Clinical, Molecular and Serological Diagnosis of Canine Leishmaniosis: An Integrated Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Collection of Samples
2.2. Molecular Analysis
2.3. Serological Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Signs
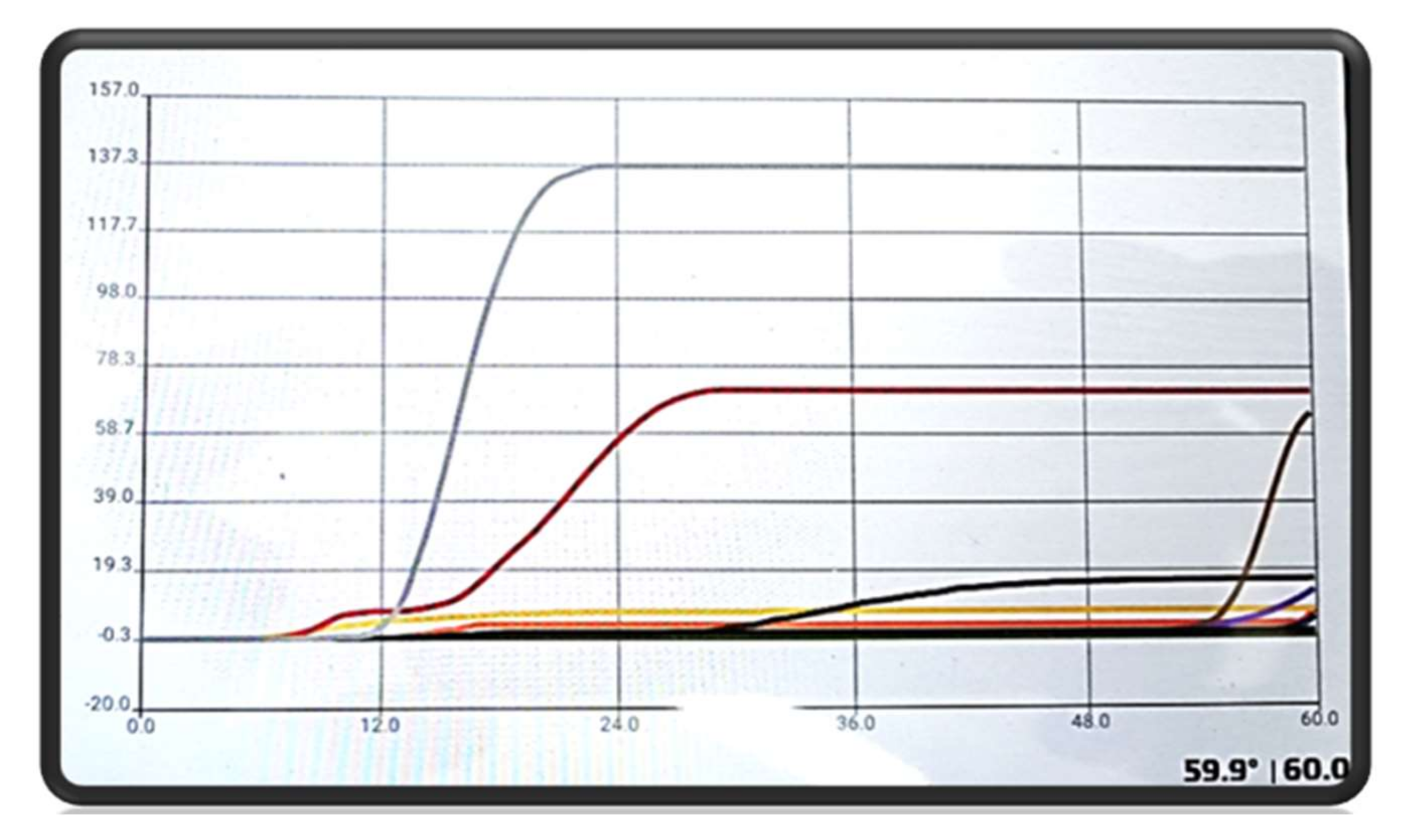
3.2. Molecular Analysis
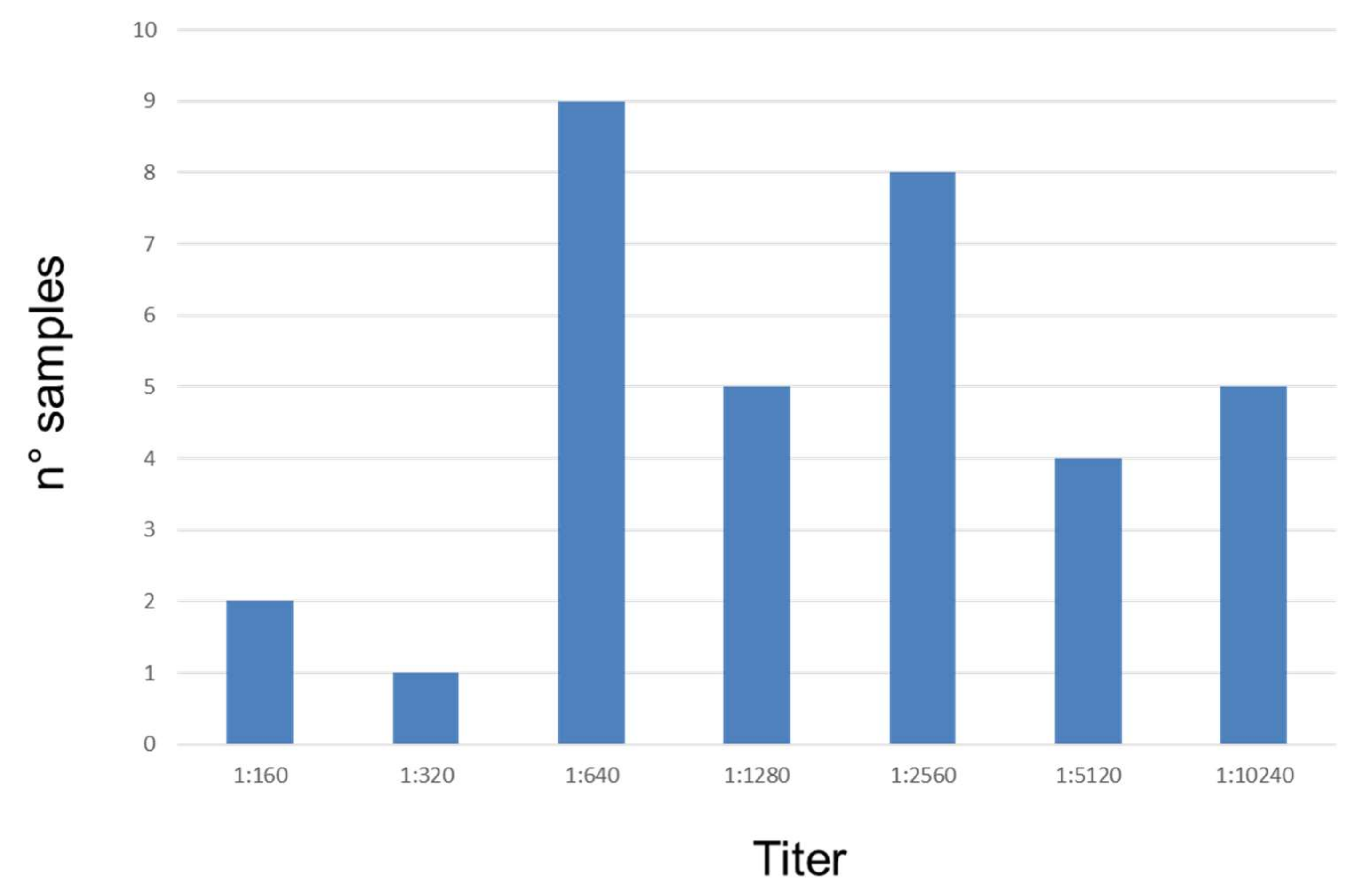
3.3. Serological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Desjeux, P. Leishmaniasis. Nat. Rev. Microbiol. 2004, 2, 692. [Google Scholar] [CrossRef] [PubMed]
- Miró, G.; Petersen, C.; Cardoso, L.; Bourdeau, P.; Baneth, G.; Solano-Gallego, L.; Pennisi, M.G.; Ferrer, L.; Oliva, G. Novel Areas for Prevention and Control of Canine Leishmaniosis. Trends Parasitol. 2017, 33, 718–730. [Google Scholar]
- Moreno, J.; Alvar, J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399. [Google Scholar] [CrossRef]
- Athanasiou, L.V.; Kontos, V.I.; Saridomichelakis, M.N.; Rallis, T.S.; Diakou, A. A cross-sectional sero-epidemiological study of canine leishmaniasis in Greek mainland. Acta Trop. 2012, 122, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Villanueva-Saz, S.; Basurco, A.; Martín, V.; Fernández, A.; Loste, A.; Verde, M.T. Comparison of a qualitative immunochromatographic test with two quantitative serological assays for the detection of antibodies to Leishmania infantum in dogs. Acta Vet. Scand. 2019, 61, 38. [Google Scholar] [CrossRef]
- Maia, C.; Campino, L. Biomarkers Associated with Leishmania infantum Exposure, Infection, and Disease in Dogs. Front. Cell. Infect. Microbiol. 2018, 8, 302. [Google Scholar] [CrossRef]
- Chukwunonso, O.N.; Hirotomo, K.; Peters, N.C. Loop-mediated isothermal amplification (LAMP): An advanced molecular point-of-care technique for the detection of Leishmania infection. PLoS Negl. Trop. Dis. 2019, 13, e0007698. [Google Scholar]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef]
- Calzada, J.E.; Saldaña, A.; Gonzalez, K.; Rigg, C.; Pineda, V.; Santamaria, A.M.; Rodriguez, I.; Gottdenker, N.L.; Laurenti, M.D.; Chaves, L.F. Cutaneous Leishmaniasis in dogs: Is high seroprevalence indicative of a reservoir role? Parasitology 2015, 142, 1202–1214. [Google Scholar] [CrossRef]
