Overt Infection with Chronic Bee Paralysis Virus (CBPV) in Two Honey Bee Colonies
Abstract
1. Introduction
2. Case Description
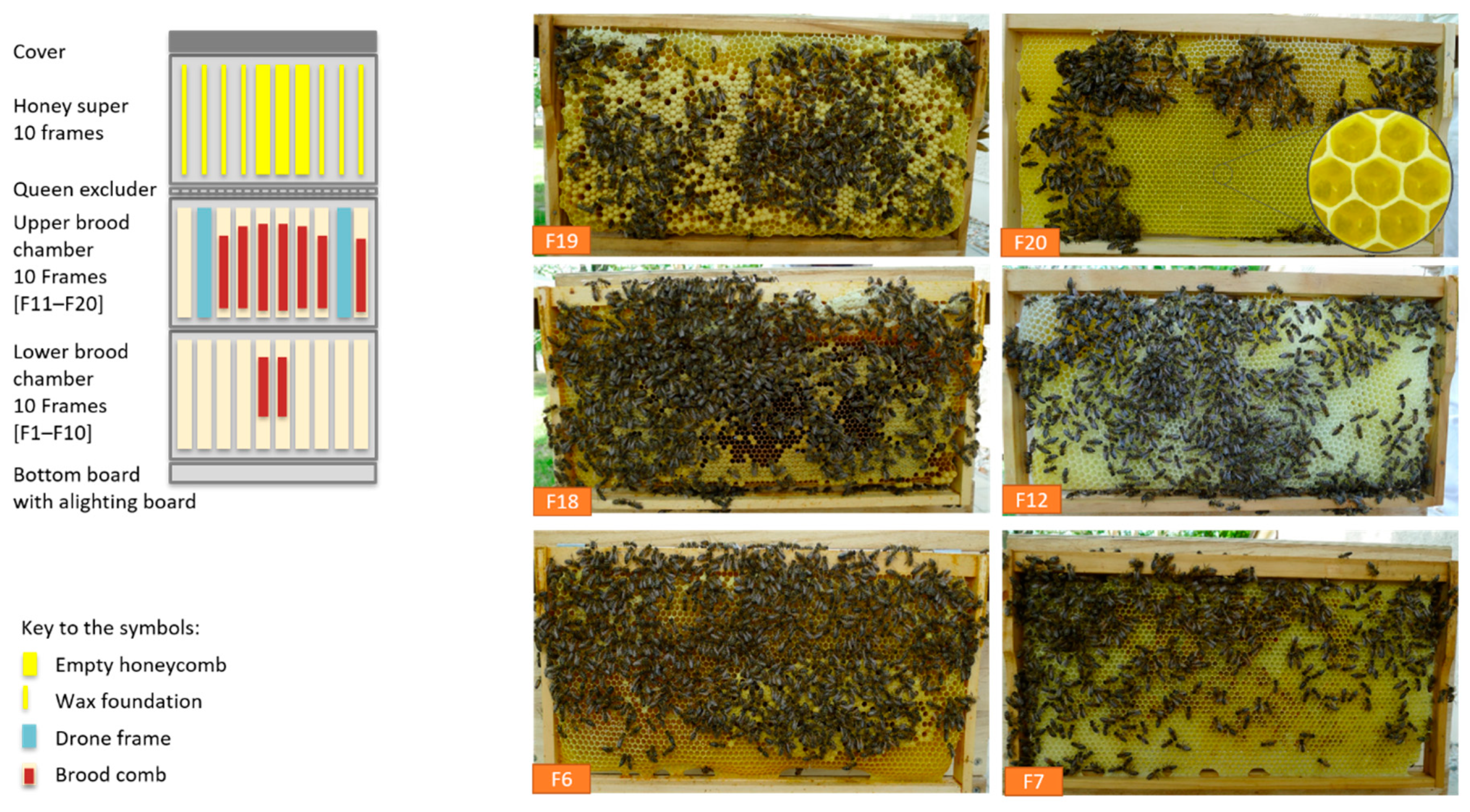
2.1. Medical History, Appearance of the Hive and Environment
2.2. Examination of the Alighting Board and Observation of the Entrance Hole
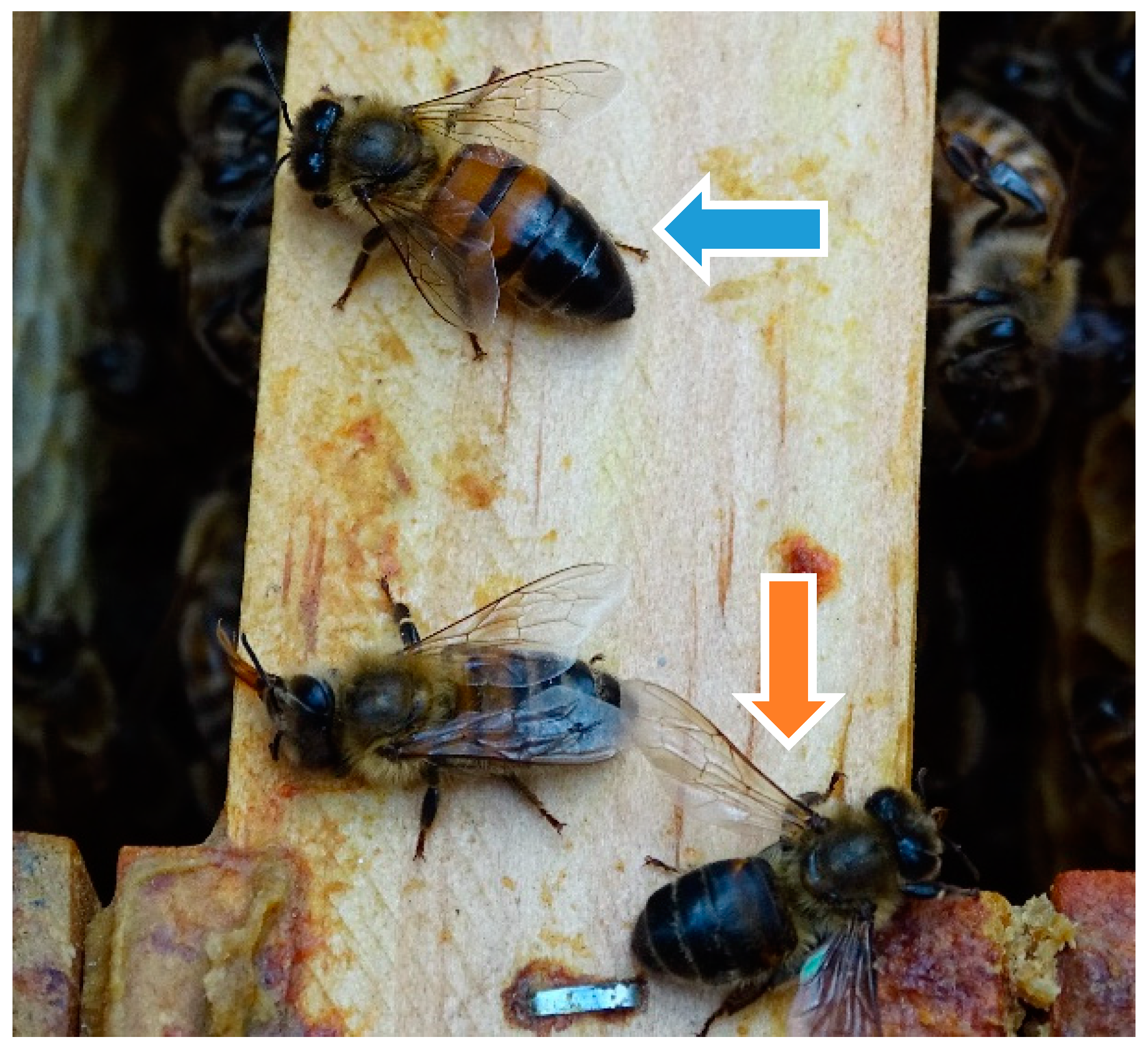
2.3. Clinical Examination of the Honey Bee Colonies and Observation of Living Bees
