Characterizing the Role of SMYD2 in Mammalian Embryogenesis—Future Directions
Abstract
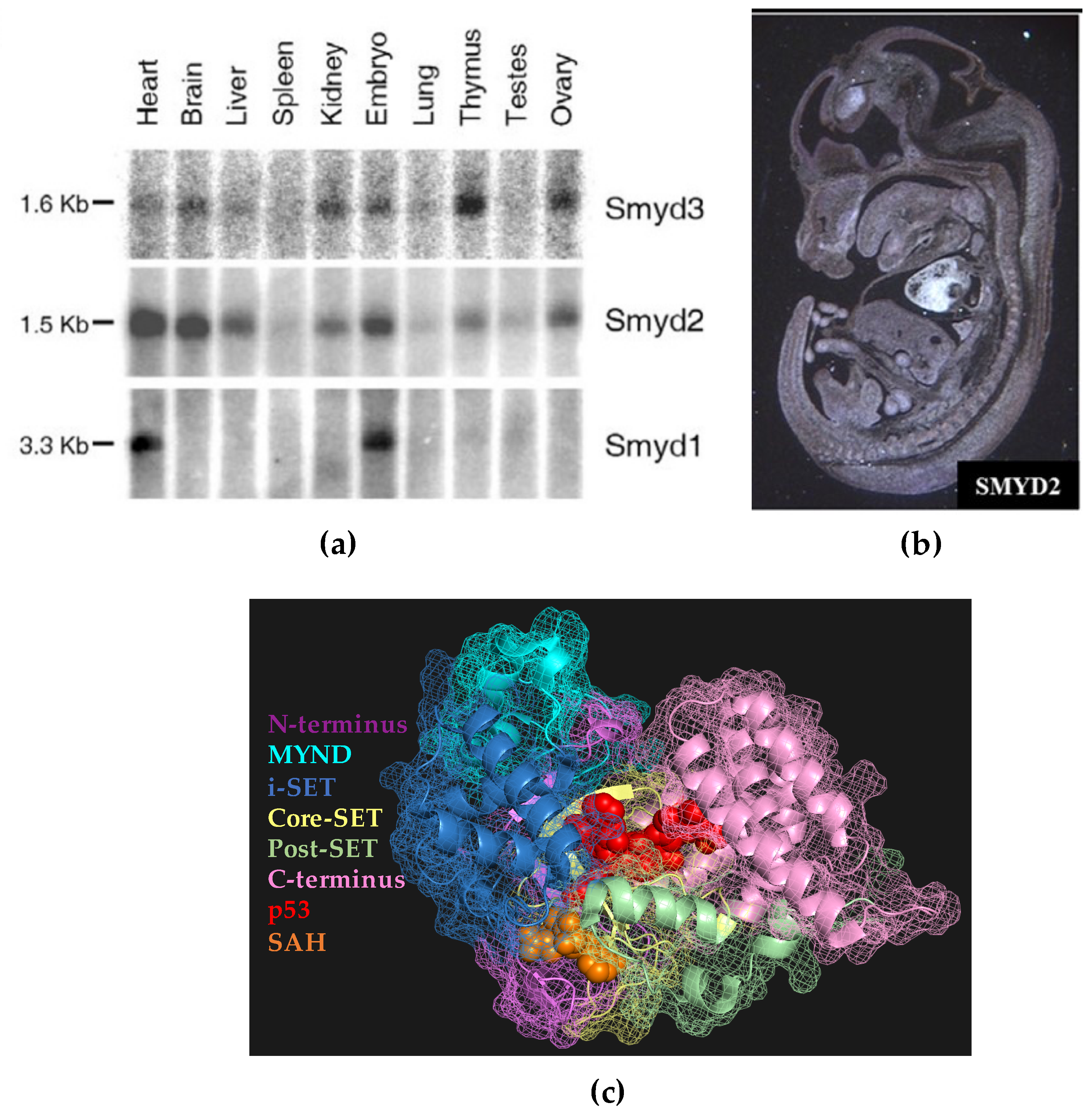
1. Introduction
2. SMYD2 in Cancer
3. SMYD2 in Embryogenesis
3.1. SMYD2 in the WNT Pathway and Mesendodermal Differentiation
3.2. SMYD2 in Hematopoiesis and Leukemia
3.3. SMYD2 in the Hypothalamus and Vomeronasal Organ (VNO)
4. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Doughan, M.; Spellmon, N.; Li, C.; Yang, Z. SMYD proteins in immunity: Dawning of a new era. AIMS Biophys 2016, 3, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Tan, X.; Zhang, J. SMYD Proteins: Key Regulators in Skeletal and Cardiac Muscle Development and Function. Anat. Rec. (Hoboken) 2014, 297, 1650–1662. [Google Scholar] [CrossRef]
- Leinhart, K.; Brown, M. SET/MYND Lysine Methyltransferases Regulate Gene Transcription and Protein Activity. Genes (Basel) 2011, 2, 210–218. [Google Scholar] [CrossRef]
- Spellmon, N.; Holcomb, J.; Trescott, L.; Sirinupong, N.; Yang, Z. Structure and function of SET and MYND domain-containing proteins. Int. J. Mol. Sci. 2015, 16, 1406–1428. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Sims, R.J., 3rd; Gottlieb, P.D.; Tucker, P.W. Identification and characterization of Smyd2: A split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol. Cancer 2006, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Zhang, H.; Luo, X.; Dai, J.; Zhou, S.; Gu, J.; Zhu, J.; Atadja, P.; Lu, C.; et al. Structure of human SMYD2 protein reveals the basis of p53 tumor suppressor methylation. J. Biol. Chem. 2011, 286, 38725–38737. [Google Scholar] [CrossRef] [PubMed]
- Diehl, F.; Brown, M.A.; van Amerongen, M.J.; Novoyatleva, T.; Wietelmann, A.; Harriss, J.; Ferrazzi, F.; Bottger, T.; Harvey, R.P.; Tucker, P.W.; et al. Cardiac deletion of Smyd2 is dispensable for mouse heart development. PLoS ONE 2010, 5, e9748. [Google Scholar] [CrossRef]
- Huang, J.; Perez-Burgos, L.; Placek, B.J.; Sengupta, R.; Richter, M.; Dorsey, J.A.; Kubicek, S.; Opravil, S.; Jenuwein, T.; Berger, S.L. Repression of p53 activity by Smyd2-mediated methylation. Nature 2006, 444, 629–632. [Google Scholar] [CrossRef]
- Clappier, E.; Gerby, B.; Sigaux, F.; Delord, M.; Touzri, F.; Hernandez, L.; Ballerini, P.; Baruchel, A.; Pflumio, F.; Soulier, J. Clonal selection in xenografted human T cell acute lymphoblastic leukemia recapitulates gain of malignancy at relapse. J. Exp. Med. 2011, 208, 653–661. [Google Scholar] [CrossRef]
- Cho, H.S.; Hayami, S.; Toyokawa, G.; Maejima, K.; Yamane, Y.; Suzuki, T.; Dohmae, N.; Kogure, M.; Kang, D.; Neal, S.; et al. RB1 methylation by SMYD2 enhances cell cycle progression through an increase of RB1 phosphorylation. Neoplasia 2012, 14, 476–486. [Google Scholar] [CrossRef]
