Efficacy of Live Attenuated Vaccine and Commercially Available Lectin against Avian Pathogenic E. coli Infection in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Chicks
2.2. Vaccine and Medication
2.3. E. coli Challenge Isolates
2.4. Experiment Design
2.4.1. Performance Parameters Evaluation
2.4.2. Clinical Signs and Lesion Scoring
2.4.3. Histopathological Examination
2.4.4. E. coli Count on Eosin Methylene Blue (EMB) Agar
2.5. Statistical Analysis
3. Results
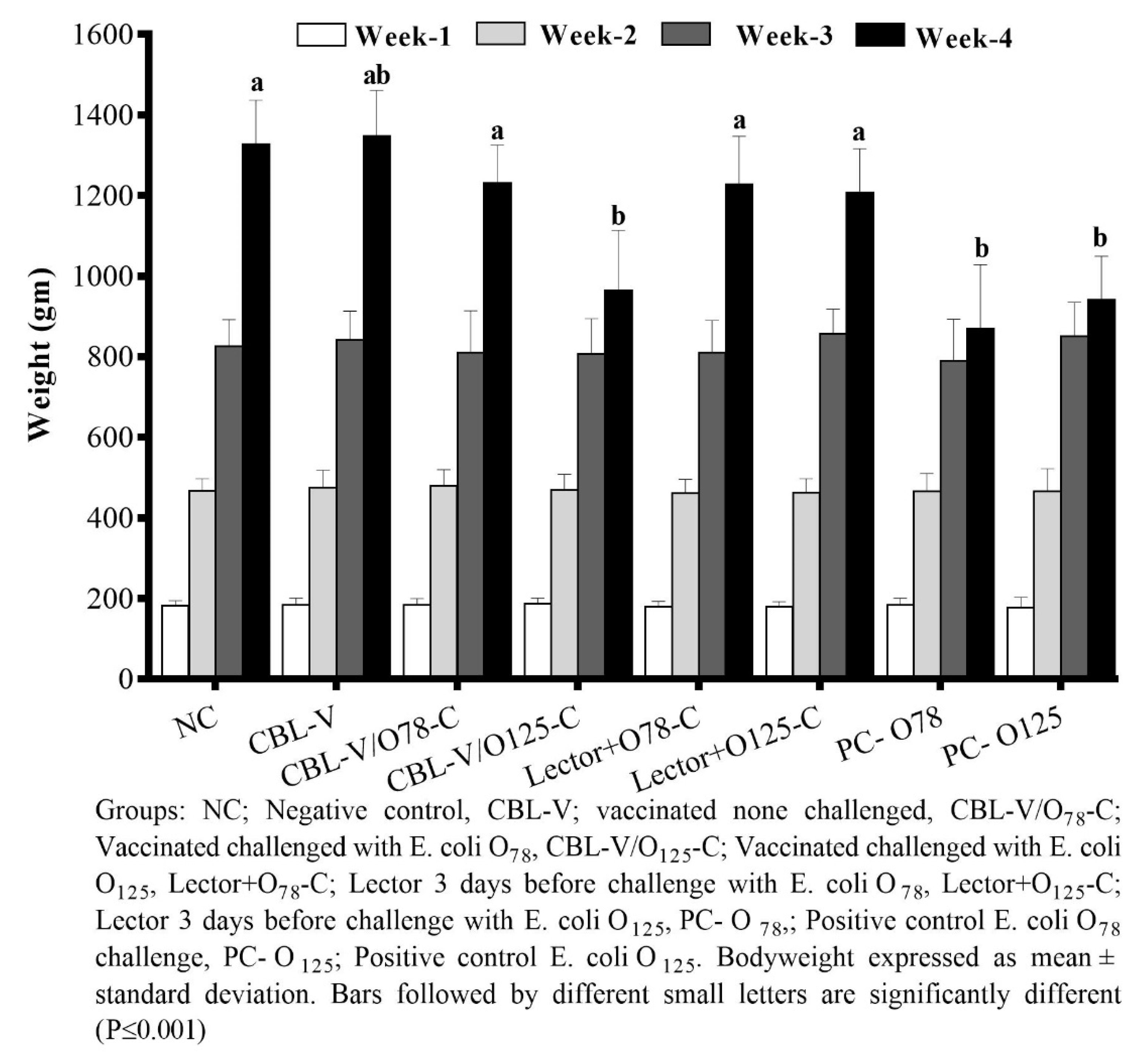
3.1. Performance Evaluation
3.2. Clinical and Lesion Scores
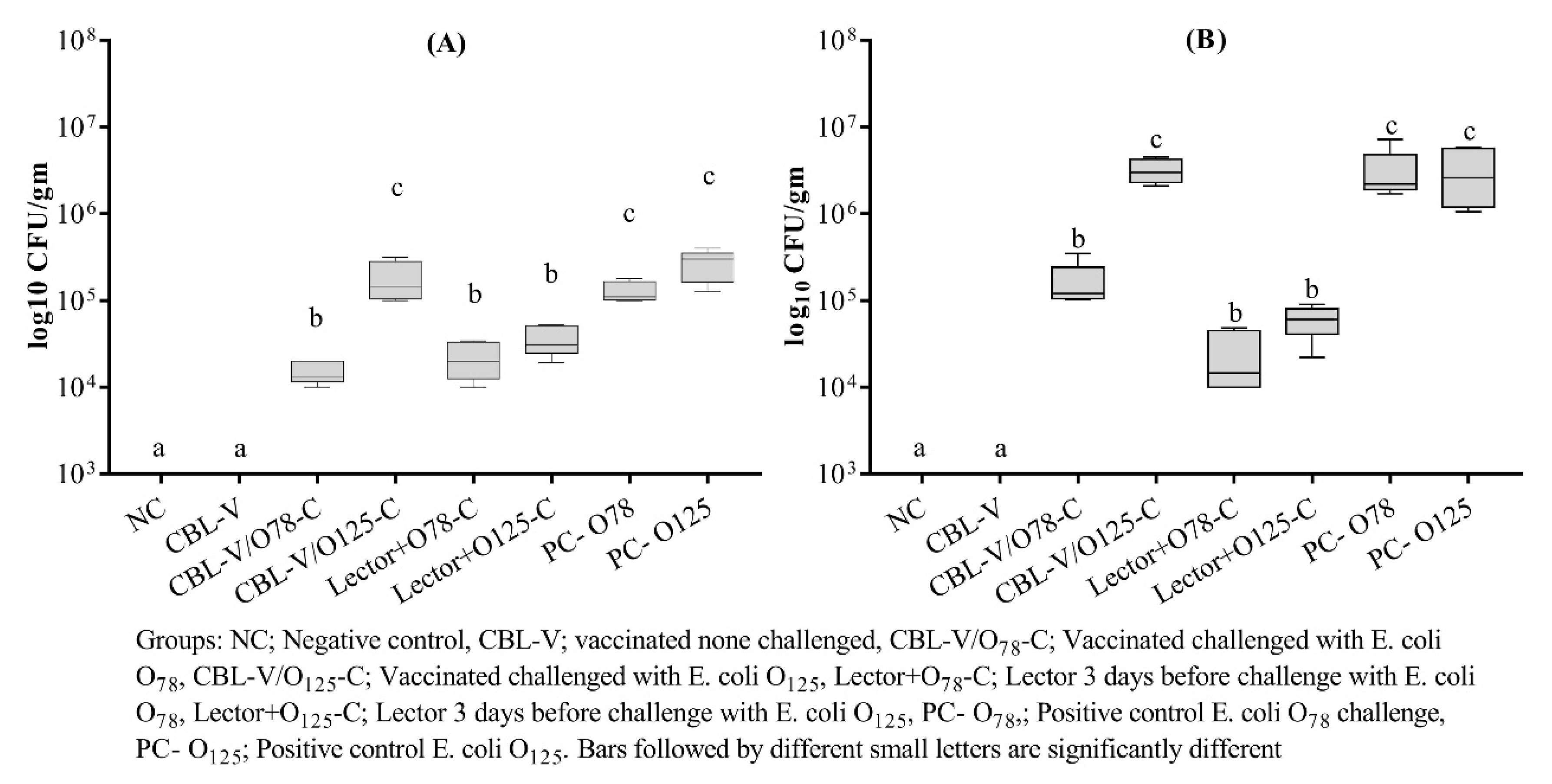
3.3. APEC Recovery from Vaccinated and Treated Groups
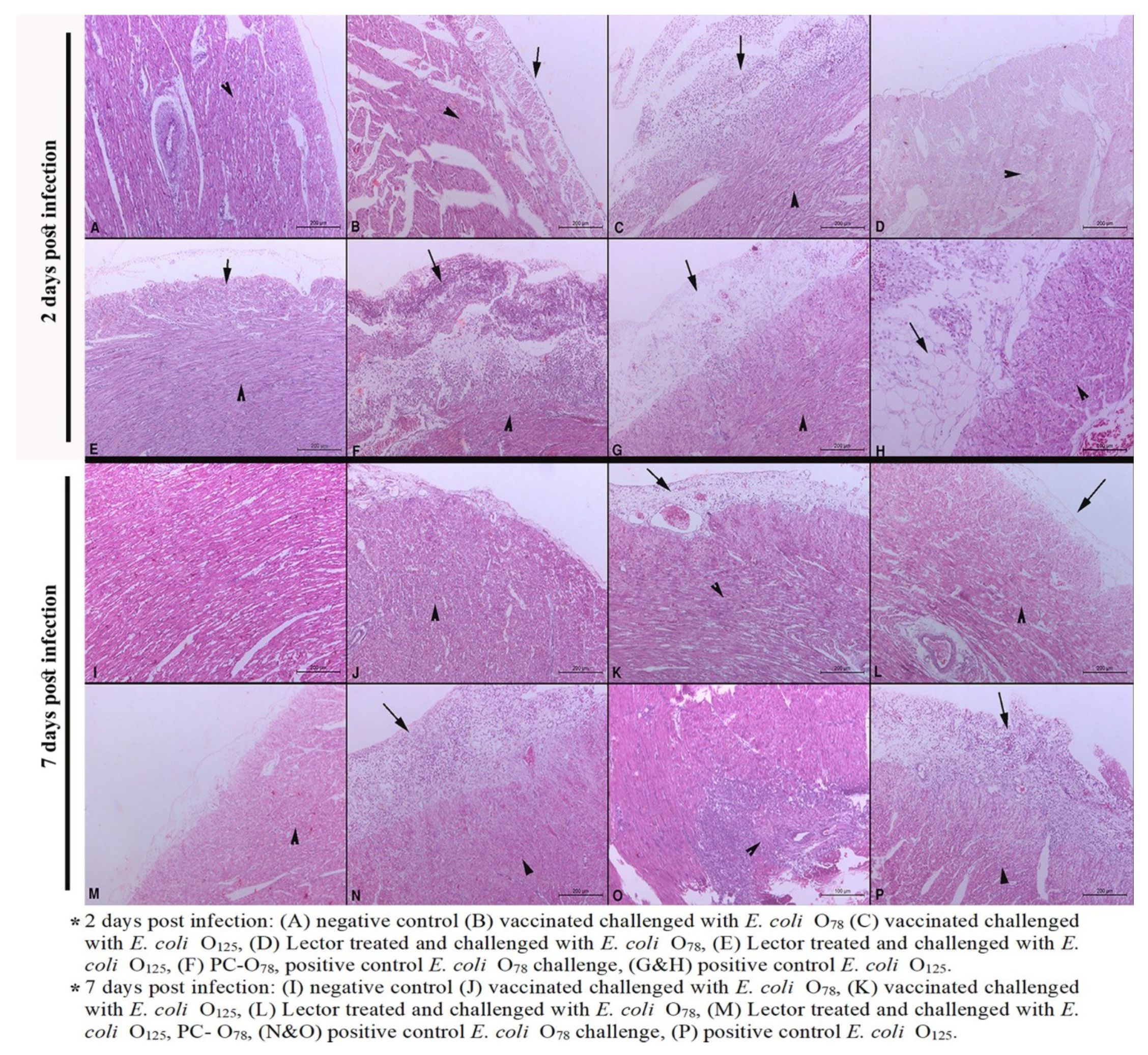
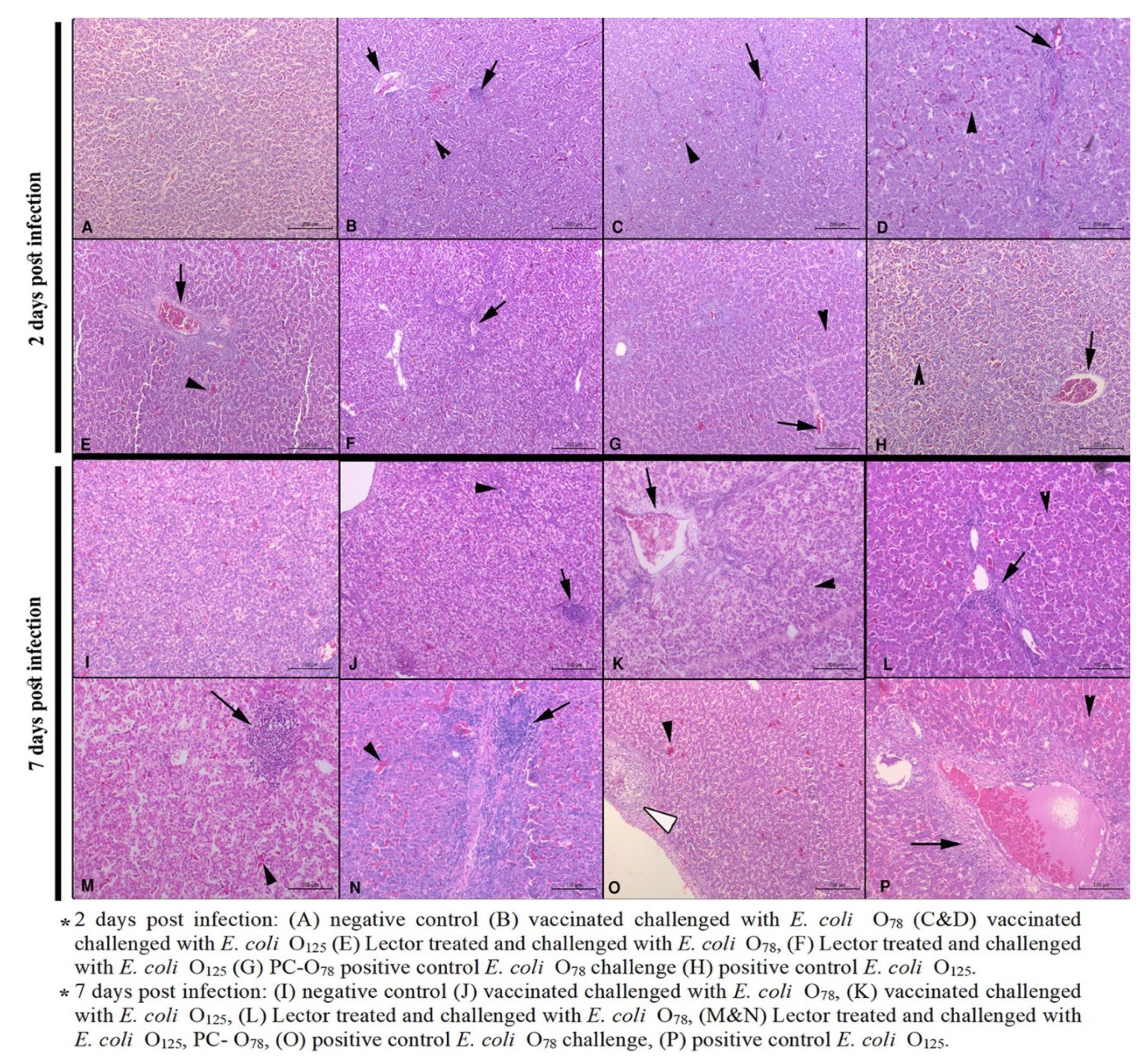
3.4. Histopathological Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belanger, L.; Garenaux, A.; Harel, J.; Boulianne, M.; Nadeau, E.; Dozois, C.M. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 2011, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Janssen, T.; Wieler, L.H. Avian pathogenic Escherichia coli (APEC). Berliner und Munchener tierarztliche Wochenschrift 2003, 116, 381–395. [Google Scholar] [PubMed]
- Kolb, M.; Schieder, D.; Faulstich, M.; Sieber, V. Analysis of lignocellulose derived phenolic monomers by headspace solid-phase microextraction and gas chromatography. J. Chromatogr. A 2013, 1307, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Younis, G.; Awad, A.; Mohamed, N. Phenotypic