- Albuquerque, A.; Campino, L.; Cardoso, L.; Cortes, S. Evaluation of four molecular methods to detect Leishmania infection in dogs. Parasites Vectors 2017, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Hara-Kudo, Y.; Konishi, N.; Ohtsuka, K.; Hiramatsu, R.; Tanaka, H.; Konuma, H.; Takatori, K. Detection of verotoxigenic Escherichia coli O157 and O26 in food by plating methods and LAMP method: A collaborative study. Int. J. Food Microbiol. 2008, 122, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, L.; Yang, Q.; Prinyawiwatkul, W.; Ge, B. Rapid and specific detection of Escherichiacoli serogroups O26, O45, O103, O111, O121, O145, and O157 in ground beef, beef trim, and produce by loop-mediated isothermal amplification. Appl. Environ. Microbiol. 2012, 78, 2727–2736. [Google Scholar] [CrossRef]
- Yamazaki, W.; Ishibashi, M.; Kawahara, R.; Inoue, K. Development of a loop mediated isothermal amplification assay for sensitive and rapid detection of Vibrio parahaemolyticus. BMC Microbiol. 2008, 8, 163. [Google Scholar] [CrossRef]
- Ren, W.; Renault, T.; Cai, Y.; Wang, C. Development of a loop-mediated isothermal amplification assay for rapid and sensitive detection of ostreid herpesvirus 1 DNA. J. Virol. Methods 2010, 170, 30–36. [Google Scholar] [CrossRef]
- Ikadai, H.; Tanaka, H.; Shibahara, N.; Matsuu, A.; Uechi, M.; Itoh, N.; Oshiro, S.; Kudo, N.; Igarashi, I.; Oyamada, T. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J. Clin. Microbiol. 2004, 42, 2465–2469. [Google Scholar] [CrossRef]
- Poon, L.L.; Wong, B.W.; Ma, E.H.; Chan, K.H.; Chow, L.M.; Abeyewickreme, W.; Tangpukdee, N.; Yuen, K.Y.; Guan, Y.; Looareesuwan, S.; et al. Sensitive and inexpensive molecular test for falciparum malaria: Detecting Plasmodium falciparum DNA directly from heat-treated blood by loopmediated isothermal amplification. Clin. Chem. 2006, 52, 303–306. [Google Scholar] [CrossRef]
- Karanis, P.; Thekisoe, O.; Kiouptsi, K.; Ongerth, J.; Igarashi, I.; Inoue, N. Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of cryptosporidium oocysts in fecal and water samples. Appl. Environ. Microbiol. 2007, 73, 5660–5662. [Google Scholar] [CrossRef]
- Njiru, Z.K.; Mikosza, A.S.; Armstrong, T.; Enyaru, J.C.; Ndung’u, J.M.; Thompson, A.R. Loop-mediated isothermal amplification (LAMP) method for rapid detection of Trypanosoma brucei rhodesiense. PLoS Negl. Trop. Dis. 2008, 2, e147. [Google Scholar] [CrossRef]
