Rahman, M.M.; Chowdhury, M.N.; Nine, H.S.; Rahman, M.K.; Järhult, J.D.; Hoque, M.A. Serological Evidence of Avian Influenza in Captive Wild Birds in a Zoo and Two Safari Parks in Bangladesh. Vet. Sci. 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S. Recent trends in the climate of Bangladesh. Clim. Res. 2010, 42, 185–193. [Google Scholar] [CrossRef]
- Montecino-Latorre, D.; Barker, C.M. Overwintering of West Nile virus in a bird community with a communal crow roost. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-Y.; Park, J.-Y.; Ostlund, E.N. Serologic evidence of West Nile Virus in wild ducks captured in major inland resting sites for migratory waterfowl in South Korea. Vet. Microbiol. 2011, 154, 96–103. [Google Scholar] [CrossRef]
- Khan, S.A.; Dutta, P.; Khan, A.M.; Chowdhury, P.; Borah, J.; Doloi, P.; Mahanta, J. West nile virus infection, Assam, India. Emerg. Infect. Dis. 2011, 17, 947. [Google Scholar] [CrossRef]
- Mishra, N.; Kalaiyarasu, S.; Nagarajan, S.; Rao, M.V.S.; George, A.; Sridevi, R.; Behera, S.P.; Dubey, S.C.; McCracken, T.; Newman, S.H. Serological evidence of West Nile virus infection in wild migratory and resident water birds in Eastern and Northern India. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 591–598. [Google Scholar] [CrossRef]
- Zohaib, A.; Saqib, M.; Beck, C.; Hussain, M.; Lowenski, S.; Lecollinet, S.; Sial, A.; Asi, M.; Mansoor, M.; Saqalein, M. High prevalence of West Nile virus in equines from the two provinces of Pakistan. Epidemiol. Infect. 2015, 143, 1931–1935. [Google Scholar] [CrossRef]
- Khan, E.; Barr, K.L.; Farooqi, J.Q.; Prakoso, D.; Abbas, A.; Khan, Z.; Ashi, S.; Imtiaz, K.; Aziz, Z.; Malik, F.; et al. Human West Nile Virus Disease Outbreak in Pakistan, 2015–2016. Front. Public Health 2018, 6, 20. [Google Scholar] [CrossRef]
- Islam, M.; Das, B.; Hossain, K.; Lucky, N.; Mostafa, M. A study on the occurrence of poultry diseases in Sylhet region of Bangladesh. Int. J. Poult. Sci. 2003, 2, 354–356. [Google Scholar]
- Elahi, R.; Islam, A.; Hossain, M.S.; Mohiuddin, K.; Mikolon, A.; Paul, S.K.; Hosseini, P.R.; Daszak, P.; Alam, M.S. Prevalence and diversity of avian haematozoan parasites in wetlands of Bangladesh. J. Parasitol. Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Hoque, M.A.; Ujvari, B.; Klaassen, M. Live bird markets in Bangladesh as a potentially important source for Avian Influenza Virus transmission. Prev. Vet. Med. 2018, 156, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Basler, C.F.; García-Sastre, A.; Palese, P. Mutation of Neuraminidase Cysteine Residues Yields Temperature-Sensitive Influenza Viruses. J. Virol. 1999, 73, 8095–8103. [Google Scholar] [CrossRef]
- Curry, P.S.; Ribble, C.; Sears, W.C.; Hutchins, W.; Orsel, K.; Godson, D.; Lindsay, R.; Dibernardo, A.; Kutz, S.J. Blood collected on filter paper for wildlife serology: Detecting antibodies to Neospora caninum, West Nile virus, and five bovine viruses in reindeer. J. Wildl. Dis. 2014, 50, 297–307. [Google Scholar] [CrossRef]