and genotypic characterization of antimicrobial susceptibility of avian pathogenic Escherichia coli isolated from broiler chickens. Vet. World 2017, 10, 1167–1172. [Google Scholar] [CrossRef]
- Ali, A.; Abd El-Mawgoud, A.I.; Dahshan, A.M.; EL-Sawah, A.A.; Nasef, S.A. Escherichia coli in broiler chickens in Egypt, its virulence traits and vaccination as an intervention strategy. Nov. Res. Microbiol. J. 2019, 3, 415–427. [Google Scholar] [CrossRef]
- Ali, A.; AbdEl-Mawgoud, A.I.; Dahshan, A.M.; El-Sawah, A.A.; Nasef, S.A.; Ibrahim, M. Virulence Gene Constellations Associated with Lethality in Avian Pathogenic E. coli Recovered from Broiler Chickens. Adv. Anim. Vet. Sci. 2019, 7, 1076–1082. [Google Scholar] [CrossRef]
- Wang, J.; Tang, P.; Tan, D.; Wang, L.; Zhang, S.; Qiu, Y.; Dong, R.; Liu, W.; Huang, J.; Chen, T.; et al. The Pathogenicity of Chicken Pathogenic Escherichia coli is Associated with the Numbers and Combination Patterns of Virulence-Associated Genes. Open J. Vet. Med. 2015, 05, 243–254. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Reuland, E.A.; Wintermans, B.B.; Al Naiemi, N.; Koek, A.; Abdelwahab, A.M.; Ammar, A.M.; Mohamed, A.A.; Vandenbroucke-Grauls, C.M. Extended-Spectrum beta-Lactamases and/or Carbapenemases-Producing Enterobacteriaceae Isolated from Retail Chicken Meat in Zagazig, Egypt. PLoS ONE 2015, 10, e0136052. [Google Scholar] [CrossRef]
