A Preliminary Study of the Cross-Reactivity of Canine MAGE-A with Hominid Monoclonal Antibody 6C1 in Canine Mammary Gland Tumors: An Attractive Target for Cancer Diagnostic, Prognostic and Immunotherapeutic Development in Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Immunohistochemistry
2.3. Protein Extraction
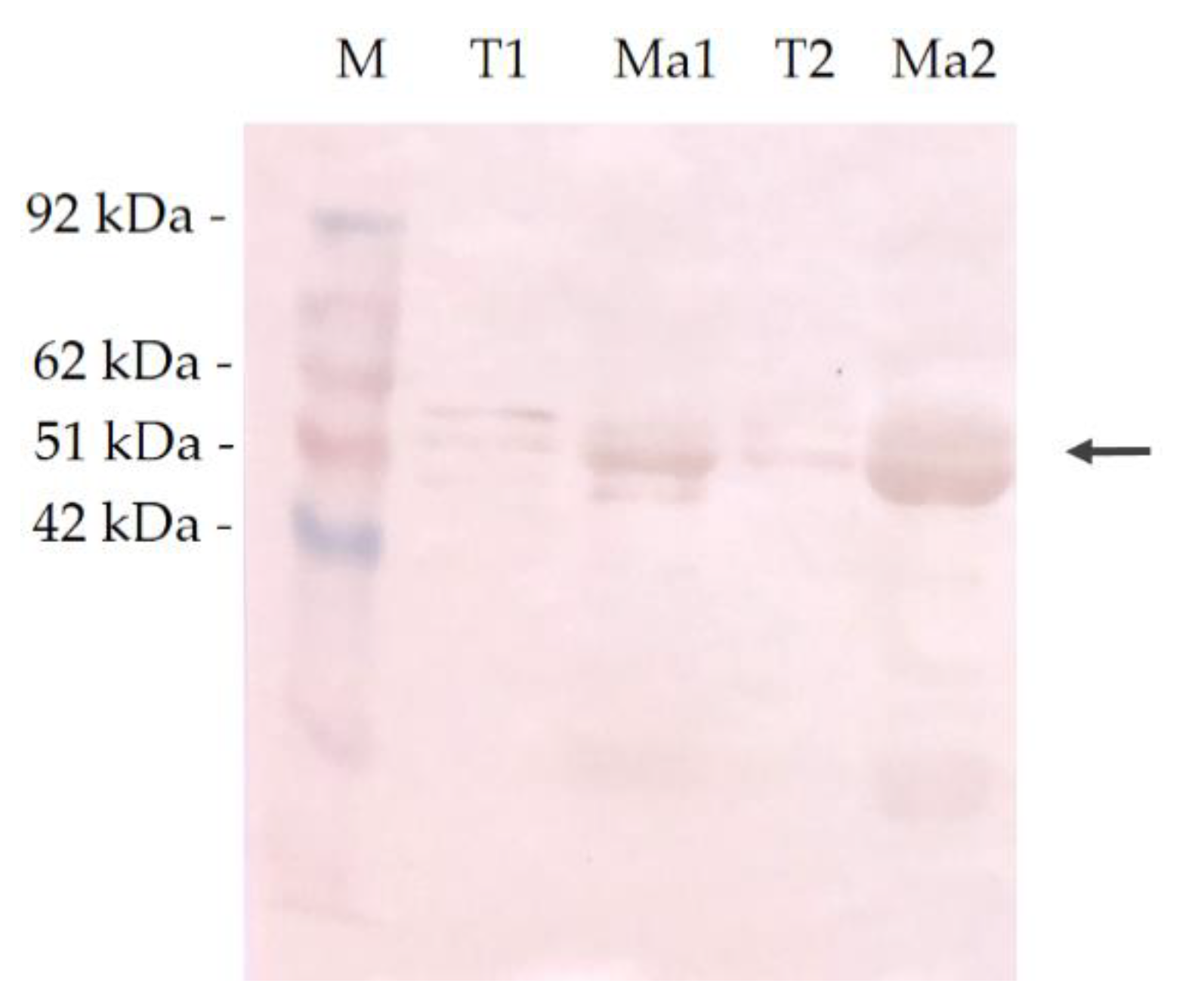
2.4. SDS-PAGE and Western Immunoblotting Analysis
2.5. Data Analysis
3. Results
3.1. CMT Classification and Grading
3.2. Canine MAGE-A Antigens Recognized by Human MAb 6C1
3.2.1. MAGE-A Antigens Recognized by MAb 6C1 in Canine Testicular Tissue
3.2.2. MAGE-A Antigens Recognized by MAb 6C1 in CMT Tissues
3.3. Molecular Mass and Immunoreactivity of Canine MAGE-A Antigen
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statements
References
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: A growing animal health problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018, 15, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Lian, Y.; Zhou, X.; Shan, B. MAGE-A family: Attractive targets for cancer immunotherapy. Vaccine 2011, 29, 8496–8500. [Google Scholar] [CrossRef] [PubMed]
- Schooten, E.; Di Maggio, A.D.; Van Bergen En Henegouwen, P.M.P.; Kijanka, M.M. MAGE-A antigens as targets for cancer immunotherapy. Cancer Treat. Rev. 2018, 67, 54–62. [Google Scholar] [CrossRef]
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar] [CrossRef]
- Barker, P.A.; Salehi, A. The MAGE proteins: Emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J. Neurosci. Res. 2002, 67, 705–712. [Google Scholar] [CrossRef]
- Caballero, O.L.; Chen, Y.T. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009, 100, 2014–2021. [Google Scholar] [CrossRef]