Examination of Dead Bees
2.4. Laboratory Diagnosis
2.4.1. PCR-Diagnosis of CBPV and Further Viruses
2.4.2. Diagnostics of Further Diseases
Monitoring the Varroa Infestation
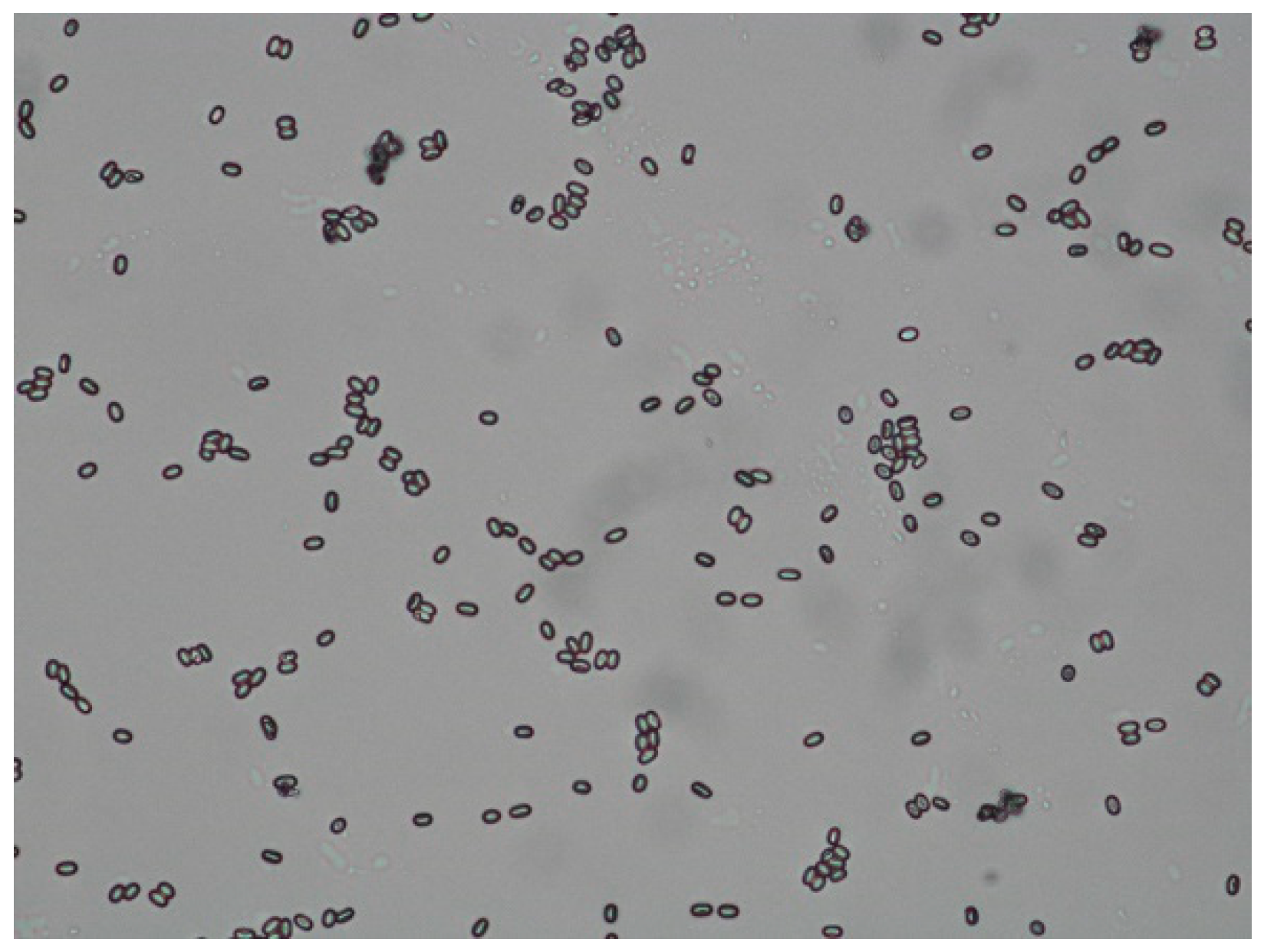
Detection of Nosemosis
2.5. List of Medical Issues and Diagnoses
2.6. Therapeutic Measures and Outcome
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Rosenkranz, P.; von der Ohe, W.; Schäfer, M.; Genersch, E.; Büchler, R.; Berg, S.; Otten, C. Zwischenbericht 2018 Deutsches Bienenmonitoring–DeBiMo Projektlaufzeit 1.1.2017–31.12.2019, Berichtszeitraum 1.1.2018–31.12.2018. Available online: https://bienenmonitoring.uni-hohenheim.de/88584?tx_ttnews%5Btt_news%5D=44864&cHash=b943160d769aa565de89b191d468bd9e (accessed on 11 June 2020).