- El-Shazly, D.A.; Nasef, S.A.; Mahmoud, F.F.; Jonas, D. Expanded spectrum beta-lactamase producing Escherichia coli isolated from chickens with colibacillosis in Egypt. Poult. Sci. 2017, 96, 2375–2384. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Neubauer, H.; Ehricht, R.; Monecke, S.; Tomaso, H.; Hafez, H.M.; Roesler, U.; El-Adawy, H. Antimicrobial resistance in Enterobacteriaceae from healthy broilers in Egypt: Emergence of colistin-resistant and extended-spectrum beta-lactamase-producing Escherichia coli. Gut. Pathog. 2018, 10, 39. [Google Scholar] [CrossRef]
- Projahn, M.; Pacholewicz, E.; Becker, E.; Correia-Carreira, G.; Bandick, N.; Kaesbohrer, A. Reviewing Interventions against Enterobacteriaceae in Broiler Processing: Using Old Techniques for Meeting the New Challenges of ESBL E. coli? Biomed. Res. Int. 2018, 2018, 7309346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, X.; Wang, L.; Lv, X.; Li, D.; Liu, C.; Feng, Z. Two-Step Isolation, Purification, and Characterization of Lectin from Zihua Snap Bean (Phaseolus vulgaris) Seeds. Polymers (Basel) 2019, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Ghunaim, H.; Abu-Madi, M.A.; Kariyawasam, S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: Potentials and limitations. Vet. Microbiol. 2014, 172, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.J.; Poretz, R.D. Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In The Lectins, Properties, Functions and Applications in Biology and Medicine; Liener, I.E., Sharon, N., Goldstein, I.J., Eds.; Academic Press: Orlando, FL, USA, 1986; pp. 32–246. [Google Scholar]