- Yildirim, Y.; Yilmaz, V.; Yazici, K.; Ozic, C.; Ozkul, A. Molecular and serological investigation of West Nile virus (WNV) infection in donkeys, horses and native geese in Turkey. Revue Méd. Vét 2018, 169, 87–92. [Google Scholar]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Lecollinet, S.; Pancewicz, S.A.; Kozdruń, W.; Czekaj, H. Occurrence of West Nile virus antibodies in wild birds, horses, and humans in Poland. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Druce, J.; Garcia, K.; Tran, T.; Papadakis, G.; Birch, C. Evaluation of Swabs, Transport Media, and Specimen Transport Conditions for Optimal Detection of Viruses by PCR. J. Clin. Microbiol. 2012, 50, 1064–1065. [Google Scholar] [CrossRef]
- Moureau, G.; Temmam, S.; Gonzalez, J.; Charrel, R.; Grard, G.; De Lamballerie, X. A real-time RT-PCR method for the universal detection and identification of flaviviruses. Vector-Borne Zoonotic Dis. 2007, 7, 467–478. [Google Scholar] [CrossRef]
- Anthony, S.J.; Islam, A.; Johnson, C.; Navarrete-Macias, I.; Liang, E.; Jain, K.; Hitchens, P.L.; Che, X.; Soloyvov, A.; Hicks, A.L. Non-random patterns in viral diversity. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Anthony, D.S.; Goldstein, D.T.; Rejmanek, D.D.; Sanchez, M.; Seimon, D.T.; Fair, D.J.; Schneider, D.B.; Epstein, D.J.; Lipkin, D.I. Laboratory Protocols for PREDICT Surveillance. PREDICT USAID Columbia Univ. Version 2013, 2, 9. [Google Scholar]
- Barros, S.C.; Ramos, F.; Fagulha, T.; Duarte, M.; Henriques, M.; Luís, T.; Fevereiro, M. Serological evidence of West Nile virus circulation in Portugal. Vet. Microbiol. 2011, 152, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; Van Der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Osa, Y.; Asakawa, M. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: Risk assessment of invasive flaviviruses. Vector-Borne Zoonotic Dis. 2009, 9, 253–258. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Feeroz, M.M.; Krauss, S.; Vogel, P.; McKenzie, P.; Webby, R.J.; Webster, R.G. Replication and pathogenic potential of influenza A virus subtypes H3, H7, and H15 from free-range ducks in Bangladesh in mammals. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Panella, N.A.; Burns, J.E.; Dusza, S.W.; Mascarenhas, T.M.; Talbot, T.O. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg. Infect. Dis. 2001, 7, 621. [Google Scholar] [CrossRef]
- Torres, A.R.; Steel, J.J.; Varian-Ramos, C.W. The Prevalence of West Nile Virus Antibodies in Blood Samples from Song Birds Collected from the Fountain Creek Region of Colorado. El Río A Stud. Res. J. 2018, 1, 39–48. [Google Scholar]
- Beveroth, T.A.; Ward, M.P.; Lampman, R.L.; Ringia, A.M.; Novak, R.J. Changes in seroprevalence of West Nile virus across Illinois in free-ranging birds from 2001 through 2004. Am. J. Trop. Med. Hyg. 2006, 74, 174–179. [Google Scholar] [CrossRef]
- Shukla, J.; Saxena, D.; Rathinam, S.; Lalitha, P.; Joseph, C.R.; Sharma, S.; Soni, M.; Rao, P.; Parida, M. Molecular detection and characterization of West Nile virus associated with multifocal retinitis in patients from southern India. Int. J. Infect. Dis. 2012, 16, e53–e59. [Google Scholar] [CrossRef]
- Dhama, K.; Mahendran, M.; Tomar, S. Pathogens transmitted by migratory birds: Threat perceptions to poultry health and production. Int. J. Poult. Sci. 2008, 7, 516–525. [Google Scholar] [CrossRef]
- Mackenzie, J.; Williams, D. The zoonotic flaviviruses of Southern, South-Eastern and Eastern Asia, and Australasia: The potential for emergent viruses. Zoonoses Public health 2009, 56, 338–356. [Google Scholar] [CrossRef]