- Beumer, T.L.; Roepers-Gajadien, H.L.; Gademan, I.S.; Van Buul, P.P.W.; Gil-Gomez, G.; Rutgers, D.H.; De Rooij, D.G. The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ. 1998, 5, 669–677. [Google Scholar] [CrossRef]
- Vamolri, D.; Gildaei, U.; Servis, C.; Dunbar, P.R.; Cerottini, J.; Romeo, P.; Cerudoro, V.; Lévy, F. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide Derived from the Tumor Antigen MAGE-3. J. Exp. Med. 1999, 189, 895–906. [Google Scholar]
- Duffour, M.T.; Chaux, P.; Lurquin, C.; Cornelis, G.; Boon, T.; Van der Bruggen, P. A MAGE-A4 peptide presented by HLA-A2 is recognized by cytolytic T lymphocytes. Eur. J. Immunol. 1999, 29, 3329–3337. [Google Scholar] [CrossRef]
- Pascolo, S.; Schirle, M.; Gückel, B.; Dumrese, T.; Stumm, S.; Kayser, S.; Moris, A.; Wallwiener, D.; Rammensee, H.G.; Stevanovic, S. A MAGE-A1 HLA-A A*0201 epitope identified by mass spectrometry. Cancer Res. 2001, 61, 4072–4077. [Google Scholar] [PubMed]
- Ottaviani, S.; Colau, D.; Van der Bruggen, P.; Van der Bruggen, P. A new MAGE-4 antigenic peptide recognized by cytolytic T lymphocytes on HLA-A24 carcinoma cells. Cancer Immunol. Immun. 2006, 55, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, N.; Wargo, J.A.; Yu, Z.; Rao, M.; Frankel, T.L.; Riley, J.P.; Hong, J.J.; Parkhurst, M.R.; Feldman, S.A.; Schrump, D.S.; et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol. 2011, 186, 685–696. [Google Scholar] [CrossRef]
- Kuramoto, T. Detection of MAGE-1 tumor antigen in brain tumor. Kurume Med. J. 1997, 44, 43–51. [Google Scholar] [CrossRef]
- Sugita, M.; Geraci, M.; Gao, B.; Powell, R.L.; Hirsch, F.R.; Johnson, G.; Lapadat, R.; Gabrielson, E.; Bremnes, R.; Bunn, P.A.; et al. Combined use of oligonucleotide and tissue microarrays identifies cancer/testis antigens as biomarkers in lung carcinoma. Cancer Res. 2002, 62, 3971–3979. [Google Scholar] [PubMed]