- Saddic, L.A.; West, L.E.; Aslanian, A.; Yates, J.R., 3rd; Rubin, S.M.; Gozani, O.; Sage, J. Methylation of the retinoblastoma tumor suppressor by SMYD2. J. Biol. Chem. 2010, 285, 37733–37740. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.B.; Cao, X.J.; Han, B.; Chen, L.H.; Horvath, A.; Richardson, T.I.; Campbell, R.M.; Garcia, B.A.; Nguyen, H. Quantitative Profiling of the Activity of Protein Lysine Methyltransferase SMYD2 Using SILAC-Based Proteomics. Mol. Cell Proteomics 2016, 15, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Hamamoto, R.; Vougiouklakis, T.; Wang, R.; Yoshioka, Y.; Suzuki, T.; Dohmae, N.; Matsuo, Y.; Park, J.H.; Nakamura, Y. Critical roles of SMYD2-mediated beta-catenin methylation for nuclear translocation and activation of Wnt signaling. Oncotarget 2017, 8, 55837–55847. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, Z.; Wang, W.; Hu, X.; Chen, P.; Li, J.; Feng, X.; Wong, J.; Du, J.X. The lysine methyltransferase SMYD2 methylates the kinase domain of type II receptor BMPR2 and stimulates bone morphogenetic protein signaling. J. Biol. Chem. 2017, 292, 12702–12712. [Google Scholar] [CrossRef]
- Nakakido, M.; Deng, Z.; Suzuki, T.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. Dysregulation of AKT Pathway by SMYD2-Mediated Lysine Methylation on PTEN. Neoplasia 2015, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Kang, D.; Suzuki, T.; Masuda, A.; Dohmae, N.; Nakamura, Y.; Hamamoto, R. The histone methyltransferase SMYD2 methylates PARP1 and promotes poly(ADP-ribosyl)ation activity in cancer cells. Neoplasia 2014, 16, 257–264.e2. [Google Scholar] [CrossRef]
- Zhang, X.; Tanaka, K.; Yan, J.; Li, J.; Peng, D.; Jiang, Y.; Yang, Z.; Barton, M.C.; Wen, H.; Shi, X. Regulation of estrogen receptor alpha by histone methyltransferase SMYD2-mediated protein methylation. Proc. Natl. Acad. Sci. USA 2013, 110, 17284–17289. [Google Scholar] [CrossRef] [PubMed]
- Cowen, S.D.; Russell, D.; Dakin, L.A.; Chen, H.; Larsen, N.A.; Godin, R.; Throner, S.; Zheng, X.; Molina, A.; Wu, J.; et al. Design, Synthesis, and Biological Activity of Substrate Competitive SMYD2 Inhibitors. J. Med. Chem. 2016, 59, 11079–11097. [Google Scholar] [CrossRef] [PubMed]
- Eggert, E.; Hillig, R.C.; Koehr, S.; Stockigt, D.; Weiske, J.; Barak, N.; Mowat, J.; Brumby, T.; Christ, C.D.; Ter Laak, A.; et al. Discovery and Characterization of a Highly Potent and Selective Aminopyrazoline-Based in Vivo Probe (BAY-598) for the Protein Lysine Methyltransferase SMYD2. J. Med. Chem. 2016, 59, 4578–4600. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Zhou, J.X.; Calvet, J.P.; Godwin, A.K.; Jensen, R.A.; Li, X. Lysine methyltransferase SMYD2 promotes triple negative breast cancer progression. Cell Death Dis. 2018, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Allali-Hassani, A.; Antonysamy, S.; Chang, S.; Chen, L.H.; Curtis, C.; Emtage, S.; Fan, L.; Gheyi, T.; Li, F.; et al. LLY-507, a Cell-active, Potent, and Selective Inhibitor of Protein-lysine Methyltransferase SMYD2. J. Biol. Chem. 2015, 290, 13641–13653. [Google Scholar] [CrossRef] [PubMed]