- Chatterjee, A.; Ratner, D.M.; Ryan, C.M.; Johnson, P.J.; O’Keefe, B.R.; Secor, W.E.; Anderson, D.J.; Robbins, P.W.; Samuelson, J. Anti-Retroviral Lectins Have Modest Effects on Adherence of Trichomonas vaginalis to Epithelial Cells In Vitro and on Recovery of Tritrichomonas foetus in a Mouse Vaginal Model. PLoS ONE 2015, 10, e0135340. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Lectins as Promising Therapeutics for the Prevention and Treatment of HIV and Other Potential Coinfections. Biomed. Res. Int. 2018, 2018, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Iordache, F.; Ionita, M.; Mitrea, L.I.; Fafaneata, C.; Pop, A. Antimicrobial and antiparasitic activity of lectins. Curr. Pharm. Biotechnol. 2015, 16, 152–161. [Google Scholar] [CrossRef] [PubMed]
- She, Q.B.; Ng, T.B.; Liu, W.K. A novel lectin with potent immunomodulatory activity isolated from both fruiting bodies and cultured mycelia of the edible mushroom Volvariella volvacea. Biochem. Biophys. Res. Commun. 1998, 247, 106–111. [Google Scholar] [CrossRef]
- Nagano, T.; Kitahara, R.; Nagai, S. An attenuated mutant of avian pathogenic Escherichia coli serovar O78: A possible live vaccine strain for prevention of avian colibacillosis. Microbiol. Immunol. 2012, 56, 605–612. [Google Scholar] [CrossRef]