- Chen, Y.T.; Ross, D.S.; Chiu, R.; Zhou, X.K.; Chen, Y.Y.; Lee, P.; Hoda, S.A.; Simpson, A.J.; Old, L.J.; Caballero, O.; et al. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS ONE 2011, 6, e17876. [Google Scholar] [CrossRef]
- Curigliano, G.; Viale, G.; Ghioni, M.; Jungbluth, A.A.; Bagnardi, V.; Spagnoli, G.C.; Neville, A.M.; Nolè, F.; Rotmensz, N.; Goldhirsch, A. Cancer-testis antigen expression in triple-negative breast cancer. Ann. Oncol. 2011, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ayyoub, M.; Scarlata, C.M.; Hamaï, A.; Pignon, P.; Valmori, D. Expression of MAGE-A3/6 in primary breast cancer is associated with hormone receptor negative status, high histologic grade, and poor survival. J. Immunother. Cancer 2014, 37, 73–76. [Google Scholar] [CrossRef]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. Suppl. 2001, 57, 439–443. [Google Scholar]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef]
- Bronson, R.T. Variation in age at death of dogs of different sexes and breeds. Am. J. Vet. Res. 1982, 43, 2057–2059. [Google Scholar] [PubMed]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Ani. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, T.; Kobayashi, T.; Takahashi, K.; Sugiyama, M. Influence of ovariectomy at the time of mastectomy on the prognosis for canine malignant mammary tumours. J. Small Ani. Pract. 1996, 37, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Histological grading and prognosis in dogs with mammary carcinomas: Application of a human grading method. J. Comp. Pathol. 2005, 133, 246–252. [Google Scholar] [CrossRef]