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Celle, O.; Blanchard, P.; Olivier, V.; Schurr, F.; Cougoule, N.; Faucon, J.-P.; Ribiere, M. Detection of Chronic bee paralysis virus (CBPV) genome and its replicative RNA form in various hosts and possible ways of spread. Virus Res. 2008, 133, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Two viruses from adult honey bees (Apis mellifera Linnaeus). Virology 1963, 21, 390–395. [Google Scholar] [CrossRef]
- Aristotle. Aristotle´s History of Animals in Ten Books; Cresswell, R., Translator; George Bell & Sons: London, UK, 1902; pp. 262–263. [Google Scholar]
- Amiri, E.; Meixner, M.; Büchler, R.; Kryger, P. Chronic bee paralysis virus in honeybee queens: Evaluating susceptibility and infection routes. Viruses 2014, 6, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Olivier, V.; Blanchard, P. Chronic bee paralysis: A disease and a virus like no other? J. Invertebr. Pathol. 2010, 103, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Rinderer, T.E.; Rothenbuhler, W.C. The fate and effects of hairs removed from honeybees with hairless-black syndrome. J. Invertebr. Pathol. 1975, 26, 305–308. [Google Scholar] [CrossRef]
- Bailey, L. Paralysis of the honey bee, Apis mellifera Linnaeus. J. Invertebr. Pathol. 1965, 7, 132–140. [Google Scholar] [CrossRef]
- Budge, G.E.; Simcock, N.K.; Holder, P.J.; Shirley, M.D.F.; Brown, M.A.; Van Weymars, P.S.M.; Evans, D.J.; Rushton, S.P. Chronic bee paralysis as a serious emerging threat to honey bees. Nat. Commun. 2020, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Rennich, K.; Forsgren, E.; Rose, R.; Pettis, J.; Kunkel, G.; Madella, S.; Evans, J.; Lopez, D.; van Engelsdorp, D. Multiyear survey targeting disease incidence in US honey bees. Apidologie 2016, 47, 325–347. [Google Scholar] [CrossRef]
- Porrini, C.; Mutinelli, F.; Bortolotti, L.; Granato, A.; Laurenson, L.; Roberts, K.; Gallina, A.; Silvester, N.; Medrzycki, P.; Renzi, T.; et al. The status of honey bee health in italy: Results from the nationwide bee monitoring network. PLoS ONE 2016, 11, e0155411. [Google Scholar] [CrossRef] [PubMed]
- Imdorf, A.; Buehlmann, G.; Gerig, L.; Klichenmann, V.; Wille, H. Überprüfung der schätzmethode zur ermitllung der brutfläche und der anzahl arbeiterinnen in freifliegenden bienenvölkern. Apidologie 1987, 18, 137–146. [Google Scholar] [CrossRef]
- Deutsches Bienenjournal: Bienenvolk Schätzen: Volksstärken Genau Erfassen (20 Oktober 2016). Available online: https://www.bienenjournal.de/imkerpraxis/ratgeber/volksstaerken-erfassen-quiz/ (accessed on 24 May 2020).
- Dittes, J.; Schäfer, M.O.; Aupperle-Lellbach, H.; Mülling, C.K.W.; Emmerich, I.U. Veterinary diagnostic approach of common virus diseases in adult honey bees. Vet. Sci. 2020, in press. [Google Scholar]
- Blanchard, P.; Ribière, M.; Celle, O.; Lallemand, P.; Schurr, F.; Olivier, V.; Iscache, A.L.; Faucon, J.P. Evaluation of a real-time two-step RT-PCR assay for quantitation of Chronic bee paralysis virus (CBPV) genome in experimentally-infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods 2007, 141, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Naquet, N. Honeybee Veterinary Medicine: Apis mellifera L., 1st ed.; 5m Books Ltd.: Sheffield, UK, 2015; pp. 71–75. [Google Scholar]
- Ritter, W. Diagnostik und Bekämpfung der Bienenkrankheiten, 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1996; p. 51. [Google Scholar]
- Burnside, C.E. Preliminary Observations on “Paralysis” of Honeybees. J. Econ. Entomol. 1933, 26, 162–168. [Google Scholar] [CrossRef]
- Burnside, C.E. The cause of paralysis of bees. Am. BeeJ. 1945, 85, 354–355. [Google Scholar]
- Deutschlandwetter im Mai 2019. Available online: https://www.dwd.de/DE/presse/pressemitteilungen/DE/2019/20190529_deutschlandwetter_mai_news.hthtm (accessed on 29 May 2019).
