Incidence of Gastroesophageal Reflux in Dogs Undergoing Orthopaedic Surgery or Endoscopic Evaluation of the Upper Gastrointestinal Tract
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anaesthetic Protocol
2.3. Anaesthetic Monitoring
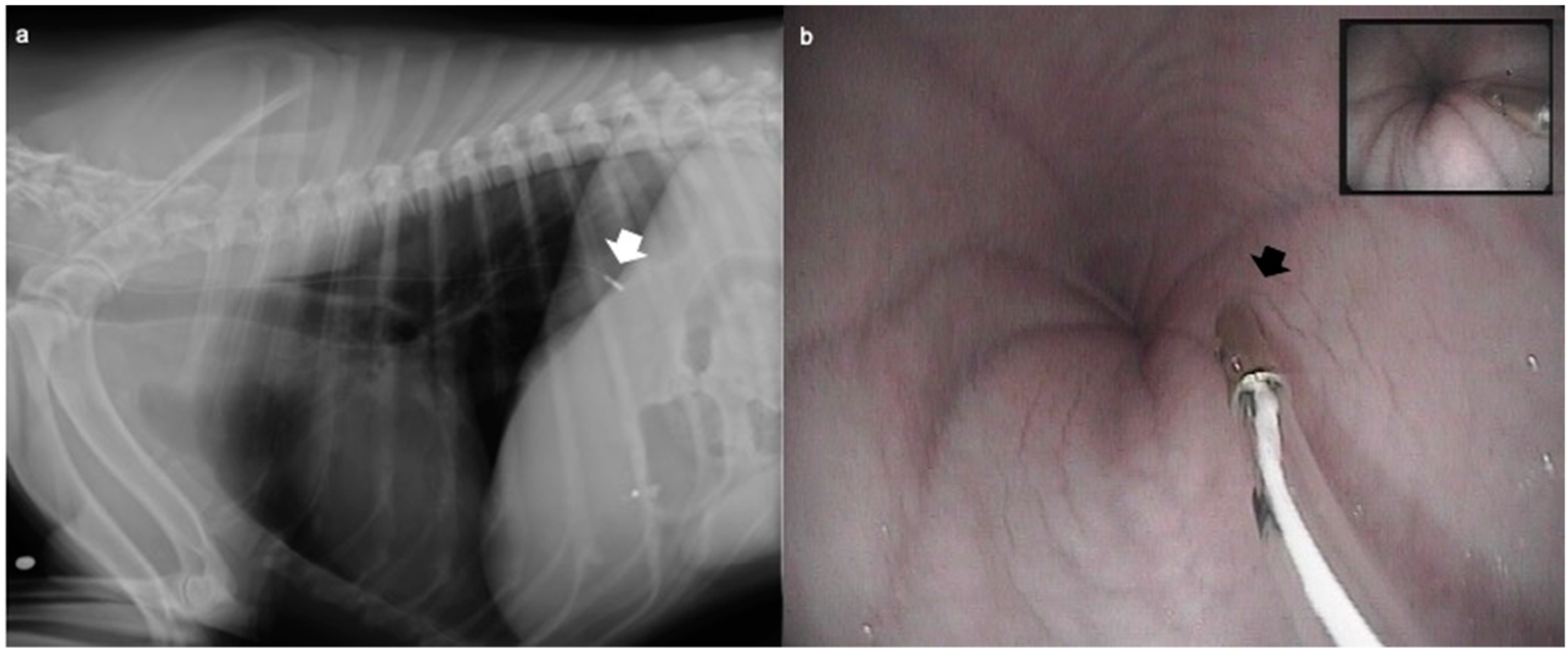
2.4. Oesophageal pH Measurement
2.5. Statistical Analysis
3. Results
3.1. Animals and Procedures
3.2. Oesophageal pH Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galatos, A.D.; Raptopoulos, D. Gastro-oesophageal reflux during anaesthesia in the dog, the effect of preoperative fasting and premedication. Vet. Rec. 1995, 137, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Galatos, A.D.; Raptopoulos, D. Gastro-oesophageal reflux during anaesthesia in the dog, the effect of age; positioning and type of surgical procedure. Vet. Rec. 1995, 137, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V.; Evans, A.T.; Miller, R. Effects of preanesthetic administration of morphine on gastroesophageal reflux and regurgitation during anesthesia in dogs. Am. J. Vet. Res. 2005, 66, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Favarato, E.S.; de Souza, M.V.; Costa, P.R.; Pompermayer, L.G.; Favarato, L.S.; Ribeiro Júnior, J.I. Ambulatory esophageal pHmetry in healthy dogs with and without the influence of general anesthesia. Vet. Res. Commun. 2011, 35, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Adamama-Moraitou, K.K.; Rallis, T.S.; Prassinos, N.N.; Galatos, A.D. Benign oesophageal stricture in the dog and cat: A retrospective study of 20 cases. Can. J. Vet. Res. 2002, 66, 55–59. [Google Scholar] [PubMed]
- Sellon, R.K.; Willard, M.D. Esophagitis and esophageal strictures. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 945–967. [Google Scholar] [CrossRef]
- Wilson, D.V.; Walshaw, R. Postanesthetic esophageal dysfunction in 13 dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 455–460. [Google Scholar] [CrossRef]
- Gawad, K.A.; Wachowiak, R.; Rempf, C.; Tiefenbacher, W.J.; Strate, T.; Achilles, E.G.; Blöchle, C.; Izbicki, J.R. Ambulatory long-term pH monitoring in pigs. Surg. Endosc. 2003, 17, 1556–1560. [Google Scholar] [CrossRef]
- Savvas, I.; Rallis, T.; Raptopoulos, D. The effect of pre-anaesthetic fasting time and type of food on gastric content volume and acidity in dogs. Vet. Anaesth. Analg. 2009, 36, 539–546. [Google Scholar] [CrossRef]
- Anagnostou, T.L.; Savvas, I.; Kazakos, G.M.; Ververidis, H.N.; Psalla, D.; Kostakis, C.; Skepastianos, P.; Raptopoulos, D. The effect of the stage of the ovarian cycle (anoestrus or dioestrus) and of pregnancy on the incidence of gastro-oesophageal reflux in dogs undergoing ovariohysterectomy. Vet. Anaesth. Analg. 2015, 42, 502–511. [Google Scholar] [CrossRef]