- Stratmann, N.; Failing, K.; Richter, A.; Wehrend, A. Mammary tumor recurrence in bitches after regional mastectomy. Vet. Sur. 2008, 37, 82–86. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Adjuvant post-operative chemotherapy in bitches with mammary cancer. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 85–96. [Google Scholar] [CrossRef]
- Simon, D.; Schoenrock, D.; Baumgärtner, W.; Nolte, I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J. Vet. Int. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef]
- Marconato, L.; Lorenzo, R.M.; Abramo, F.; Ratto, A.; Zini, E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet. Comp. Oncol. 2008, 6, 90–101. [Google Scholar] [CrossRef]
- Lavalle, G.E.; De Campos, C.B.; Bertagnolli, A.C.; Cassali, G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo 2012, 26, 375–379. [Google Scholar]
- Estrela-Lima, A.; Araújo, M.S.S.; Costa-Neto, J.M.; Teixeira-Carvalho, C.; Barrouin-Melo, M.; Cardoso, S.V.; Martins-Filho, O.A.; Serakides, R.; Cassali, G.D. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer 2010, 10, 256. [Google Scholar] [CrossRef]
- Kim, J.H.; Chon, S.K.; Im, K.S.; Kim, N.H.; Cho, K.W.; Sur, J.H. Correlation of Foxp3 positive regulatory T cells with prognostic factors in canine mammary carcinomas. Vet. J. 2012, 193, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar]
- Schneider, R. Comparison of age, sex, and incidence rates in human and canine breast cancer. Cancer 1970, 26, 419–426. [Google Scholar] [CrossRef]
- Mottolese, M.; Morelli, L.; Agrimi, U.; Benevolo, M.; Sciarretta, F.; Antonucci, G.; Natali, P.G. Spontaneous canine mammary tumors. A model for monoclonal antibody diagnosis and treatment of human breast cancer. Lab. Investig. 1994, 71, 182–187. [Google Scholar]
