Connexin 26 and Connexin 43 in Canine Mammary Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
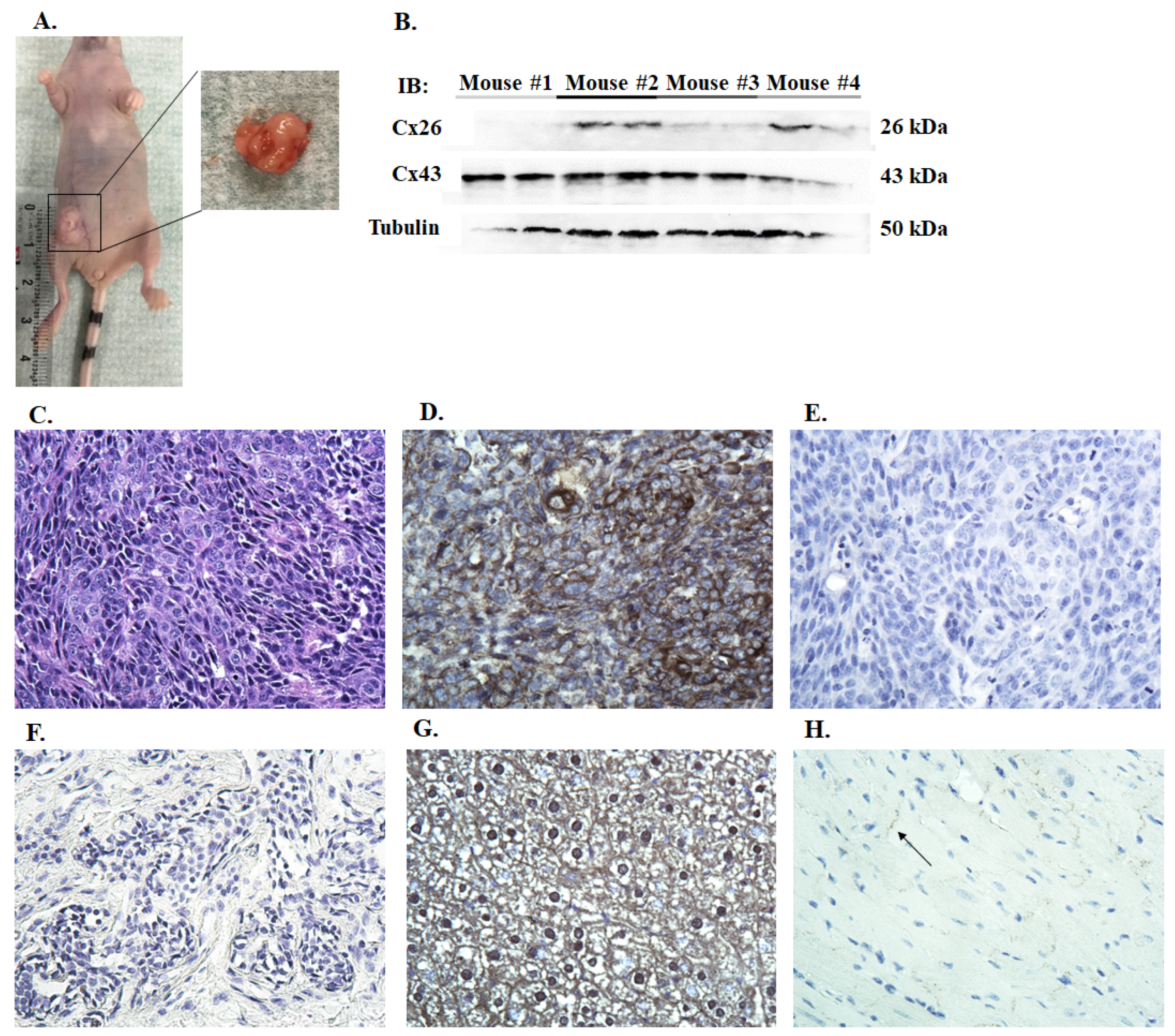
