A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis)
Abstract
1. Introduction
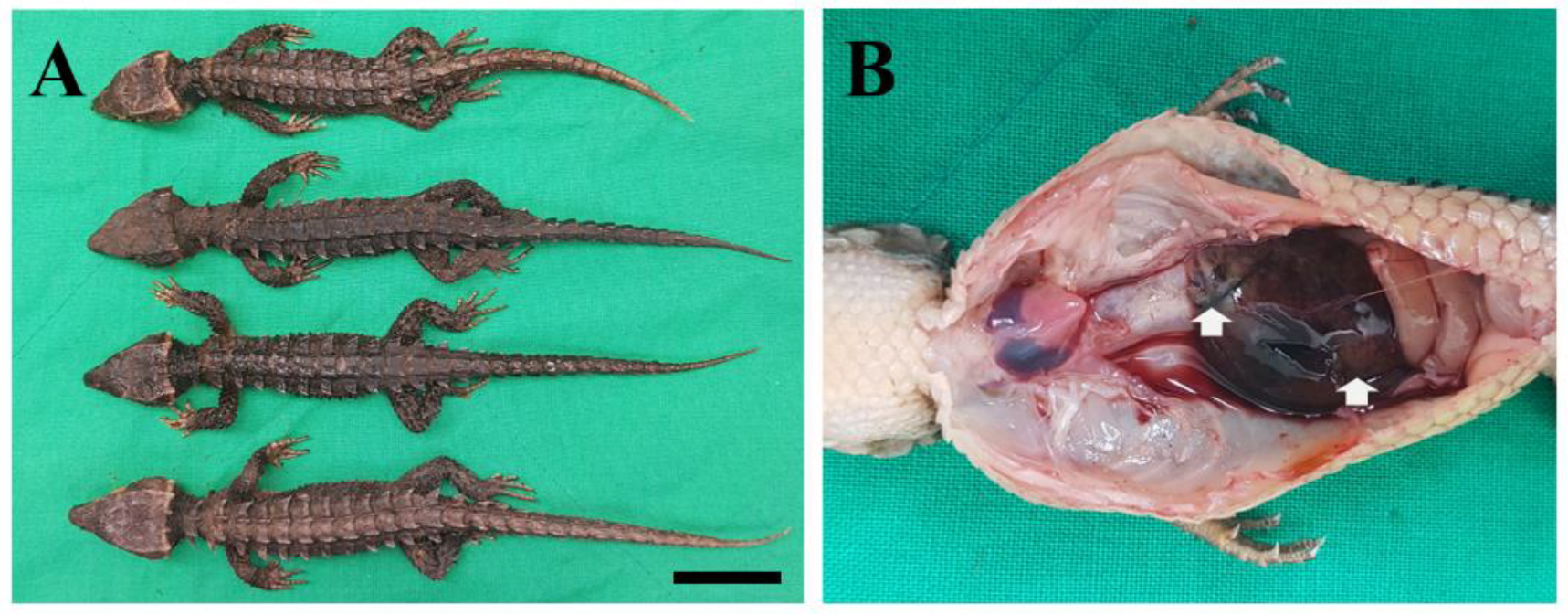
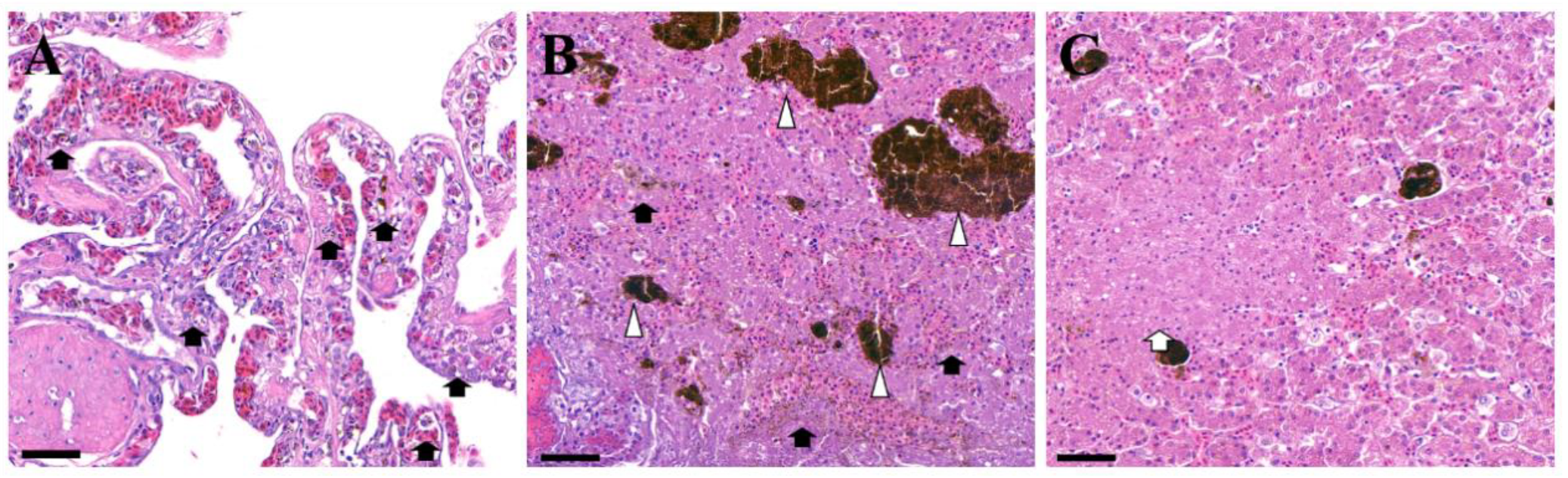
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Araujo, R.M.; Arribas, R.M.; Pares, R. Distribution of Aeromonas species in waters with different levels of pollution. J. Appl. Bacteriol. 1991, 71, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Palumbo, S.A. AEROMONAS HYDROPHILA and AEROMONAS SOBRIA as POTENTIAL FOOD POISONING SPECIES: A REVIEW 1. J. Food Saf. 1985, 7, 15–29. [Google Scholar] [CrossRef]
- Pasteris, S.E.; Bühler, M.I.; Nader-Macias, M.E. Microbiological and histological studies of farmed-bullfrog (Rana catesbeiana) tissues displaying red-leg syndrome. Aquaculture 2006, 251, 11–18. [Google Scholar] [CrossRef]
- Wright, K.M.; Whitaker, B.R. Amphibian Medicine and Captive Husbandry; Krieger Publishing Company: Malabar, FL, USA, 2001; pp. 161–165. [Google Scholar]
- Bhunia, A.K. Foodborne Microbial Pathogens; Springer: New York, NY, USA, 2018; pp. 343–350. [Google Scholar]
- Jiravanichpaisal, P.; Roos, S.; Edsman, L.; Liu, H.; Söderhäll, K. A highly virulent pathogen, Aeromonas hydrophila, from the freshwater crayfish Pacifastacus leniusculus. J. Invertebr. Pathol. 2009, 101, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Khajanchi, B.K.; Fadl, A.A.; Borchardt, M.A.; Berg, R.L.; Horneman, A.J.; Stemper, M.E.; Joseph, S.W.; Moyer, N.P.; Sha, J.; Chopra, A.K. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: Suggestive evidence of water-to-human transmission. Appl. Environ. Microb. 2010, 76, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Khan, S.A.; Khan, A.A.; Sung, K.; Tran, Q.; Kerdahi, K.; Steele, R. Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol. 2010, 27, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Auliya, M. A review of the live reptile trade in the European Union in the 1990s with a focus on Germany. In Hot Trade in Cool Creatures; TRAFFIC Europe: Brussel, Belgium, 2003; pp. 42–59. [Google Scholar]
- Farkas, S.L.; Gál, J. Adenovirus and Mycoplasma infection in an ornate box turtle (Terrapene ornata ornata) in Hungary. Vet. Microbiol. 2009, 138, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Marschang, R.E.; Becher, P.; Posthaus, H.; Wild, P.; Thiel, H.J.; Müller-Doblies, U.; Kalet, E.F.; Bacciarini, L.N. Isolation and characterization of an iridovirus from Hermann’s tortoises (Testudo hermanni). Arch. Virol. 1999, 144, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Van Devanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar]