- Rawiwet, V.; Chansiripornchai, N. The efficacy of Escherichia coli aroa-live vaccine in broilers against avian E. coli serotype O78 infection. Thai J. Vet. Med. 2009, 39, 337–342. [Google Scholar]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Technique; Churchl Livingstone: Edinburgh, UK, 1996. [Google Scholar]
- Lau, G.L.; Sieo, C.C.; Tan, W.S.; Hair-Bejo, M.; Jalila, A.; Ho, Y.W. Efficacy of a bacteriophage isolated from chickens as a therapeutic agent for colibacillosis in broiler chickens. Poult. Sci. 2010, 89, 2589–2596. [Google Scholar] [CrossRef]
- Kwaga, J.K.; Allan, B.J.; van der Hurk, J.V.; Seida, H.; Potter, A.A. A carAB mutant of avian pathogenic Escherichia coli serogroup O2 is attenuated and effective as a live oral vaccine against colibacillosis in turkeys. Infect. Immun. 1994, 62, 3766–3772. [Google Scholar] [CrossRef] [PubMed]
- Peighambari, S.M.; Hunter, D.B.; Shewen, P.E.; Gyles, C.L. Safety, immunogenicity, and efficacy of two Escherichia coli cya crp mutants as vaccines for broilers. Avian Dis. 2002, 46, 287–297. [Google Scholar]
- Mombarg, M.; Bouzoubaa, K.; Andrews, S.; Vanimisetti, H.B.; Rodenberg, J.; Karaca, K. Safety and efficacy of an aroA-deleted live vaccine against avian colibacillosis in a multicentre field trial in broilers in Morocco. Avian Pathol. 