- Plutzer, J.; Karanis, P. Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitol. Res. 2009, 104, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Furushima-Shimogawara, R.; Ohmae, H.; Wang, T.P.; Lu, S.; Chen, R.; Wen, L.; Ohta, N. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am. J. Trop. Med. Hyg. 2010, 83, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.; La Carbona, S.; Geffard, A.; Possenti, A.; Dubey, J.P.; Lalle, M. Comparative evaluation of loop-mediated isothermal amplification (LAMP) vs qPCR for detection of Toxoplasma gondii oocysts DNA in mussels. Exp. Parasitol. 2019, 28, 107809. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.H.; Ding, D.; Wang, J.Y.; Steverding, D.; Wang, X.; Yang, Y.T.; Shi, F. Development of a LAMP assay for detection of Leishmania infantum infection in dogs using conjunctival swab samples. Parasites Vectors 2015, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.K.; Verma, S.; Singh, O.P.; Singh, D.; Singh, A.P.; Gupta, R.; Negi, N.S.; Das, P.; Sundar, S.; Singh, R.; et al. Validation of SYBR green I based closed tube loop mediated isothermal amplification (LAMP) assay and simplified direct-blood-lysis (DBL)-LAMP assay for diagnosis of visceral leishmaniasis (VL). PLoS Negl. Trop. Dis. 2018, 12, e0006922. [Google Scholar] [CrossRef]
- De Lima Celeste, J.L.L.; Caldeira, R.L.; Pires, S.D.F.; Silveira, K.D.; Soares, R.P.; de Andrade, H.M. Development and evaluation of a loop-mediated isothermal amplification assay for rapid detection of Leishmania amazonensis in skin samples. Exp. Parasitol. 2019, 203, 23–29. [Google Scholar] [CrossRef]
- de Avelar, D.M.; Carvalho, D.M.; Rabello, A. Development and Clinical Evaluation of Loop-Mediated Isothermal Amplification (LAMP) Assay for the Diagnosis of Human Visceral Leishmaniasis in Brazil. Biomed. Res. Int. 2019, 24, 8240784. [Google Scholar] [CrossRef]
- Chaouch, M.; Mhadhbi, M.; Limam, S.; Darghouth, M.A.; Benabderrazak, S. Development and Evaluation of a Loop-mediated Isothermal Amplification Assay for Rapid Detection of Theileria annulata Targeting the Cytochrome B Gene. Iran. J. Parasitol. 2018, 13, 225–234. [Google Scholar]