- Sternberger, L.A.; Hardy, P.H., Jr.; Cuculis, J.J.; Meyer, H.G. The unlabeled antibody enzyme method of immunohistochemistry: Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J. Histochem. Cytochem. 1970, 18, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Misdorp, W.; Else, R.W.; Hellmén, E.; Lipscomb, T.P. Histological Classification of Mammary Tumors of the Dogs and the Cats, 2nd ed.; Armed Forces Institute of Pathology: Washington, DC, USA, 1999. [Google Scholar]
- Misdorp, W. Tumor of the mammary gland. In Tumors in Domestic Animals, 4th ed.; Meuten, D.J., Ed.; Iowa State Press: Ames, Iowa, 2002. [Google Scholar]
- Chen, Y.C.; Hsu, W.L.; Chiu, C.Y.; Liao, J.W.; Chang, C.C.; Chang, S.C. Expression of MAGE--A restricted to testis and ovary or to various cancers in dogs. Vet. Immunol. Immunopathol. 2013, 153, 26–34. [Google Scholar] [CrossRef]
- Rimoldi, D.; Salvi, S.; Schultz-Thater, E.; Spagnoli, G.C.; Cerottini, J.C. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int. J. Cancer 2000, 86, 749–751. [Google Scholar] [CrossRef]
- Guo, H.; Liu, W.; Ju, Z.; Tamboli, P.; Jonasch, E.; Mills, G.B.; Lu, Y.; Hennessy, B.T.; Tsavachidou, D. An efficient procedure for protein extraction from formalin-fixed, paraffin-embedded tissues for reverse phase protein arrays. Proteome Sci. 2012, 10, 56. [Google Scholar] [CrossRef]
- Van Diest, P.J.; Van Dam, P.; Henzen-Logmans, S.C.; Berns, E.; Van der Burg, M.E.; Green, J.; Vergote, I. A scoring system for immunohistochemical staining: Consensus report of the task force for basic research of the EORTC-GCCG. European organization for research and treatment of cancer-gynaecological cancer cooperative group. J Clin Pathol. 1997, 50, 801–804. [Google Scholar] [CrossRef]