2014, 43, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sadeyen, J.R.; Wu, Z.; Davies, H.; van Diemen, P.M.; Milicic, A.; La Ragione, R.M.; Kaiser, P.; Stevens, M.P.; Dziva, F. Immune responses associated with homologous protection conferred by commercial vaccines for control of avian pathogenic Escherichia coli in turkeys. Vet. Res. 2015, 46, 5. [Google Scholar] [CrossRef]
- Schouler, C.; Schaeffer, B.; Bree, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef]
- Gharib, A.A.; Hamouda, A.M.; Abdel-Wahab, A.A.M.; Fawzy, M.F. Protective Efficacy of a Commercial Live Attenuated aroA mutant Vaccine Against Avian Pathogenic Escherichia coli Challenge in Broilers. Zagazig Vet. J. 2017, 45, 366–375. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Bakhit, B.M.; Ibrahim, A.A.; Saleh, M. Evaluation of The Living Escherichia coli-O78 Deleted aroA Vaccine Against Homologous and Heterologous E. coli Challenge in Broiler Chickens. J. Adv. Vet. Res. 2016, 6, 89–92. [Google Scholar]
- Kariyawasam, S.; Wilkie, B.N.; Gyles, C.L. Construction, characterization, and evaluation of the vaccine potential of three genetically defined mutants of avian pathogenic Escherichia coli. Avian Dis. 2004, 48, 287–299. [Google Scholar] [CrossRef]
- Fan, H.H.; Kumar, M.; Marcello, R.; Woodward, M.J. Avian E. coli vaccine for protection against colibacillosis. US patent No. US7357935B2, 2009. Available online: https://patents.google.com/patent/US7357935B2/en (accessed on 13 April 2020).