- Zoran, D.L. Gastroduodenoscopy in the Dog and Cat. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 631–656. [Google Scholar] [CrossRef]
- Simpson, K.W.; Jergens, A.E. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Slovak, J.; Wang, C.; Morrison, J.; Deitz, K.; LeVine, D.; Otoni, C.; King, R.; Gerber, L.; Hanson, K.; Lundberg, A.; et al. Endoscopic Assessment of the Duodenum in Dogs with Inflammatory Bowel Disease. J. Vet. Int. Med. 2014, 28, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Tams, T.R. Gastroscopy. In Small Animal Endoscopy, 3rd ed.; Tams, T.R., Rawling, C.A., Eds.; Elsevier: St. Luis, MO, USA, 2011; pp. 97–171. [Google Scholar]
- Sherding, R.G.; Johnson, S.E. Esophagoscopy. In Small Animal Endoscopy, 3rd ed.; Tams, T.R., Rawling, C.A., Eds.; Elsevier: St. Luis, MO, USA, 2011; pp. 41–95. [Google Scholar]
- Waterman, A.E.; Hashim, M.A. Measurement of the length and position of the lower oesophageal sphincter by correlation of external measurements and radiographic estimations in dogs. Vet. Rec. 1991, 129, 261–264. [Google Scholar] [CrossRef]
- Pietra, M.; Gianella, P. Bowel endoscopic examination in chronic enteropathy of dog and cat. Veterinaria (Cremona) 2018, 32, 197–208. [Google Scholar]
- Nimmo, W.S. Effect of anaesthesia on gastric motility and emptying. Br. J. Anaesth. 1984, 56, 29–36. [Google Scholar] [CrossRef]
- Hardy, J.F. Large volume gastroesophageal reflux, a rationale for risk reduction in the perioperative period. Can. J. Anaesth. 1988, 35, 162–173. [Google Scholar] [CrossRef]
- Strombeck, D.R.; Harrold, D. Effects of atropine; acepromazine; meperidine; and xylazine on gastroesophageal sphincter pressure in the dog. Am. J. Vet. Res. 1985, 46, 963–965. [Google Scholar]
- Hall, J.A.; Magne, M.L.; Twedt, D.C. Effect of acepromazine; diazepam; fentanyl-droperidol; and oxymorphone on gastroesophageal sphincter pressure in healthy dogs. Am. J. Vet. Res. 1987, 48, 556–557. [Google Scholar]
- Sternini, C.; Patierno, S.; Selmer, I.S.; Kirchgessner, A. The opioid system in the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16, 3–16. [Google Scholar] [CrossRef]
- Rattan, S.; Goyal, R.K. Identification and localization of opioid receptors in the opossum lower esophageal sphincter. J. Pharmacol. Exp. Ther. 1983, 224, 391–397. [Google Scholar] [PubMed]