- Fiszer, D.; Kurpisz, M. Major histocompatibility complex expression on human, male germ cells: A review. Am. J. Reprod. Immunol. 1998, 40, 172–176. [Google Scholar] [CrossRef]
- Addissie, S.; Klingemann, H. Cellular Immunotherapy of canine cancer. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef]
- Takahashi, K.; Shichijo, S.; Noguchi, M.; Hirohata, M.; Itoh, K. Identification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res. 1995, 55, 3478–3482. [Google Scholar] [PubMed]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Marcar, L.; MacLaine, N.J.; Hupp, T.R.; Meek, D.W. Mage-A cancer/testis antigens inhibit p53 function by blocking its interaction with chromatin. Cancer Res. 2010, 70, 10362–10369. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Meng, L.; Ding, P.; Sang, M. Epigenetic regulation of MAGE family in human cancer progression-DNA methylation, histone modification, and non-coding RNAs. Clin. Epigenetics 2018, 10, 115. [Google Scholar] [CrossRef]
- Monte, M.; Simonatto, M.; Peche, L.Y.; Bublik, D.R.; Gobessi, S.; Pierotti, M.A.; Rodolfo, M.; Schneider, C. MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. Proc. Natl. Acad. Sci. USA 2006, 103, 11160–11165. [Google Scholar] [CrossRef]
- Marcar, L.; Ihrig, B.; Hourihan, J.; Bray, S.E.; Quinlan, P.R.; Jordan, L.B.; Thompson, A.M.; Hup, T.R.; Meek, D.W. MAGE-A cancer/testis antigens inhibit MDM2 ubiquitylation function and promote increased levels of MDM4. PLoS ONE 2015, 10, e0127713. [Google Scholar] [CrossRef]