- Ribière, M.; Lallemand, P.; Iscache, A.-L.; Schurr, F.; Celle, O.; Blanchard, P.; Olivier, V.; Faucon, J.P. Spread of infectious chronic bee paralysis virus by honeybee (Apis mellifera L.) feces. Appl. Environ. Microbiol. 2007, 73, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Toplak, I.; Ciglenečki, U.J.; Aronstein, K.; Gregorc, A. Chronic bee paralysis virus and nosema ceranae experimental co-infection of winter honey bee workers. Viruses 2013, 5, 2282–2297. [Google Scholar] [CrossRef] [PubMed]



| Virus | Titres for Colony 1 | Titres for Colony 2 |
|---|---|---|
| CBPV | ++++ (Ct = 13.45) | ++++ (Ct = 13.08) |
| DWV-B | +++ (Ct = 25.31) | +++ (Ct = 23.10) |
| BQCV | ++++ (Ct = 18.27) | +++ (Ct = 27.56) |
| ABPV | + (Ct = 34.65) | - (N/A) |
| Plastic Drawer | Natural Mite Fall | |
|---|---|---|
| Into the Hive | Out of the Hive | |
| 23 July | 25 July | 0m/2d → 0m/d |
| 16 August | 19 August | 0m/3d → 0m/d |
| 11 September | 13 September | 6m/2d → 3m/d |
| 25 November | 28 November | 0m/3d → 0m/d |
| Colony 1 | Colony 2 |
|---|---|
for both colonies:
| |
| moderate infestation of Nosema spores | slight infestation of Nosema spores |
| loss of the queen | |
| laying worker bees and large number of drones | |
| other viruses (without symptoms): DWV-B, BQCV, ABPV (exact Cts in Table 1) | other viruses (without symptoms): DWV-B, BQCV, ABPV (exact Cts in Table 1) |
| Date | Applied Measures/Methods and Development of Colony 1 |
|---|---|
| 29 May 2019 | removal of the old queen from the hive, bees start raising a new queen |
| 06 June 2019 | destruction of all of the queen cells, insertion of a queen cage with the new queen (Nr. 71, marked green) and some food, gentle acclimatization while workers gnaw through the food |
| 18 June 2019 | hive check: many eggs and young brood in the brood combs, queen Nr. 71 with a larger abdomen |
| 19 June 2019 | shook swarm method: migration to a new hive body with new pathogen free frames and wax foundations, protection of the queen in a queen cage, bees shaken off in front of the new hive onto a base to enter the new hive by themselves. |
| 21 June 2019 | treatment with 5.7% Oxalic acid dihydrate (OXUVAR® 5.7%, Andermatt Biovet GmbH, Lörrach, BW, D)) diluted to a 3.5% solution by spraying; Feeding with sugar syrup (2 kg sugar) |
| 25 June 2019 | decreased symptoms of trembling and paralysis or black abdomens |
| 28 June 2019 | feeding with sugar syrup (2 kg sugar) |
| 1 July 2019 | feeding with sugar syrup (2 kg sugar) |
| 17 July 2019 | feeding with sugar syrup (2 kg sugar) |
| 21 August 2019 | feeding with sugar syrup (2 kg sugar) |
| 16 September 2019 | late summer treatment with formic acid (Ameisensäure 60% ad us. vet., Serumwerk Bernburg AG, Bernburg, ST, D) after the last honey harvest: evaporation of 140 mL of formic acid in the hive using a Liebig Dispenser; observed–mite load (Table 1): about 3 mites per day = medium infestation rate [18] |
| October 2019–April 2020 | overwintering of the colony with only one brood chamber, no application of a winter treatment with oxalic acid because of a low mite infestation rate in November (Table 1) |
| 12 April 2020 | successful hibernation; enlargement of the hive with a second brood chamber. |
| 28 April 2020 | brood nest out of 9 brood combs during apple blossom |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittes, J.; Schäfer, M.O.; Aupperle-Lellbach, H.; Mülling, C.K.W.; Emmerich, I.U. Overt Infection with Chronic Bee Paralysis Virus (CBPV) in Two Honey Bee Colonies. Vet. Sci. 2020, 7, 142. https://doi.org/10.3390/vetsci7030142
Dittes J, Schäfer MO, Aupperle-Lellbach H, Mülling CKW, Emmerich IU. Overt Infection with Chronic Bee Paralysis Virus (CBPV) in Two Honey Bee Colonies. Veterinary Sciences. 2020; 7(3):142. https://doi.org/10.3390/vetsci7030142
Chicago/Turabian StyleDittes, Julia, Marc O. Schäfer, Heike Aupperle-Lellbach, Christoph K. W. Mülling, and Ilka U. Emmerich. 2020. "Overt Infection with Chronic Bee Paralysis Virus (CBPV) in Two Honey Bee Colonies" Veterinary Sciences 7, no. 3: 142. https://doi.org/10.3390/vetsci7030142
APA StyleDittes, J., Schäfer, M. O., Aupperle-Lellbach, H., Mülling, C. K. W., & Emmerich, I. U. (2020). Overt Infection with Chronic Bee Paralysis Virus (CBPV) in Two Honey Bee Colonies. Veterinary Sciences, 7(3), 142. https://doi.org/10.3390/vetsci7030142





