Exploring the Mental Model of Cattle Farmers in Disease Prevention and Control Practices
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Farmers’ Characteristics
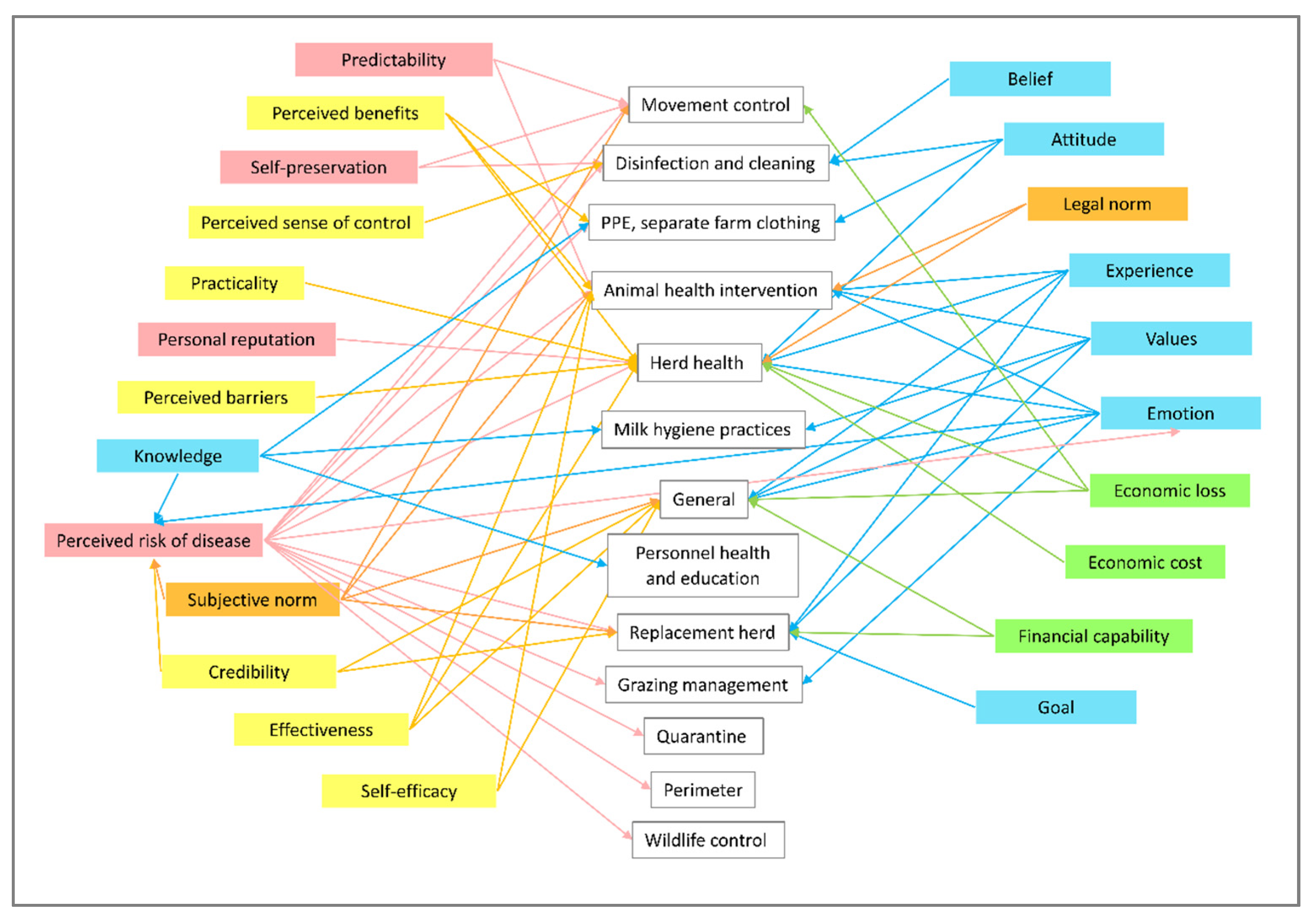
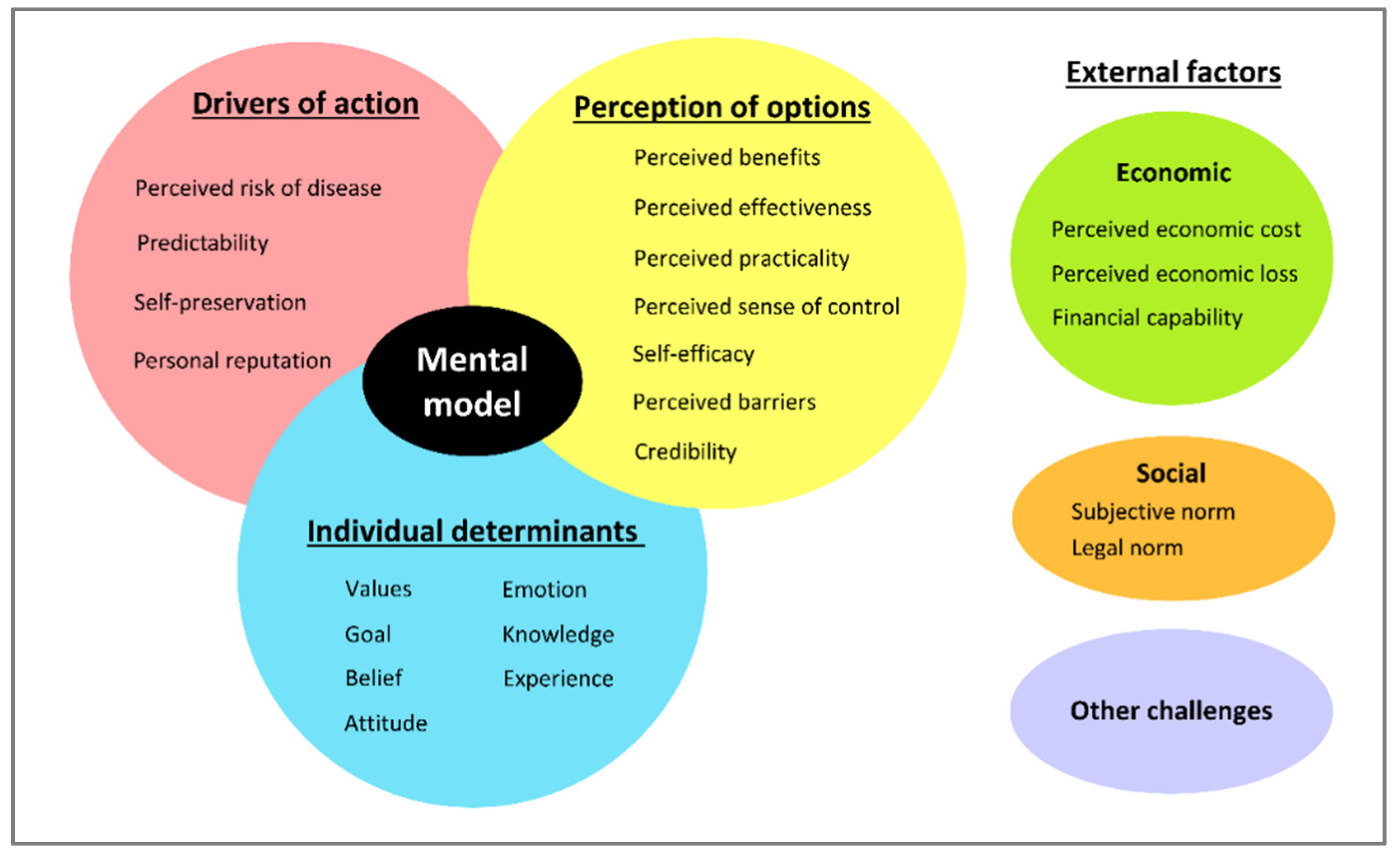
3.2. Mental Model
3.2.1. Drivers of Action
Must be careful. When you buy new cows and bulls, you have to do blood test first. Some people have Brucella, they won’t tell you. Then the sick animals come to your farm, breed with our cows, they will get Brucella.(F2)
You see, first is you cannot buy diseased animals. If the animal is carrying disease, the disease is there. Get good animals, clean animals, from abroad, not local. You cannot source animals locally now.(F5)
We cannot bring animals from outside. We just keep whatever we have and we test them again. We must cull the infected ones. Other than that, we can’t do much. Or, we can cull the entire herd.(F1)
Every day we make sure that we throw all feces away to make sure the floor is clean and wash their legs and nails because diseases come from their nails.(F4)
When outbreaks are happening, we will definitely put some sacks of lime and all at the main entrance.(F5)
Wild cows can jump a 4-foot drain. I make big perimeter drains and STILL they come in! And then there’s a lot of wild boars. Wild boars carry a lot of diseases, blood parasite diseases. So it’s very challenging.(F3)
No grazing, I cut and carry. Too scared to let them out to graze. When you know this group of friends has all kinds of sickness, you won’t want to mix with them. You know that all the village cows are there, why would you want to let your cows graze?(F3)
3.2.2. Perception of Practice Options
I think the effectiveness of vaccines and drugs are important. But the vaccines for FMD are not practical. The hassle of catching every cattle for vaccination every six months is too much work. You need 5 people to catch because there’s no proper cattle crush.(F7)