- Mukhtar, M.; Ali, S.S.; Boshara, S.A.; Albertini, A.; Monnerat, S.; Bessell, P.; Mori, Y.; Kubota, Y.; Ndung’u, J.M.; Cruz, I. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP). PLoS Negl. Trop. Dis. 2018, 12, e0006264. [Google Scholar] [CrossRef]
- Ibarra-Meneses, A.V.; Cruz, I.; Chicharro, C.; Sánchez, C.; Biéler, S.; Broger, T.; Moreno, J.; Carrillo, E. Evaluation of fluorimetry and direct visualization to interpret results of a loop-mediated isothermal amplification kit to detect Leishmania DNA. Parasites Vectors 2018, 11, 250. [Google Scholar] [CrossRef]
- Adams, E.R.; Schoone, G.; Versteeg, I.; Gomez, M.A.; Diro, E.; Mori, Y.; Perlee, D.; Downing, T.; Saravia, N.; Assaye, A.; et al. Development and Evaluation of a Novel Loop-Mediated Isothermal Amplification Assay for Diagnosis of Cutaneous and Visceral Leishmaniasis. J. Clin. Microbiol. 2018, 56, e00386–18. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.F.H.; Hu, R.V.P.; Kent, A.D.; van Loenen, M.; Menting, S.; Picado, A.; Oosterling, Z.; Cruz, I. Evaluation of point of care tests for the diagnosis of cutaneous leishmaniasis in Suriname. BMC Infect. Dis. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Vitale, F.; Reale, S.; Vitale, M.; Petrotta, E.; Torina, A.; Caracappa, S. TaqMan-based detection of Leishmania infantum DNA using canine samples. Ann. N. Y. Acad. Sci. 2004, 1026, 139–143. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Rutjes, A.W.S.; Khan, K.S.; Coomarasamy, A.; Bossuyt, P.M. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J. Clin. Epidemiol. 2009, 62, 797e806. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd: London, UK, 2007. [Google Scholar]
- OIE. Terrestrial Manual. Leishmaniosis, 8th ed.; OIE: Paris, France, 2018; Available online: https://www.oie.int/standard-setting/terrestrial-manual/access-online/ (accessed on 8 December 2019).
- Farahmand, M.; Nahrevanian, H. Application of Recombinant Proteins for Serodiagnosis of Visceral Leishmaniasis in Humans and Dogs. Iran. Biomed. J. 2016, 20, 128–134. [Google Scholar]
- Branscum, A.J.; Gardner, I.A.; Johnson, W.O. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Prev. Vet. Med. 2005, 68, 145–163. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Reale, S.; Giudice, S.L.; Masucci, M.; Caracappa, S.; Vitale, M.; Vitale, F. Real-time PCR in dogs treated for leishmaniasis with allopurinol. Vet. Res. Commun. 2005, 29, 301–303. [Google Scholar] [CrossRef]
- Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP): Recent progress in research and development. J. Infect. Chemother. 2013, 19, 404–411. [Google Scholar] [CrossRef]
- Sriworarat, C.; Phumee, A.; Mungthin, M.; Leelayoova, S.; Siriyasatien, P. Development of loop-mediated isothermal amplification (LAMP) for simple detection of Leishmania infection. Parasites Vectors 2015, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Castagnaro, M.; Crotti, A.; Fondati, A.; Gradoni, L.; Lubas, G.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Solano-Gallego, L.; Roura, X.; et al. Leishmaniosi canina: Linee guida su diagnosi, stadiazione, terapia, monitoraggio e prevenzione. Parte I: Approccio diagnostico e classificazione del paziente leishmaniotico e gestione del paziente proteinurico. Veterinaria 2007, 21, 19–32. [Google Scholar]
| Alterations | Prevalence (%) | 95% CI |
|---|---|---|
| Lymphadenopathy | 82.3 | 64.8–92.6 |
| Increasing of total proteins and low A/G ratio | 70.6 | 52.2–84.3 |
| Skin lesions | 58.8 | 40.8–74.9 |
| Ocular lesions | 50.0 | 32.8–67.3 |
| Non-regenerative anemia | 50.0 | 32.8–67.3 |
| Thrombocytopenia | 50.0 | 32.8–67.3 |
| Renal parameters (AZO, CRE, alterations proteins/urinary creatinine) | 20.6 | 9.3–38.4 |
| Weight loss | 17.7 | 7.4–35.2 |
| Onycogryphosis | 11.8 | 3.8–28.4 |
| Leukocytosis/leukopenia | 11.8 | 3.8–28.4 |
| Increasing of transaminases | 5.9 | 1.0–21.1 |
| Arthritis | 2.9 | 0.2–17.1 |
| Epistaxis | 2.9 | 0.2–17.1 |
| Splenomegaly | 2.9 | 0.2–17.1 |
| Performance | Molecular Techniques on Lymph Node Samples | |
|---|---|---|
| LAMP | Rt-PCR | |
| % (95% CI) | % (95% CI) | |
| Specificity | 96.8% (81.5–99.8) | 100% (86.3–99.7) |
| Sensitivity | 86.2% (67.4–95.5) | 96.6% (80.4–99.8) |
| NPV | 88.2% (71.6–96.2) | 96.9% (82.0–99.8) |
| PPV | 96.2% (74.4–99.8) | 100% (85.0–99.7) |
| Time to obtain results | 1 h | 1 h, 15 min |
| Lymph Nodes | Blood | Conjunctival Swabs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rt-PCR | Rt-PCR | Rt-PCR | |||||||
| LAMP | Positive | Negative | Total | Positive | Negative | Total | Positive | Negative | Total |
| Positive | 24 | 2 | 26 | 0 | 0 | 0 | 10 | 2 | 12 |
| Negative | 4 | 30 | 34 | 3 | 57 | 60 | 1 | 47 | 48 |