- Sweis, R.F.; Wang, Z.; Algire, M.; Arrowsmith, C.H.; Brown, P.J.; Chiang, G.G.; Guo, J.; Jakob, C.G.; Kennedy, S.; Li, F.; et al. Discovery of A-893, A New Cell-Active Benzoxazinone Inhibitor of Lysine Methyltransferase SMYD2. ACS Med. Chem. Lett. 2015, 6, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Thomenius, M.J.; Totman, J.; Harvey, D.; Mitchell, L.H.; Riera, T.V.; Cosmopoulos, K.; Grassian, A.R.; Klaus, C.; Foley, M.; Admirand, E.A.; et al. Small molecule inhibitors and CRISPR/Cas9 mutagenesis demonstrate that SMYD2 and SMYD3 activity are dispensable for autonomous cancer cell proliferation. PLoS ONE 2018, 13, e0197372. [Google Scholar] [CrossRef] [PubMed]
- Fabini, E.; Manoni, E.; Ferroni, C.; Rio, A.D.; Bartolini, M. Small-molecule inhibitors of lysine methyltransferases SMYD2 and SMYD3: Current trends. Future Med. Chem. 2019, 11, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, T.; Andresen, C.; Unger, A.; Just, S.; Rottbauer, W.; Linke, W.A. Lysine methyltransferase Smyd2 regulates Hsp90-mediated protection of the sarcomeric titin springs and cardiac function. Biochim. Biophys. Acta 2013, 1833, 812–822. [Google Scholar] [CrossRef]
- Donlin, L.T.; Andresen, C.; Just, S.; Rudensky, E.; Pappas, C.T.; Kruger, M.; Jacobs, E.Y.; Unger, A.; Zieseniss, A.; Dobenecker, M.W.; et al. Smyd2 controls cytoplasmic lysine methylation of Hsp90 and myofilament organization. Genes Dev. 2012, 26, 114–119. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Hamamoto, R.; Toyokawa, G.; Nakakido, M.; Ueda, K.; Nakamura, Y. SMYD2-dependent HSP90 methylation promotes cancer cell proliferation by regulating the chaperone complex formation. Cancer Lett. 2014, 351, 126–133. [Google Scholar] [CrossRef]
- Li, L.X.; Fan, L.X.; Zhou, J.X.; Grantham, J.J.; Calvet, J.P.; Sage, J.; Li, X. Lysine methyltransferase SMYD2 promotes cyst growth in autosomal dominant polycystic kidney disease. J. Clin. Investig. 2017, 127, 2751–2764. [Google Scholar] [CrossRef]
- Bai, H.J.; Zhang, P.; Ma, L.; Liang, H.; Wei, G.; Yang, H.T. SMYD2 Drives Mesendodermal Differentiation of Human Embryonic Stem Cells Through Mediating the Transcriptional Activation of Key Mesendodermal Genes. Stem Cells 2019, 37, 1401–1415. [Google Scholar] [CrossRef]
- Komatsu, S.; Ichikawa, D.; Hirajima, S.; Nagata, H.; Nishimura, Y.; Kawaguchi, T.; Miyamae, M.; Okajima, W.; Ohashi, T.; Konishi, H.; et al. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis 2009, 30, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Reynoird, N.; Mazur, P.K.; Stellfeld, T.; Flores, N.M.; Lofgren, S.M.; Carlson, S.M.; Brambilla, E.; Hainaut, P.; Kaznowska, E.B.; Arrowsmith, K.; et al. Coordination of stress signals by the lysine methyltransferase SMYD2 promotes pancreatic cancer. Genes Dev. 2016, 30, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Ichikawa, D.; Hirajima, S.; Nagata, H.; Nishimura, Y.; Kawaguchi, T.; Miyamae, M.; Okajima, W.; Ohashi, T.; Konishi, H.; et al. Overexpression of SMYD2 contributes to malignant outcome in gastric cancer. Br. J. Cancer 2015, 112, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Lambert, J.P.; Al-Madhoun, A.S.; Elisma, F.; Skerjanc, I.S.; Figeys, D. The tale of two domains: Proteomics and genomics analysis of SMYD2, a new histone methyltransferase. Mol. Cell Proteomics 2008, 7, 560–572. [Google Scholar] [CrossRef]
- Brown, M.A.; Edwards, M.A.; Alshiraihi, I.; Geng, H.; Dekker, J.D.; Tucker, H.O. The lysine methyltransferase SMYD2 is required for normal lymphocyte development and survival of hematopoietic leukemias. Genes Immun. 2020, 21, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Sakamoto, L.H.; Andrade, R.V.; Felipe, M.S.; Motoyama, A.B.; Pittella Silva, F. SMYD2 is highly expressed in pediatric acute lymphoblastic leukemia and constitutes a bad prognostic factor. Leuk. Res. 2014, 38, 496–502. [Google Scholar] [CrossRef]
- Ou, H.; Chen, Z.; Xiang, L.; Fang, Y.; Xu, Y.; Liu, Q.; Hu, Z.; Li, X.; Huang, Y.; Yang, D. Frizzled 2-induced epithelial-mesenchymal transition correlates with vasculogenic mimicry, stemness, and Hippo signaling in hepatocellular carcinoma. Cancer Sci. 2019, 110, 1169–1182. [Google Scholar] [CrossRef]
- Wang, R.; Deng, X.; Yoshioka, Y.; Vougiouklakis, T.; Park, J.H.; Suzuki, T.; Dohmae, N.; Ueda, K.; Hamamoto, R.; Nakamura, Y. Effects of SMYD2-mediated EML4-ALK methylation on the signaling pathway and growth in non-small-cell lung cancer cells. Cancer Sci. 2017, 108, 1203–1209. [Google Scholar] [CrossRef]
- Sese, B.; Barrero, M.J.; Fabregat, M.C.; Sander, V.; Izpisua Belmonte, J.C. SMYD2 is induced during cell differentiation and participates in early development. Int. J. Dev. Biol. 2013, 57, 357–364. [Google Scholar] [CrossRef]
- Bagislar, S.; Sabo, A.; Kress, T.R.; Doni, M.; Nicoli, P.; Campaner, S.; Amati, B. Smyd2 is a Myc-regulated gene critical for MLL-AF9 induced leukemogenesis. Oncotarget 2016, 7, 66398–66415. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Termglinchan, V.; Shao, N.Y.; Itzhaki, I.; Liu, C.; Ma, N.; Tian, L.; Wang, V.Y.; Chang, A.C.Y.; Guo, H.; et al. A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles. Cell Stem Cell 2019, 24, 802–811.e5. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef]
- Taciak, B.; Pruszynska, I.; Kiraga, L.; Bialasek, M.; Krol, M. Wnt signaling pathway in development and cancer. J. Physiol. Pharmacol. 2018, 69. [Google Scholar]
- Bagger, F.O.; Sasivarevic, D.; Sohi, S.H.; Laursen, L.G.; Pundhir, S.; Sonderby, C.K.; Winther, O.; Rapin, N.; Porse, B.T. BloodSpot: A database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res. 2016, 44, D917–D924. [Google Scholar] [CrossRef]