The tube well is very expensive. But it’s okay, I’m only thinking of improving the quality of the milk, the quality of the cows, their life, you know, give them the best.(F3)
During an outbreak, we will spend on medicine and vitamins. We will increase vitamin supplementation to increase their antibody based on their body weight. We are confident to give this because we have a weighing scale.(F4)
3.2.3. Individual Determinants
We don’t buy from neighboring countries. We only buy from local reputable suppliers with proper records. Because animals that we bought last time died. We don’t know why. It looks physically healthy. When we bought, it did not have any records of drugs given and health.(F4)
We better grow ourselves, buy animals ourselves without assistance. My farm had tuberculosis from the pregnant heifers introduced under the previous animal subsidized scheme. Foot-and-mouth disease broke out in the first week, and later many had tuberculosis.(F5)
If you go to any farm, you must be clean, you must have medicine, and most important is to spray disinfectant. If this farm has disease, the germs sometimes touch your things, your boots, and your shoes. So when you go to their farm, the germs will spread. FMD can spread very fast. You can see the signs in 12 h–10 h.(F2)
Workers must go for medical check-ups. Sometimes, foreigners have tuberculosis. Sometimes, the cows can also be infected. When we spit, maybe the cow comes in contact.(F2)
I’ve learned about (brucellosis) from my time studying at university but I’ve never seen a case. But it’s a serious disease, so it’s important that people are aware and that my workers are aware.(F7)
If I have my old cows here, and I add in new cows, all these cows will also get sick! I am also very worried. When you get a headache, you take Panadol, isn’t it? Headache gone. That’s what I’m doing now. I’m making myself feel comfortable that I’m bringing in new cows, all disease free.(F3)