- La Ragione, R.M.; Woodward, M.J.; Kumar, M.; Rodenberg, J.; Fan, H.; Wales, A.D.; Karaca, K. Efficacy of a live attenuated Escherichia coli O78:K80 vaccine in chickens and turkeys. Avian Dis 2013, 57, 273–279. [Google Scholar] [CrossRef]
- Gibbs, P.S.; Petermann, S.R.; Wooley, R.E. Comparison of several challenge models for studies in avian colibacillosis. Avian Dis. 2004, 48, 751–758. [Google Scholar] [CrossRef]
- Ramos, D.D.M.; Gomes, F.S.; Napoleão, T.H.; Paiva, P.M.G.; Silva, M.D.C.d.; Coelho, L.C.B.B. Antimicrobial Activity of Cladonia verticillaris Lichen Preparations on Bacteria and Fungi of Medical Importance. Chin. J. Biol. 2014, 2014. [Google Scholar] [CrossRef]
- Wellman-Labadie, O.; Lakshminarayanan, R.; Hincke, M.T. Antimicrobial properties of avian eggshell-specific C-type lectin-like proteins. FEBS Lett. 2008, 582, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Liu, W.K.; Ng, T.B.; Ooi, V.E.; Chang, S.T. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 1996, 31, 205–211. [Google Scholar] [CrossRef]
- Amer, M.M.; Bastamy, M.A.; Ibrahim, H.M.; Salim, M.M. Isolation and characterization of avian pathogenic Escherichia coli from broiler chickens in some Governorates of Egypt. Vet. Med. J. Giza 2015, 61, 1–6. [Google Scholar]
- Dahshan, A.M.; Mohamed, A.A. Vaccination against some E. coli Serotypes Isolated from Diseased Broiler Chickens with Chronic Respiratory Disease (CRD). J. Vet. Med. Res. 2016, 23, 243–248. [Google Scholar] [CrossRef]
| Groups (25 Birds/Group) | CBL® 1 Vaccine Day Old by Spray | Lector 0.5 mL/L in Drinking Water (18, 19 and 20 Days Old) | Challenge 2 with APEC O78 at Day 21— 0.5 mL of 108 CFU/mL Bird | Challenge with APEC O125 at Day 21 —0.5 mL of 108 CFU/mL Bird | |
|---|---|---|---|---|---|
| 1 | Negative control (NC) | - | - | - | - |
| 2 | Vaccinated nonchallenged (CBL-V) | + | - | - | - |
| 3 | Vaccinated challenged with APEC O78 (CBL-V/ O78-C) | + | - | + | - |
| 4 | Vaccinated challenged with APEC O125 (CBL-V/O O125-C) | + | - | - | + |
| 5 | Lector before challenge with APEC O78 (Lector+O78-C) | - | + | + | - |
| 6 | Lector before challenge with APEC O125 (Lector+O125-C) | - | + | - | + |
| 7 | Positive control APEC O78 challenge (PC-O78) | - | - | + | - |
| 8 | Positive control APEC O125 (PC-O125) | - | - | - | + |
| Groups 1 | Clinical Score 2 | Lesion Score 2 | Mortality | FCR 3 | |||
|---|---|---|---|---|---|---|---|
| Heart | Liver | Air Sacs | 7 dpi | Cumulative (1–28 Days) | |||
| NC | 0 a | 0 a | 0 a | 0 a | 0% | 1.50 | 1.39 |
| CBL-V | 0 a | 0 a | 0 a | 0 a | 0% | 1.50 | 1.38 |
| CBL-V/O78-C | 1.04 ± 1.48 abd | 0.71 ± 0.69 a | 0.28 ± 0.45 ab | 0.35 ± 0.47 a | 16% | 1.70 | 1.45 |
| CBL-V/O125-C | 1.68 ± 1.7 bd | 1.35 ± 0.68 bc | 0.64 ± 0.47 bc | 1.23 ± 0.42 b | 28% | 2.50 | 1.53 |
| Lector+O78-C | 0.96 ± 1.56 abd | 0.60 ± 0.71 a | 0.26 ± 0.44 ab | 0.33 ± 0.47 a | 20% | 1.60 | 1.38 |