- Barrett, T.; Troup, D.B.; Wilhite, S.E.; Ledoux, P.; Rudnev, D.; Evangelista, C.; Kim, I.F.; Soboleva, A.; Tomashevsky, M.; Edgar, R. NCBI GEO: Mining tens of millions of expression profiles—Database and tools update. Nucleic Acids Res. 2007, 35, D760–D765. [Google Scholar] [CrossRef]
- Geng, H.; Brennan, S.; Milne, T.A.; Chen, W.Y.; Li, Y.; Hurtz, C.; Kweon, S.M.; Zickl, L.; Shojaee, S.; Neuberg, D.; et al. Integrative epigenomic analysis identifies biomarkers and therapeutic targets in adult B-acute lymphoblastic leukemia. Cancer Discov. 2012, 2, 1004–1023. [Google Scholar] [CrossRef]
- Juric, D.; Lacayo, N.J.; Ramsey, M.C.; Racevskis, J.; Wiernik, P.H.; Rowe, J.M.; Goldstone, A.H.; O’Dwyer, P.J.; Paietta, E.; Sikic, B.I. Differential gene expression patterns and interaction networks in BCR-ABL-positive and -negative adult acute lymphoblastic leukemias. J. Clin. Oncol. 2007, 25, 1341–1349. [Google Scholar] [CrossRef]
- Tobet, S.A.; Schwarting, G.A. Minireview: Recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology 2006, 147, 1159–1165. [Google Scholar] [CrossRef]
- Wierman, M.E.; Kiseljak-Vassiliades, K.; Tobet, S. Gonadotropin-releasing hormone (GnRH) neuron migration: Initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front. Neuroendocrinol. 2011, 32, 43–52. [Google Scholar] [CrossRef] [PubMed]
| Target Protein | Protein Function | Associated Cancers | Refs. |
|---|---|---|---|
| Heat shock protein 90 (HSP90) | Titin stability and sarcomere assembly in muscle tissue, drives handling of several oncoproteins | Lung, breast, urothelial, colorectal, bladder carcinomas | [25,26,27,28] |
| Signal transducer and activator of transcription-3 (STAT3) | Becomes activated in complex with SMYD2, p65, and NF-kB, transcription activator, feed-forward SMYD2 expression, increased proliferation, stem cell self-renewal | Breast carcinomas, leukemias | [29] |
| Histone 3 (K4) | Interacts with RNA Polymerase II, RNA helicase, drives gene transcription at promotor regions | Lung, breast, glioblastomas | [7,28,30] |
| Histone 3 (K36) | Associated with gene bodies, defines exons, recruits HDACs, modulates transcription | Embryonic kidney and fibroblast carcinomas | [5,28,31,32,33] |
| p53 (K370) | Cell cycle arrest | p53 dysfunction detected in most cancer types | [7,8,31,33,34] |
| β-catenin (K133) | Methylation promotes nuclear translocation and activation of WNT signaling | B-cell precursor acute lymphoblastic leukemia (ALL), colon, breast, and hepatocellular carcinomas | [13,35,36] |
| Retinoblastoma tumor suppressor protein (RB1; K810) | Promotes cell cycle progression; G1/S transition | Bladder carcinomas | [10,11] |
| Ahnak nucleoprotein 2 | Cell migration and invasion | Chronic myeloid leukemia (CML), Mixed lineage leukemia rearranged adult B-acute lymphoblastic leukemia (MLLr-B-ALL), adult B-acute lymphoblastic leukemia (B-ALL), and T-cell acute lymphoblastic leukemia (T-ALL) | [18,19,37] |