3.2.4. External (Social and Economic)
Usually our animal department will have workshops or programs with vets and lecturers from UPM. The measures you must take to prevent, etc. I mainly get my advice from UPM doctors. I can call them anytime and they can advise me to do this, do that.(F3)
We also had to slaughter some that were not having diseases because it was recommended by the veterinary authority, just dispose.(F5)
Through word of mouth, my friends will tell me careful of some cows, there’s disease.(F3)
We join a lot of breeders Facebook groups. From there we get information when they update on current disease status.(F4)
When we have diseases, we have to put animals to sleep. It will affect my milk production and income. I have to source for milk outside and profit margin will be less. If we don’t spend to prevent diseases now, we will regret later. The loss will be bigger, very big!(F1)
So after I buy the new cows, I will be more careful. I won’t even let students come inside. Because you all visit a lot of farms also, you can carry the disease, you see. And you know, the cows are very expensive, RM 8000, 9000, 10,000. Loss of livestock is a big deal for me. We have no insurance. If anything happens, we cannot get compensation.(F3)
This is not really a business [for me]. I lose money every month so nothing matters to me. But if the stock is wiped out then I’ll be very upset.(F7)
I lived abroad for a few years and I like animal farming. I think the cattle farms are very beautiful. So I wanted something like that.(F7)
3.2.5. External (Other Challenges)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department of Veterinary Services. DVS Perangkaan Ternakan. In Department of Veterinary Services, Malaysia. Available online: http://www.dvs.gov.my/dvs/resources/user_1/2019/BP/Perangkaan Ternakan/3._Msia__Perangkaan_ternakan_M_Surat_1-15_.pdf (accessed on 9 October 2019).