| Lector+O125-C | 0.80 ± 1.46 abd | 0.71 ± 0.69 a | 0.21 ± 0.41 ab | 0.42 ± 0.49 a | 16% | 1.55 | 1.36 |
| PC-O78 | 3.40 ± 0.80 c | 1.52 ± 0.49 c | 0.84 ± 0.61 c | 1.52 ± 0.64 b | 60% | 4.00 | 1.59 |
| PC-O125 | 2.04 ± 1.70 d | 1.44 ± 0.49 c | 0.83 ± 0.50 c | 1.27 ± 0.44 b | 32% | 3.48 | 1.55 |
| DPI 1 | Group 2 | Heart Lesion Scores 3 | Liver Lesion Scores | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pericarditis | Myocarditis | Myocardial Necrosis (Hyalinosis) | Necrosis of Hepatocytes | Inflammation in the Portal Area | Inflammation in the Hepatic Parenchyma | Congestion | Glisson’s Capsule Leucocytic Infiltration | ||
| 2 | NC | - | - | + | - | - | - | + | - |
| CBL-V/O78-C | + | + | + | + | +/++ | +/++ | + | - | |
| CBL-V/O125-C | ++ | ++ | +++ | + | +/++ | +/++ | ++ | - | |
| Lector/O78-C | - | - | + | ++ | ++ | ++ | ++ | - | |
| Lector/O125-C | -/+ | + | + | ++ | ++ | ++ | ++ | - | |
| PC-O78 | +++ | + | ++ | ++ | ++ | ++ | ++ | - | |
| PC-125 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | - | |
| 7 | NC | - | - | -/+ | -/+ | - | - | -/+ | - |
| CBL-V/O78-C | -/+ | + | -/+ | +/++ | +/++ | +/++ | ++ | - | |
| CBL-V/O125-C | +/++ | +/++ | +/++ | ++ | ++ | ++ | ++ | - | |
| Lector/O78-C | + | -/+ | + | ++ | ++ | ++ | +++ | - | |
| Lector/O125-C | -/+ | + | + | ++ | ++ | +++ | ++ | - | |
| PC-O78 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | |
| PC-125 | +++ | ++ | +++ | +++ | +++ | +++ | +++ | ++ | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Mawgoud, A.I.; El-Nahass, E.-S.; Shany, S.A.S.; EL-Sawah, A.A.; Dahshan, A.-H.M.; Nasef, S.A.; Ali, A. Efficacy of Live Attenuated Vaccine and Commercially Available Lectin against Avian Pathogenic E. coli Infection in Broiler Chickens. Vet. Sci. 2020, 7, 65. https://doi.org/10.3390/vetsci7020065
Abd El-Mawgoud AI, El-Nahass E-S, Shany SAS, EL-Sawah AA, Dahshan A-HM, Nasef SA, Ali A. Efficacy of Live Attenuated Vaccine and Commercially Available Lectin against Avian Pathogenic E. coli Infection in Broiler Chickens. Veterinary Sciences. 2020; 7(2):65. https://doi.org/10.3390/vetsci7020065
Chicago/Turabian StyleAbd El-Mawgoud, Ahmed I., El-Shayma El-Nahass, Salama A.S. Shany, Azza A. EL-Sawah, Al-Hussien M. Dahshan, Soad A. Nasef, and Ahmed Ali. 2020. "Efficacy of Live Attenuated Vaccine and Commercially Available Lectin against Avian Pathogenic E. coli Infection in Broiler Chickens" Veterinary Sciences 7, no. 2: 65. https://doi.org/10.3390/vetsci7020065
APA StyleAbd El-Mawgoud, A. I., El-Nahass, E.-S., Shany, S. A. S., EL-Sawah, A. A., Dahshan, A.-H. M., Nasef, S. A., & Ali, A. (2020). Efficacy of Live Attenuated Vaccine and Commercially Available Lectin against Avian Pathogenic E. coli Infection in Broiler Chickens. Veterinary Sciences, 7(2), 65. https://doi.org/10.3390/vetsci7020065