- González, E.S.; Bellver, V.O.; Jaime, F.C.; Cortés, J.A.; Gil, V.G. Opioid-induced lower esophageal sphincter dysfunction. J. Neurogastroenterol. Motil. 2015, 21, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, K.; Evander, A.; Walther, B.; Skinner, D.B. Influence of morphine on the distal oesophagus and the lower oesophageal sphincter—A manometric study. Gut 1985, 26, 802–806. [Google Scholar] [CrossRef] [PubMed]
- McFadzean, W.J.; Hall, E.J.; van Oostrom, H. Effect of premedication with butorphanol or methadone on ease of endoscopic duodenal intubation in dogs. Vet. Anaesth. Analg. 2017, 44, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Critical review of butorphanol. In Dissertation 34th Meeting of the Expert Committee on Drug Dependence 4.1; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Lombardo, L.; Ruggia, O.; Crocellà, L.; Masoero, G.; Foti, M.; Mambrini, S.; Palombo, D.; Melchiorri, C.; Lupo, M.; Pera, A. Epidural plus general anesthesia vs general anesthesia alone for elective aortic surgery: Effects on gastric electrical activity and serum gastrin secretion. Minerva Anestesiol. 2009, 75, 109–115. [Google Scholar]
- Jean, G.; Cortambert, F.; Roy, P.; Foussat, C.; Moussa, M.; Dodat, H.; Bertrix, L. Reflux gastrooesophagien sous anesthésie caudale et halothane au masque chez l’enfant [Gastroesophageal reflux with combined caudal and halothane anesthesia in children]. Ann. Fr. Anesth. Reanim. 1992, 11, 3–7. [Google Scholar] [CrossRef]
- Flouraki, E.; Savvas, I.; Kazakos, G.; Anagnostou, T.; Bourgazli, A. Aspiration of gastric contents following a gastro-oesophageal reflux episode during general anaesthesia in a dog. Hell. J. Comp. Anim. Med. 2020, 9, 104–110. [Google Scholar]
- Fuchs, K.H.; DeMeester, T.R.; Albertucci, M. Specificity and sensitivity of objective diagnosis of gastroesophageal reflux disease. Surgery 1987, 36, 575–580. [Google Scholar]
- Bredenoord, A.J. Impedance-pH monitoring: New standard for measuring gastro-oesophageal reflux. Neurogastroenterol. Motil. 2008, 20, 434–439. [Google Scholar] [CrossRef]
- Silny, J. Intraluminal Multiple Electric Impedance Procedure for Measurement of Gastrointestinal Motility. Neurogastroenterol. Motil. 1991, 3, 151–162. [Google Scholar] [CrossRef]
- Frazzoni, M.; de Bortoli, N.; Frazzoni, L.; Tolone, S.; Savarino, V.; Savarino, E. Impedance-ph monitoring for diagnosis of reflux disease: New perspectives. Dig Dis. Sci. 2017, 62, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Zacuto, A.C.; Marks, S.L.; Osborn, J.; Douthitt, K.L.; Hollingshead, K.L.; Hayashi, K.; Kapatkin, A.S.; Pypendop, B.H.; Belafsky, P.C. The influence of esomeprazole and cisapride on gastroesophageal reflux during anesthesia in dogs. J. Vet. Int. Med. 