- Serin, T.; Hashim, F.A.H. Status and demand of technology for selected beef cattle poducers in Peninsular Malaysia. Econom. Tech. Manag. Rev. 2010, 5, 21–26. [Google Scholar]
- Jamaludin, M.H.; Hassan, M.H.; Amin, M.R.; Zulhisyam, A.K. The Future of the Malaysian Beef Industry. J. Trop. Resour. Sustain. Sci. 2014, 2, 23–29. [Google Scholar]
- Nor Amna, A.M.N.; Rosali, M.H.; Mohd Syauqi, N.; Ahmad Zairy, Z.A.; Nurul Huda, S. Adoption of Technology in Malaysia’s Livestock Industry. Available online: http://ap.fftc.agnet.org/ap_db.php?id=946 (accessed on 28 October 2019).
- Muhayat, F. Animal Feed Resources and Management in Malaysia. In FAO-APHCA Regional Workshop on Animal Feed Resources and their Management in the Asia-Pacific Region; Department of Veterinary Services: Bangkok, Malaysia, 2013. [Google Scholar]
- Economic Planning Unit. Strategy Paper 20. Driving Modernisation in Agro-food. Eleventh Malaysia Plan 2016–2020. Available online: https://www.talentcorp.com.my/clients/TalentCorp_2016_7A6571AE-D9D0-4175-B35D-99EC514F2D24/contentms/img/publication/RMKe-11%20Book.pdf (accessed on 9 October 2019).
- Perry, B.D.; Grace, D.; Sones, K. Current drivers and future directions of global livestock disease dynamics. Proc. Nat. Acad. Sci. USA 2006, 110, 20871–20877. [Google Scholar] [CrossRef]
- Anka, M.S.; Hassan, L.; Adzhar, A.; Khairani-Bejo, S.; Mohamad, R.B.; Zainal, M.A. Bovine brucellosis trends in Malaysia between 2000 and 2008. BMC Vet. Res. 2013, 9, 230. [Google Scholar] [CrossRef]
- Chandrawathani, P.; Tariq, J.; Saira Banu, M.R.; Norasyikin, A.; Rohana, A.B.; Faizah Hanim, M.S.; Zulkifli, A.; Santhi, M.; Marzuki, Z. Zoonotic diseases diagnosed from Jan 2016 to Aug 2017 in Regional Veterinary Laboratories, Department Of Veterinary Services, Malaysia. Malays. J. Vet. Res. 2018, 9, 115–121. [Google Scholar]
- Anka, M.S.; Hassan, L.; Khairani-Bejo, S.; Zainal, M.A.; Mohamad, R.B.; Salleh, A.; Adzhar, A. A case-control study of risk factors for bovine brucellosis seropositivity in peninsular Malaysia. PLoS ONE 2014, 9, e108673. [Google Scholar] [CrossRef]
- Zamri-Saad, M.; Kamarudin, M.I. Control of animal brucellosis: The Malaysian experience. Asian Pac. J. Trop. Med. 2016, 9, 1136–1140. [Google Scholar] [CrossRef]
- Zainuddin, M.Z.; Mustafa, S. Lembu, Kerbau Seludup Dikhuatiri Bawa Penyakit. Available online: https://www.bharian.com.my/berita/nasional/2017/08/318666/lembu-kerbau-seludup-dikhuatiri-bawa-penyakit (accessed on 10 October 2019).