- Murata, R.; Hashiguchi, K.; Yoshii, K.; Kariwa, H.; Nakajima, K.; Ivanov, L.I.; Leonova, G.N.; Takashima, I. Seroprevalence of West Nile virus in wild birds in far eastern Russia using a focus reduction neutralization test. Am. J. Trop. Med. Hyg. 2011, 84, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bocanegra, I.; Busquets, N.; Napp, S.; Alba, A.; Zorrilla, I.; Villalba, R.; Arenas, A. Serosurvey of West Nile virus and other flaviviruses of the Japanese encephalitis antigenic complex in birds from Andalusia, southern Spain. Vector-Borne Zoonotic Dis. 2011, 11, 1107–1113. [Google Scholar] [CrossRef]
- Ebel, G.D.; Dupuis, A.P.; II, D.N.; Young, D.; Maffei, J.; Kramer, L.D. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg. Infect. Dis. 2002, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Bowen, R.A.; Marlenee, N.L.; Hall, R.A.; Bunning, M.L.; Beaty, B.J. Epitope-blocking enzyme-linked immunosorbent assays for detection of West Nile virus antibodies in domestic mammals. J. Clin. Microbiol. 2003, 41, 2676–2679. [Google Scholar] [CrossRef] [PubMed]
- Ain-Najwa, M.Y.; Omar, A.R.; Arshad, S.S.; Abu, J.; Mohammed, H.O.; Kumar, K.; Loong, S.K.; Rovie-Ryan, J.J.; Mohd-Kharip-Shah, A.-K. Evidence of West Nile virus infection in migratory and resident wild birds in west coast of peninsular Malaysia. One Health 2020, 10, 100134. [Google Scholar] [CrossRef] [PubMed]
| Variable | Category | N | Positive n (%) | p (Fisher’s Exact) |
|---|---|---|---|---|
| District | Chattogram | 58 | 15 (25.8) | 0.00 |
| Moulavibazar | 47 | 7 (14.8) | ||
| Rajshahi | 79 | 0 (0) | ||
| Type of birds | Resident wild bird | 140 | 15 (10.7) | 0.42 |
| Migratory wild bird | 44 | 7 (15.9) | ||
| Landscape | Plain | 137 | 15 (10.9) | 0.45 |
| Wetland | 47 | 7 (14.8) | ||
| Family | ||||
| Anatidae | Tufted duck | 7 | 2 (28.5) | |
| Lesser whistling duck | 22 | 5 (22.7) | 0.05 | |
| Northern pintail | 12 | 0 | ||
| Sturnidae | Asian pied starling | 43 | 9 (20.9) | |
| Common myna | 40 | 0 | ||
| Corvidae | House crow | 48 | 6 (12.5) | |
| Columbidae | Rock pigeon | 2 | 0 | |
| Alcedinidae | White-throated kingfisher | 2 | 0 | |
| Laridae | Seagull (Gangchil) | 3 | 0 | |
| Passeridae | House sparrow | 1 | 0 | |
| Rallidae | Common moorhen | 3 | 0 | |
| Tytonidae | Barn owl | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, A.; Islam, S.; Hossain, M.E.; Ferdous, J.; Abedin, J.; Ziaur Rahman, M.; Rahman, M.K.; Hoque, M.A.; Hassan, M.M. Serological Evidence of West Nile Virus in Wild Birds in Bangladesh. Vet. Sci. 2020, 7, 164. https://doi.org/10.3390/vetsci7040164
Islam A, Islam S, Hossain ME, Ferdous J, Abedin J, Ziaur Rahman M, Rahman MK, Hoque MA, Hassan MM. Serological Evidence of West Nile Virus in Wild Birds in Bangladesh. Veterinary Sciences. 2020; 7(4):164. https://doi.org/10.3390/vetsci7040164
Chicago/Turabian StyleIslam, Ariful, Shariful Islam, Mohammad Enayet Hossain, Jinnat Ferdous, Josefina Abedin, Mohammad Ziaur Rahman, Md. Kaisar Rahman, Md. Ahasanul Hoque, and Mohammad Mahmudul Hassan. 2020. "Serological Evidence of West Nile Virus in Wild Birds in Bangladesh" Veterinary Sciences 7, no. 4: 164. https://doi.org/10.3390/vetsci7040164
APA StyleIslam, A., Islam, S., Hossain, M. E., Ferdous, J., Abedin, J., Ziaur Rahman, M., Rahman, M. K., Hoque, M. A., & Hassan, M. M. (2020). Serological Evidence of West Nile Virus in Wild Birds in Bangladesh. Veterinary Sciences, 7(4), 164. https://doi.org/10.3390/vetsci7040164