| Estrogen receptor-α (ER-α; K266) | Nuclear receptor for sex hormone estrogen, potent upstream activator of gene transcription | Breast | [17] |
| Poly-(ADP-ribose) polymerase 1 (PARP1; K528) | Enhances poly (ADP-ribose) activity | Esophageal squamous cell and bladder carcinoma, pediatric acute lymphoblastic leukemia | [16,31,33] |
| Phosphatase and tensin homologue (PTEN; K313) | Down-regulates tumor suppressor & activates the phosphatidylinositol 3-kinase-AKT pathway | Prostate, small cell lung cancers | [15] |
| Bone Morphogenic Protein Receptor-2 (BMPR2) | Methylation upregulates BMP2 signaling, important for bone development and MSC proliferation | Skin, colorectal, ovarian, breast, embryonic kidney carcinomas | [13] |
| Frizzled-2 | STAT3 interaction, epithelial-mesenchymal transition, WNT and Hippo signaling | Hepatocellular | [35,38] |
| Erythrocyte membrane protein band 4.1 like (3EBP41L3; K1610) | Tumor suppressor | Breast, non-small-cell lung, brain, ovarian cancers | [39] |
| Model | Phenotype | Refs. |
|---|---|---|
| Nkx2.5 Cre/Smyd2flox/flox (CKO) mice (cardiac-specific) | No phenotype | [7] |
| Mx-1Cre/Smyd2flox/flox (CKO) mice (hematopoetic stem cell-specific) | Reduced HSC and downstream progenitor populations via apoptosis and cell cycle blocking | [35] |
| Smyd2-/- human embryonic stem cells | Blocked mesendodermal lineage commitment, No affect on self-renewal and neurectoderm | [30] |
| morpholino-Smyd2a-/- zebrafish | Developmental delay, tail abnormalities, over-induction of Nodal pathway | [40] |
| morpholino-Smyd2a-/- zebrafish | Severe cardiac structural and sarcomeric malformation, tail abnormalities | [25] |
| Smyd2-/- (global KO) mice | No developmental phenotype, reduced susceptibility to leukemia | [41] |
| CRISPR/Cas9 SMYD2 KO human keratinocytes (in vitro) | Impaired BMP2 morphogen signaling | [14] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrell, D.K.; Hassell, K.N.; Crans, D.C.; Lanning, S.; Brown, M.A. Characterizing the Role of SMYD2 in Mammalian Embryogenesis—Future Directions. Vet. Sci. 2020, 7, 63. https://doi.org/10.3390/vetsci7020063
Jarrell DK, Hassell KN, Crans DC, Lanning S, Brown MA. Characterizing the Role of SMYD2 in Mammalian Embryogenesis—Future Directions. Veterinary Sciences. 2020; 7(2):63. https://doi.org/10.3390/vetsci7020063
Chicago/Turabian StyleJarrell, Dillon K., Kelly N. Hassell, Debbie C. Crans, Shari Lanning, and Mark A. Brown. 2020. "Characterizing the Role of SMYD2 in Mammalian Embryogenesis—Future Directions" Veterinary Sciences 7, no. 2: 63. https://doi.org/10.3390/vetsci7020063
APA StyleJarrell, D. K., Hassell, K. N., Crans, D. C., Lanning, S., & Brown, M. A. (2020). Characterizing the Role of SMYD2 in Mammalian Embryogenesis—Future Directions. Veterinary Sciences, 7(2), 63. https://doi.org/10.3390/vetsci7020063