- Bernama. Ladang Ternakan Didenda RM10,000 Jual Lembu Berpenyakit TB. Available online: Ladang ternakan didenda RM10,000 jual lembu berpenyakit TB (accessed on 9 October 2019).
- Lim, K.G.; Centre, T.M.; Taiping, J.M. Rabies Raises its Ugly Head Once More. Med. J. Malaysia. 1998, 53, 4–5. [Google Scholar]
- Rai, S.B.; Kamaludin, F.; Chow, T.S.; Yoon, C.K. First Documented Zoonotic Case of Q Fever in Penang, Malaysia. Outbreak Surveill. Investig. Rep. 2011, 4, 1–5. [Google Scholar]
- Firdaus, F.; Jesse, A.; Bitrus, A.A.; Abba, Y.; Sadiq, M.A.; Umar, I.; Lim, E.; Chung, T.; Lau, F.; Ping, P.; et al. Case Report A clinical case of bovine trypanosomosis in an endemic farm in Malaysia. J. Adv. Vet. Anim. Res. 2016, 3, 286–291. [Google Scholar]
- Daud, A.; Mohd, N.; Mohd, H.; Arshad, M.M.; Kamarudin, S.; Zahiruddin, W.M. Leptospirosis seropositivity and its serovars among cattle in Northeastern Malaysia. Vet. World 2018, 11, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.; Koh, F.; Jaafar, T.; Nizamuddin, Q.; Nizam, H.; Tay, S. Prevalence and molecular heterogeneity of Bartonella bovis in cattle and Haemaphysalis bispinosa ticks in Peninsular Malaysia. BMC Vet. Res. 2015, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Masrin, A.; Magendren, S.; Chandrawathani, P.; NurainIrzierah, I. Current status of fascioliasis in ruminants: Cases diagnosed from 2004 to 2013 in VRI, Ipoh. In Proceedings of the 51st MSPTM Annual Scientific Conference, Kuala Lumpur, Malaysia, 15–20 May 2015. [Google Scholar]
- Ritter, C.; Jansen, J.; Roche, S.; Kelton, D.F.; Adams, C.L.; Orsel, K.; Erskine, R.J.; Benedictus, G.; Lam, T.J.G.M.; Barkema, H.W. Invited review: Determinants of farmers’ adoption of management-based strategies for infectious disease prevention and control. J. Dairy Sci. 2017, 100, 3329–3347. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Abdullah, J.; Abubak, M.; Sadiq, A.R.; Ropie, A.M.; Mohammed, K.; Teik, E.L.I.M.; Bitrus, A.A.; Hanani, N.; Mat, B.T.; et al. A cross-sectional study on the association between farmers’ awareness and compliance on herd health program among five selected dairy cattle farm in Selangor and Negeri Sembilan state in Malaysia. Malays. J. Vet. Res. 2017, 8, 19–29. [Google Scholar]
- Nor Amna, A.M.N.; Mohd Syauqi, N.; Ahmad Zairy, Z.A.; Nurul Huda, S. Kajian Penilaian Tahap Penggunaan Teknologi Bagi Subsektor Pertanian Terpilih ke Arah Pertanian Moden: Lembu Tenusu. Laporan Kajian Sosioekonomi 2017 Pusat Penyelidikan Ekonomi dan Sains Sosial. 2017. Available online: http://etmr.mardi.gov.my/Content/Report/2017/Artikel%2011-Nor%20Amna%20et%20al.pdf (accessed on 10 October 2019).