2012, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.S.; Belafsky, P.C.; Della Maggiore, A.; Osborn, J.M.; Pypendop, B.H.; Pierce, T.; Walker, V.J.; Fulton, A.; Marks, S.L. Prevalence of Gastroesophageal Reflux in Cats During Anesthesia and Effect of Omeprazole on Gastric pH. J. Vet. Intern. Med. 2017, 31, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.R.; Junior, A.R.; Assis, H.M.; Campagnol, D.; Quitzan, J.G. Comparative study on the sedative effects of morphine, methadone, butorphanol or tramadol, in combination with acepromazine, in dogs. Vet. Anaesth. Analg. 2009, 36, 25–33. [Google Scholar] [CrossRef]
| Parameter | ORT | END |
|---|---|---|
| Dogs (n) | 12 | 12 |
| Age (months) | 70.7 ± 45.1 | 83.4 ± 45.1 |
| Weight (kg) | 24.7 ± 11.5 | 25.6 ± 9.4 |
| Gender (n) | 6 males/6 females | 8 males/4 females |
| Duration of anaesthesia (min) | 133.3 ± 34.6 * | 31.3 ± 9.9 * |
| Propofol (mg/kg) | 2.4 ± 0.9 | 2.3 ± 0.6 |
| Parameters | ORT | END | p Value |
|---|---|---|---|
| Animals with GER (n) | 10 (6B/4A) | 11 (9B/4A) | 0.67 (B)/0.68 (A) |
| First value (pH) | 6.8 ± 2.1 | 8.2 ± 1.5 | 0.23 |
| Minimum value (pH) | 5.3 ± 2.8 | 4.6 ± 3.3 | 0.6 |
| Maximum value (pH) | 7.5 ± 2.5 | 8.6 ± 1.4 | 0.23 |
| Maximum difference versus first value | −0.7 ± 2.5 * | −2.6 ± 3.5 * | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambertini, C.; Pietra, M.; Galiazzo, G.; Torresan, F.; Pinna, S.; Pisoni, L.; Romagnoli, N. Incidence of Gastroesophageal Reflux in Dogs Undergoing Orthopaedic Surgery or Endoscopic Evaluation of the Upper Gastrointestinal Tract. Vet. Sci. 2020, 7, 144. https://doi.org/10.3390/vetsci7040144
Lambertini C, Pietra M, Galiazzo G, Torresan F, Pinna S, Pisoni L, Romagnoli N. Incidence of Gastroesophageal Reflux in Dogs Undergoing Orthopaedic Surgery or Endoscopic Evaluation of the Upper Gastrointestinal Tract. Veterinary Sciences. 2020; 7(4):144. https://doi.org/10.3390/vetsci7040144
Chicago/Turabian StyleLambertini, Carlotta, Marco Pietra, Giorgia Galiazzo, Francesco Torresan, Stefania Pinna, Luciano Pisoni, and Noemi Romagnoli. 2020. "Incidence of Gastroesophageal Reflux in Dogs Undergoing Orthopaedic Surgery or Endoscopic Evaluation of the Upper Gastrointestinal Tract" Veterinary Sciences 7, no. 4: 144. https://doi.org/10.3390/vetsci7040144
APA StyleLambertini, C., Pietra, M., Galiazzo, G., Torresan, F., Pinna, S., Pisoni, L., & Romagnoli, N. (2020). Incidence of Gastroesophageal Reflux in Dogs Undergoing Orthopaedic Surgery or Endoscopic Evaluation of the Upper Gastrointestinal Tract. Veterinary Sciences, 7(4), 144. https://doi.org/10.3390/vetsci7040144