- Su, S.; Minges, J.T.; Grossman, G.; Blackwelder, A.J.; Mohler, J.L.; Wilson, E.M. Proto-oncogene activity of melanoma antigen-A11 (MAGE-A11) regulates retinoblastoma-related p107 and E2F1 proteins. J. Biol. Chem. 2013, 288, 24809–24824. [Google Scholar] [CrossRef]
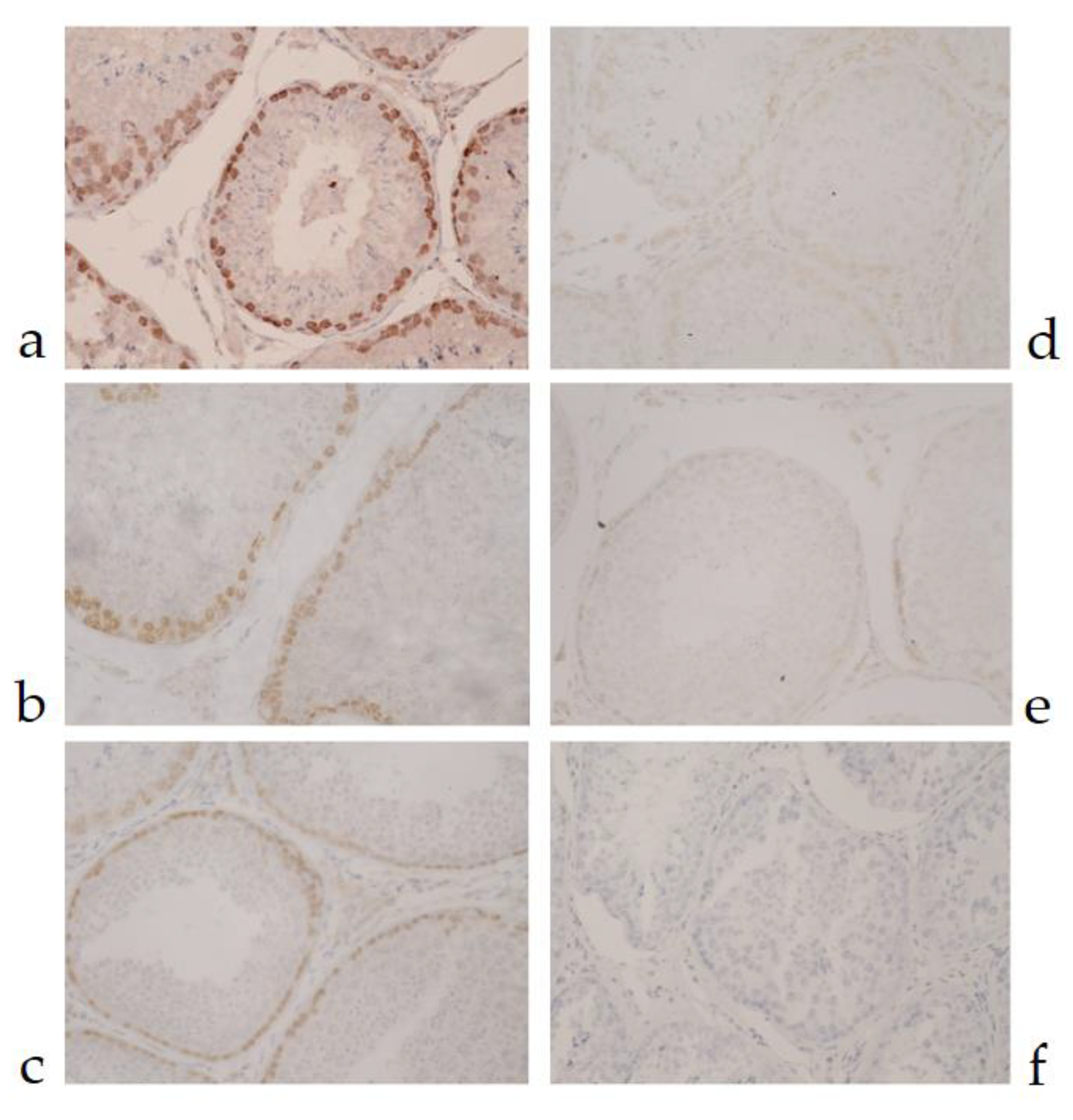
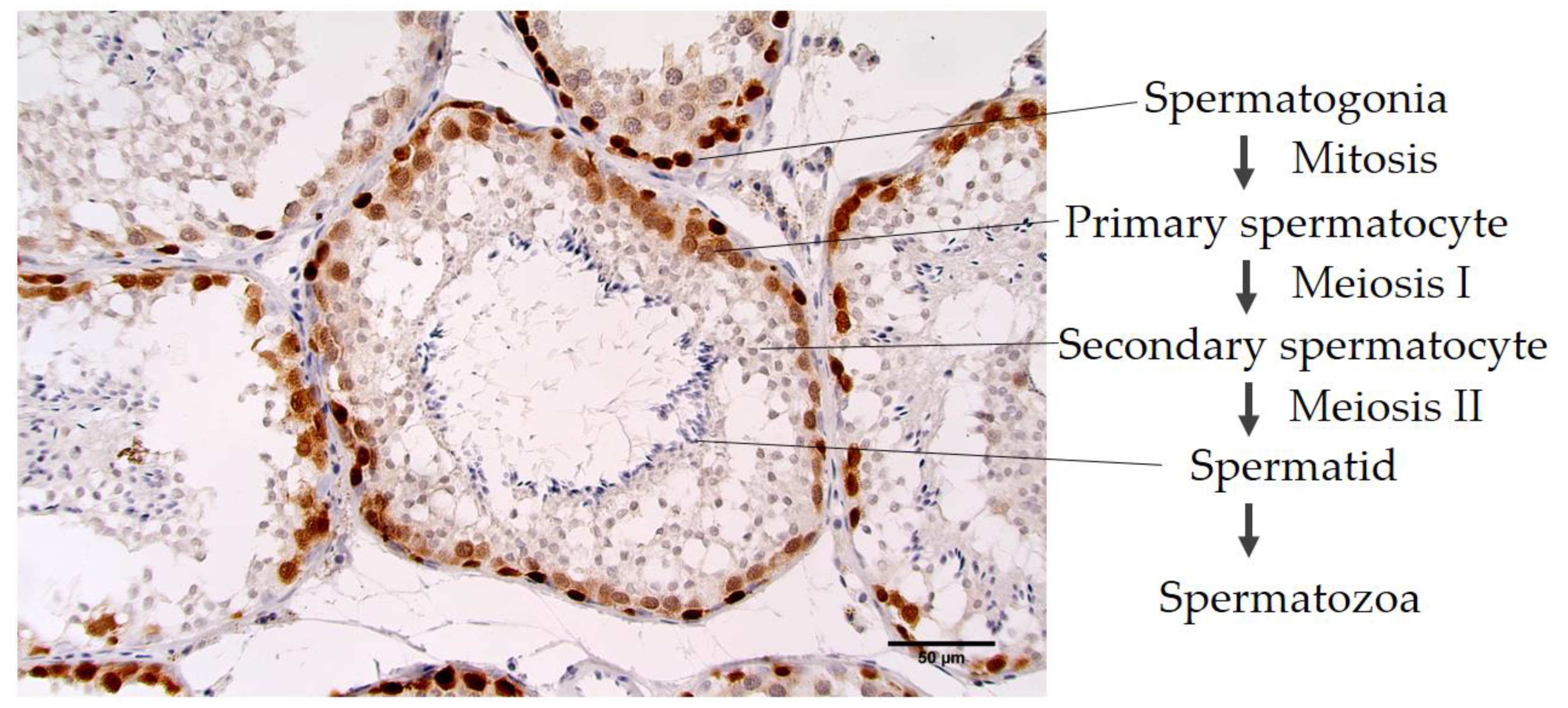
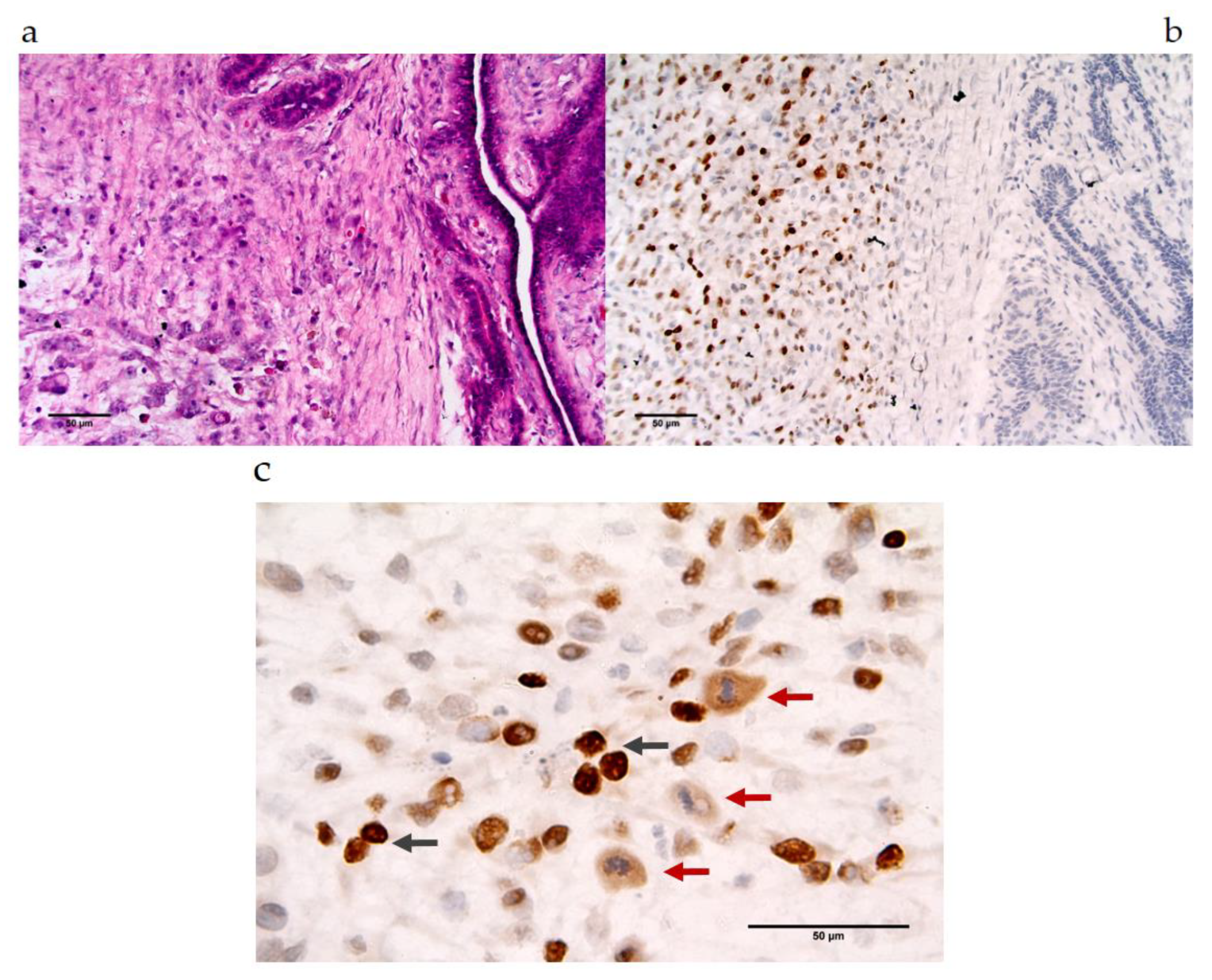
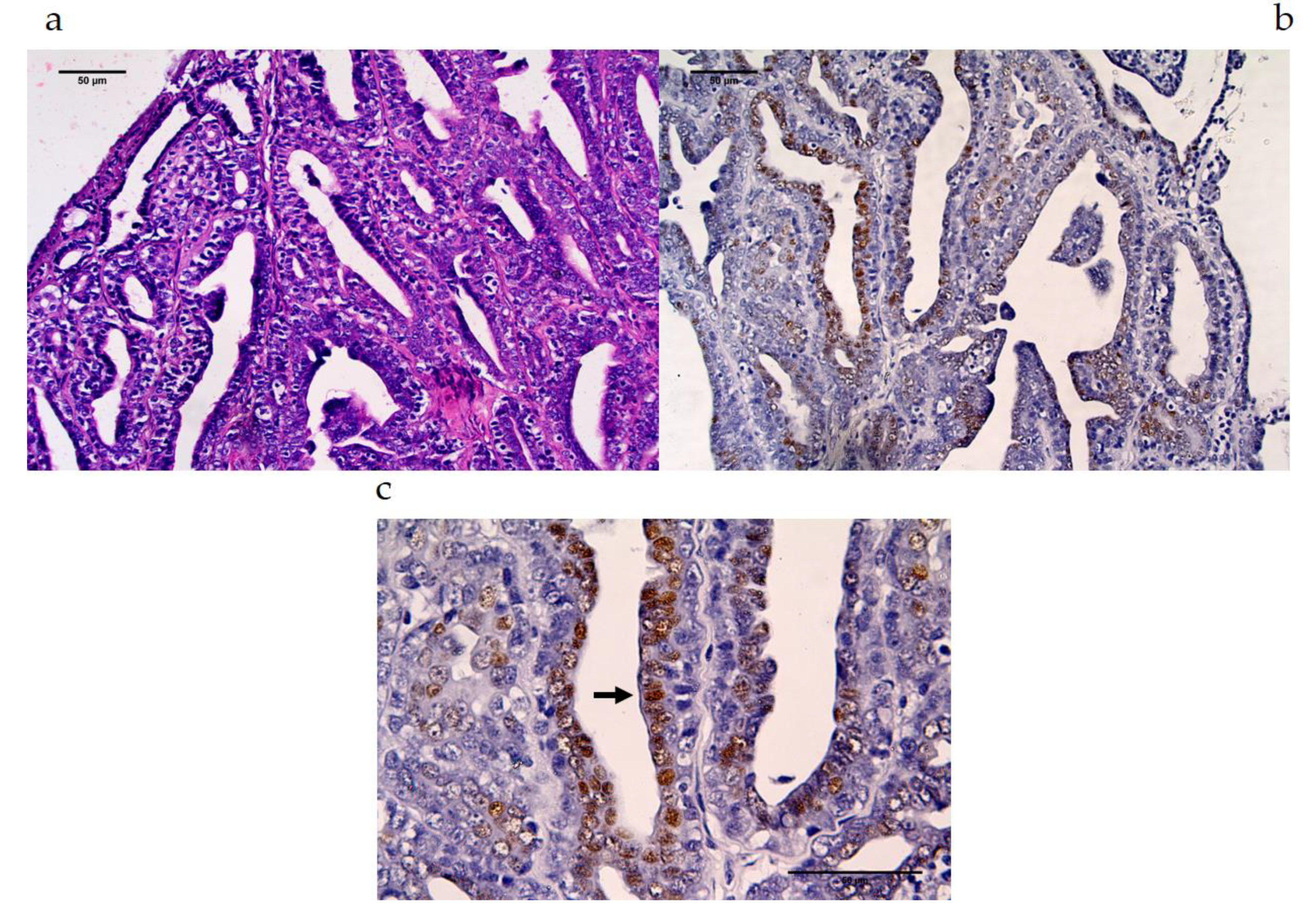
| Protein Name | NCBI No. | Peptide Sequences | Percent Homology | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6C1immunogen | – | S | D | P | A | R | Y | E | F | L | W | G | – |
| hMAGE-A10 | NP_001238757.2 | S | D | P | A | R | Y | E | F | L | W | G | 100 |
| cMAGE-A8 | XP_005642019.3 | S | D | P | A | R | Y | E | F | L | W | G | 100 |
| cMAGE-A9 | XP_005642020.1 | S | N | P | A | R | Y | E | F | L | W | G | 90.1 |
| cMAGE-A10 | XP_022272024.1 | S | D | P | A | C | Y | E | F | L | W | G | 90.1 |
| No. | Age (Years) | Breed | Type of Tumor | TNM Score | MAGE Expression Score |
|---|---|---|---|---|---|
| (1–4) | (0–3) | ||||
| B1 | 5 | Pomeranian | Benign mix tumor | 1 | 0 |
| B2 | 10 | Mixed | Complex adenoma | 1 | 0 |
| No. | Age (Years) | Breed | Type of Tumor | TNM Score | Histological Grade | MAGE Expression Score |
|---|---|---|---|---|---|---|
| (1–4) | (1–3) | (0–3) | ||||
| Ma1 | 10 | Chihuahua | Complex carcinoma | 3 | 3 | 2 |
| Ma2 | 10 | Mixed | Simple tubular carcinoma | 3 | 2 | 2 |
| Ma3 | 10 | Mixed | Anaplastic carcinoma | 3 | 3 | 0 |
| Ma4 | 12 | Pomeranian | Complex carcinoma | 3 | 1 | 0 |
| Ma5 | 12 | Poodle | Fibroadenocarcinoma | 3 | – | 0 |
| Ma6 | 14 | Miniature | Carcinosarcoma | 3 | – | 0 |
| Ma7 | 12 | Mixed | Simple Solid carcinoma | 3 | 2 | 0 |
| Ma8 | 14 | Cocker Spaniel | Simple Solid carcinoma | 3 | 3 | 0 |