- Kristensen, E.; Jakobsen, E. Evaluation of dairy herd health management. In Proceedings of the XXVI World Buiatrics Congress, Santiago, Chile, 14–18 November 2010; pp. 53–64. [Google Scholar]
- Garforth, C. Livestock keepers’ reasons for doing and not doing things which governments, vets and scientists would like them to do. Zoonoses Public Health 2015, 62, 29–38. [Google Scholar] [CrossRef]
- Mankad, A. Psychological influences on biosecurity control and farmer decision-making. A review. Agron. Sustain. Dev. 2016, 36, 40. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Cook, A.J.C.; Watson, E.; Nielen, M.; Larkin, L.; Wooldridge, M.; Hogeveen, H. Perceptions, circumstances and motivators that influence implementation of zoonotic control programs on cattle farms. Prev. Vet. Med. 2015, 93, 276–285. [Google Scholar] [CrossRef]
- Wood, M.D.; Thorne, S.; Kovacs, D.; Butte, G.; Linkov, I. An Introduction to Mental Modeling. In Mental Modeling Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–9. [Google Scholar]
- Denzau, A.; North, D. Shared Mental Models: Ideologies and Institutions. Kyklos 1994, 47, 3–31. [Google Scholar] [CrossRef]
- Eckert, E.; Bell, A. Invisible Force: Farmers’ Mental Models and How They Influence Learning and Actions. J. Ext. 2005, 43, 1–9. [Google Scholar]
- Pauen, M. Emotion, Decision and Mental Models. In Mental Models and the Mind: Current Developments in Cognitive Psychology, Neuroscience, and Philosophy of Mind; Elsevier Masson: Paris, France, 2006; Volume 138, pp. 173–188. [Google Scholar]
- Bradshaw, C.; Atkinson, S.; Doody, O. Employing a Qualitative Description Approach in Health Care Research. Glob. Qualit. Nurs. Res. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Saharee, A.A.; Jesse, F.F.; Wahid, A.H.; Ramanoon, S.Z.; Mansor, R.; Syed Hassan, S. University-Farmer Linkages: For Smallholder Ruminant Productivity and Graduate Day- One Competency. Available online: https://uctc.upm.edu.my/upload/dokumen/20170406101350ABD_AZIZ_SAHAREE.pdf (accessed on 10 October 2019).
- Krauss, S.E.; Hamzah, A.; Omar, Z.; Suandi, T.; Ismail, I.A.; Zahari, M.Z.; Nor, Z.M. Preliminary Investigation and Interview Guide Development for Studying how Malaysian Farmers Form their Mental Models of Farming. Qualit. Rep. 2009, 14, 245–260. [Google Scholar]
- Elo, S.; Kyngas, H. The qualitative content analysis process. J. Adv. Nurs. 2007, 62, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.N. An overview of qualitative research methodology for public health researchers. Int. J. Med. Public Health 2014, 4, 318–323. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Jones, J.; Turunen, H.; Snelgrove, S. Theme development in qualitative content analysis and thematic analysis. J. Nurs. Edu. Pract. 2016, 6, 100–110. [Google Scholar] [CrossRef]
- Garforth, C. Motivating farmers: Insights from social psychology. In Proceedings of the 49th Annual Meeting of the National Mastitis Council, Orlanda, FL, USA, 31 January–3 February 2010; pp. 60–67. [Google Scholar]
- Garforth, C.J.; Bailey, A.P.; Tranter, R.B. Farmers’ attitudes to disease risk management in England: A comparative analysis of sheep and pig farmers. Prev. Vet. Med. 2013, 110, 456–466. [Google Scholar] [CrossRef]
- Kristensen, E.; Jakobsen, E.B. Danish dairy farmers’ perception of biosecurity. Prev. Vet. Med. 2011, 99, 122–129. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Kelly, T.R.; Bunn, D.A.; Joshi, N.P.; Grooms, D.; Devkota, D.; Devkota, N.R.; Paudel, L.N.; Roug, A.; Wolking, D.J.; Mazet, J.A. Awareness and Practices Relating to Zoonotic Diseases Among Smallholder Farmers in Nepal. EcoHealth 2018, 15, 656–669. [Google Scholar] [CrossRef]