- Van Kuppeveld, F.J.; Van der Logt, J.T.; Angulo, A.F.; Van Zoest, M.J.; Quint, W.G.; Niesters, H.G.; Galama, J.M.; Melchers, W.J. Genus-and species-specific identification of Mycoplasmas by 16S rRNA amplification. Appl. Environ. Microb. 1992, 58, 2606–2615. [Google Scholar]
- Wellehan, J.F.; Johnson, A.J.; Harrach, B.; Benkö, M.; Pessier, A.P.; Johnson, C.M.; Garner, M.M.; Childress, A.; Jacobson, E.R. Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J. Virol. 2004, 78, 13366–13369. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Al-Shuweli, S.; Yapici, S.; Jensen, J.N.; Olsen, K.E. Identification of clinical Aeromonas species by rpoB and gyrB sequencing and development of a multiplex PCR method for detection of Aeromonas hydrophila, A. caviae, A. veronii and A. media. J. Clin. Microbiol. 2015, 53, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Rodgers, M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: A PCR identification. J. Appl. Microbiol. 2004, 97, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Cook, R.A.; Bennett, E.L.; Newcomb, J. Wildlife trade and global disease emergence. Emerg. Infect. Dis. 2005, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.M. A review of the role of antibiotic policies in the control of antibiotic resistance. J. Antimicrob. Chemoth. 1999, 43, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, L.I. Responses to rapid temperature change in vertebrate ectotherms. Am. Zool. 1979, 19, 225–237. [Google Scholar] [CrossRef]
- Munday, K.A.; Blane, G.F. Cold stress of the mammal, bird and reptile. Comp. Biochem. Physiol. 1961, 2, 8–21. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Kortet, R.; Sinclair, B.J. Eco-immunology in the cold: The role of immunity in shaping the overwintering survival of ectotherms. J. Exp. Biol. 2018, 221, jeb163873. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.K.; Cooper, E.L. Temperature effects on ectotherm immune responses. Dev. Comp. Immunol. 1981, 5, 117–122. [Google Scholar] [CrossRef]
- Zapata, A.G.; Varas, A.; Torroba, M. Seasonal variations in the immune system of lower vertebrates. Immunol. Today 1992, 13, 142–147. [Google Scholar] [CrossRef]
| Primers | Sequences | Target Gene |
|---|---|---|
| act | 5′-AGAAGGTGACCACCACCAAGAACA-3′ | cytotoxic enterotoxin |