| Total | 28 | 32 | 60 | 3 | 57 | 60 | 11 | 49 | 60 |
| Samples (n) | Leishmania amastigotes/mL | |
|---|---|---|
| Min–Max | Mean | |
| 11 | 5–100 | 26.8 |
| 8 | 101–1000 | 575.6 |
| 9 | 1001–78,000 | 30,022.2 |
| Performance | Serological Techniques | ||
|---|---|---|---|
| IFAT | ELISA | SNAP | |
| % (95% CI) | % (95% CI) | % (95% CI) | |
| Specificity | 100% (83.4–99.6) | 88.0% (67.7–96.9) | 92.0% (72.5–98.6) |
| Sensitivity | 97.2% (83.8–99.9) | 94.3% (79.5–99.0) | 62.9% (45.0–78.0) |
| NPV | 96.2% (78.4–99.8) | 91.7 (71.5–98.5) | 63.7 (46.2–78.7) |
| PPV | 100% (87.4–99.7) | 91.7% (76.4–97.8) | 91.7 (71.5–98.5) |
| Time to obtain results | 90 min | 150 min | 10 min |
| ELISA | SNAP | |||||
|---|---|---|---|---|---|---|
| IFAT | Positive | Negative | Total | Positive | Negative | Total |
| Positive | 33 | 1 | 34 | 22 | 12 | 34 |
| Negative | 3 | 23 | 26 | 2 | 24 | 26 |
| Total | 36 | 24 | 60 | 24 | 36 | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurelli, M.P.; Bosco, A.; Foglia Manzillo, V.; Vitale, F.; Giaquinto, D.; Ciuca, L.; Molinaro, G.; Cringoli, G.; Oliva, G.; Rinaldi, L.; et al. Clinical, Molecular and Serological Diagnosis of Canine Leishmaniosis: An Integrated Approach. Vet. Sci. 2020, 7, 43. https://doi.org/10.3390/vetsci7020043
Maurelli MP, Bosco A, Foglia Manzillo V, Vitale F, Giaquinto D, Ciuca L, Molinaro G, Cringoli G, Oliva G, Rinaldi L, et al. Clinical, Molecular and Serological Diagnosis of Canine Leishmaniosis: An Integrated Approach. Veterinary Sciences. 2020; 7(2):43. https://doi.org/10.3390/vetsci7020043
Chicago/Turabian StyleMaurelli, Maria Paola, Antonio Bosco, Valentina Foglia Manzillo, Fabrizio Vitale, Daniela Giaquinto, Lavinia Ciuca, Giuseppe Molinaro, Giuseppe Cringoli, Gaetano Oliva, Laura Rinaldi, and et al. 2020. "Clinical, Molecular and Serological Diagnosis of Canine Leishmaniosis: An Integrated Approach" Veterinary Sciences 7, no. 2: 43. https://doi.org/10.3390/vetsci7020043
APA StyleMaurelli, M. P., Bosco, A., Foglia Manzillo, V., Vitale, F., Giaquinto, D., Ciuca, L., Molinaro, G., Cringoli, G., Oliva, G., Rinaldi, L., & Gizzarelli, M. (2020). Clinical, Molecular and Serological Diagnosis of Canine Leishmaniosis: An Integrated Approach. Veterinary Sciences, 7(2), 43. https://doi.org/10.3390/vetsci7020043