- Lerner, J. Emotions and Decision Making. Annual Rev. Psychol. 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Chilonda, P.; Van Huylenbroeck, G. A conceptual framework for the economic analysis of factors influencing decision-making of small-scale farmers in animal health management. OIE Rev. Sci. Tech. 2001, 20, 687–700. [Google Scholar] [CrossRef]
- Jansen, J.; Lam, T.J.G.M. The Role of Communication in Improving Udder Health. Vet. North Am. Food Anim. Prac. 2012, 28, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Jakobsen, E.B. Challenging the myth of the irrational dairy farmer: Understanding decision-making related to herd health. N. Z. Vet. J. 2011, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Garforth, C.; Mckemey, K.; Yates, C.; Rana, R. Farmers’ behavioural inclinations and their influence on the anticipated response to the reform of the Common Agricultural Policy in England. J. Farm Manag. 2008, 13, 1–29. [Google Scholar]
- Lilford, R.J.; Braunholtz, D.; Lilford, R.J.; Braunholtz, D. Reconciling the Quantitative and Qualitative Traditions—The Bayesian Approach. Pub. Money Manag. 2003, 23, 203–208. [Google Scholar] [CrossRef]
| Production Type | Production System | Cattle Herd Size | Other Farmed Animals | Years of Farming Experience | Familiar Zoonotic Disease | Experienced Tb or Bru Outbreak |
|---|---|---|---|---|---|---|
| Dairy | Semi-intensive | 80 | Buffalo, goats | >30 | Tb, Bru | Yes |
| Dairy | Intensive | 60 | - | >20 | Tb | Yes |
| Dairy | Intensive | 150 | Buffalo | >40 | Tb, Bru | Yes |
| Beef | Semi-intensive | 200 | - | >40 | Tb, Bru | Yes |
| Beef | Intensive | 40 | Horse, goats | >10 | Tb, Bru | No |
| Beef & Dairy | Semi-intensive | 200 | Buffalo | >30 | Tb, Bru | No |
| Beef & Dairy | Intensive | 100 | Buffalo | >10 | Bru | No |
| No. | Practices | Category of Practices |
|---|---|---|
| 1 | Disinfection and cleaning | Biosecurity |
| 2 | Personal protective equipment (PPE)/separate farm clothing | |
| 3 | Movement control | |
| 4 | Replacement herd | |
| 5 | Quarantine | |
| 6 | Perimeter | |
| 7 | Wildlife control | |
| 8 | Grazing management | |
| 9 | Clean water | Herd health |
| 10 | Disease screening | |
| 11 | Animal care and monitoring | |
| 12 | Isolation | |
| 13 | Vaccination | |
| 14 | Veterinary | Animal health intervention |
| 15 | Supplementation | |
| 16 | Drug use | |
| 17 | Culling | |
| 18 | Milk hygiene practices | Milk hygiene practices |
| 19 | Personnel education | Personnel health and education |
| 20 | Personnel health check | |
| 21 | General disease control practices | General |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suit-B, Y.; Hassan, L.; Krauss, S.E.; Ramanoon, S.Z.; Ooi, P.T.; Yasmin, A.R.; Epstein, J. Exploring the Mental Model of Cattle Farmers in Disease Prevention and Control Practices. Vet. Sci. 2020, 7, 27. https://doi.org/10.3390/vetsci7010027
Suit-B Y, Hassan L, Krauss SE, Ramanoon SZ, Ooi PT, Yasmin AR, Epstein J. Exploring the Mental Model of Cattle Farmers in Disease Prevention and Control Practices. Veterinary Sciences. 2020; 7(1):27. https://doi.org/10.3390/vetsci7010027
Chicago/Turabian StyleSuit-B, Yong, Latiffah Hassan, Steven Eric Krauss, Siti Zubaidah Ramanoon, Peck Toung Ooi, Abd Rahaman Yasmin, and Jonathan Epstein. 2020. "Exploring the Mental Model of Cattle Farmers in Disease Prevention and Control Practices" Veterinary Sciences 7, no. 1: 27. https://doi.org/10.3390/vetsci7010027
APA StyleSuit-B, Y., Hassan, L., Krauss, S. E., Ramanoon, S. Z., Ooi, P. T., Yasmin, A. R., & Epstein, J. (2020). Exploring the Mental Model of Cattle Farmers in Disease Prevention and Control Practices. Veterinary Sciences, 7(1), 27. https://doi.org/10.3390/vetsci7010027