| 5′-AACTGACATCGGCCTTGAACT C-3′ | ||
| ast | 5′-TCTCCATGCTTCCCTTCCACT-3′ | cytotonic enterotoxin |
| 5′-GTGTAGGGATTGAAGAAGCCG-3′ | ||
| fla | 5′-TCCAACCGTYTGACC TC-3′ | flagellin |
| 5′-GMYTGGTTGCGRATG GT-3′ | ||
| gcaT | 5′-CTCCTGGAATCCCAAGTATCA G-3′ | glycerophospholipid: cholesterol acyltranferase |
| 5′-GGCAGGTTGAACAGCAGTATC T-3′ | ||
| ser | 5′-CACCGAAGTATTGGGTCAGG-3′ | serine protease |
| 5′-GGCTCATGCGTAACTCTGGT-3′ | ||
| ahyB | 5′-ACACGGTCAAGGAGATCAAC-3′ | elastase |
| 5′-CGCTGGTGTTGGCCAGCAGG-3′ | ||
| alt | 5′-TGACCCAGTCCTGGCACGGC-3′ | cytotonic enterotoxin |
| 5′-GGTGATCGATCACCACCAGC-3′ | ||
| aerA | 5′-CCTATGGCCTGAGCGAGAAG-3′ | aerolysin |
| 5′-CCAGTTCCAGTCCCACCACT-3′ | ||
| Aeromonas species specific primers | ||
| A. hyd | 5′-AGTCTGCCGCCAGTGGC-3′ | gyrB |
| 5′-CRCCCATCGCCTGTTCG-3′ | ||
| A. 16S | 5′-CGACGATCCCTAGCTGGTCT-3′ | 16S rRNA gene |
| 5′-GCCTTCGCCACCGGTAT-3′ | ||
| Antibiotics | A.hydrophila Isolates | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| IPM | I | I | I | I |
| MEM | S | S | S | I |
| VAN | R | R | R | R |
| AZM | S | S | S | S |
| CHL | S | S | S | S |
| GEN | S | S | S | S |
| TET | S | S | S | S |
| ATM | S | S | S | S |
| AMK | S | S | S | S |
| CRO | S | S | S | S |
| FEP | S | S | S | S |
| CAZ | S | S | S | S |
| SXT | S | S | S | S |
| TZP | S | S | S | S |
| LVX | S | S | S | S |
| CIP | S | S | S | S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Kim, S.G.; Kim, S.W.; Yun, S.; Kim, H.J.; Giri, S.S.; Han, S.J.; Oh, W.T.; Park, S.C. A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis). Vet. Sci. 2020, 7, 4. https://doi.org/10.3390/vetsci7010004
Kwon J, Kim SG, Kim SW, Yun S, Kim HJ, Giri SS, Han SJ, Oh WT, Park SC. A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis). Veterinary Sciences. 2020; 7(1):4. https://doi.org/10.3390/vetsci7010004
Chicago/Turabian StyleKwon, Jun, Sang Guen Kim, Sang Wha Kim, Saekil Yun, Hyoun Joong Kim, Sib Sankar Giri, Se Jin Han, Woo Teak Oh, and Se Chang Park. 2020. "A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis)" Veterinary Sciences 7, no. 1: 4. https://doi.org/10.3390/vetsci7010004
APA StyleKwon, J., Kim, S. G., Kim, S. W., Yun, S., Kim, H. J., Giri, S. S., Han, S. J., Oh, W. T., & Park, S. C. (2020). A Case of Mortality Caused by Aeromonas hydrophila in Wild-Caught Red-Eyed Crocodile Skinks (Tribolonotus gracilis). Veterinary Sciences, 7(1), 4. https://doi.org/10.3390/vetsci7010004