| Ma9 | 10 | Mixed | Fibrosarcoma | 2 | – | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisawat, W.; Nambooppha, B.; Pringproa, K.; Thongtharb, A.; Prachasilchai, W.; Sthitmatee, N. A Preliminary Study of the Cross-Reactivity of Canine MAGE-A with Hominid Monoclonal Antibody 6C1 in Canine Mammary Gland Tumors: An Attractive Target for Cancer Diagnostic, Prognostic and Immunotherapeutic Development in Dogs. Vet. Sci. 2020, 7, 109. https://doi.org/10.3390/vetsci7030109
Srisawat W, Nambooppha B, Pringproa K, Thongtharb A, Prachasilchai W, Sthitmatee N. A Preliminary Study of the Cross-Reactivity of Canine MAGE-A with Hominid Monoclonal Antibody 6C1 in Canine Mammary Gland Tumors: An Attractive Target for Cancer Diagnostic, Prognostic and Immunotherapeutic Development in Dogs. Veterinary Sciences. 2020; 7(3):109. https://doi.org/10.3390/vetsci7030109
Chicago/Turabian StyleSrisawat, Wanwisa, Boondarika Nambooppha, Kidsadagon Pringproa, Atigan Thongtharb, Worapat Prachasilchai, and Nattawooti Sthitmatee. 2020. "A Preliminary Study of the Cross-Reactivity of Canine MAGE-A with Hominid Monoclonal Antibody 6C1 in Canine Mammary Gland Tumors: An Attractive Target for Cancer Diagnostic, Prognostic and Immunotherapeutic Development in Dogs" Veterinary Sciences 7, no. 3: 109. https://doi.org/10.3390/vetsci7030109
APA StyleSrisawat, W., Nambooppha, B., Pringproa, K., Thongtharb, A., Prachasilchai, W., & Sthitmatee, N. (2020). A Preliminary Study of the Cross-Reactivity of Canine MAGE-A with Hominid Monoclonal Antibody 6C1 in Canine Mammary Gland Tumors: An Attractive Target for Cancer Diagnostic, Prognostic and Immunotherapeutic Development in Dogs. Veterinary Sciences, 7(3), 109. https://doi.org/10.3390/vetsci7030109