2.2. Xenograft Tumor Model
2.3. Ethics Statement
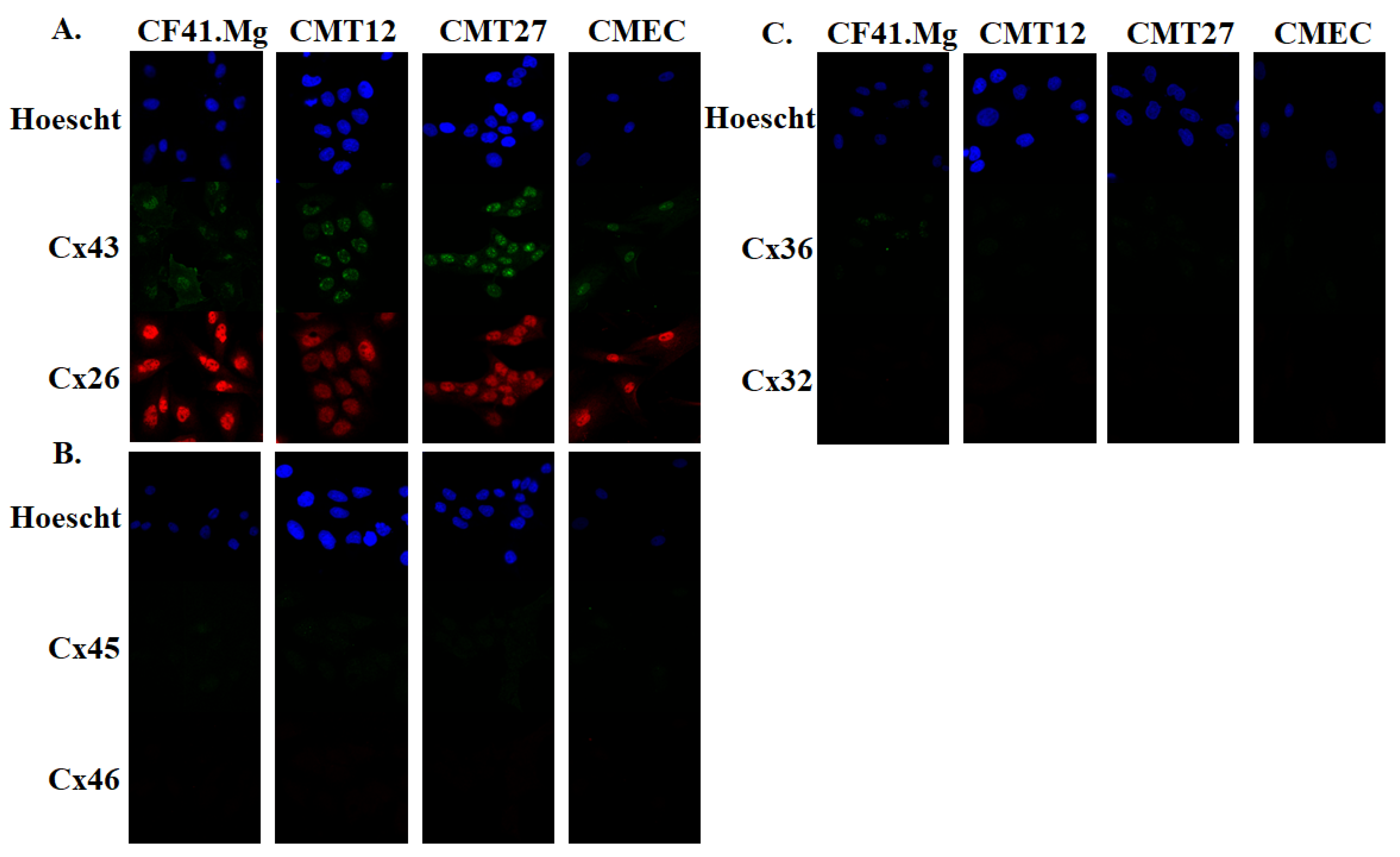
2.4. Immunofluorescence
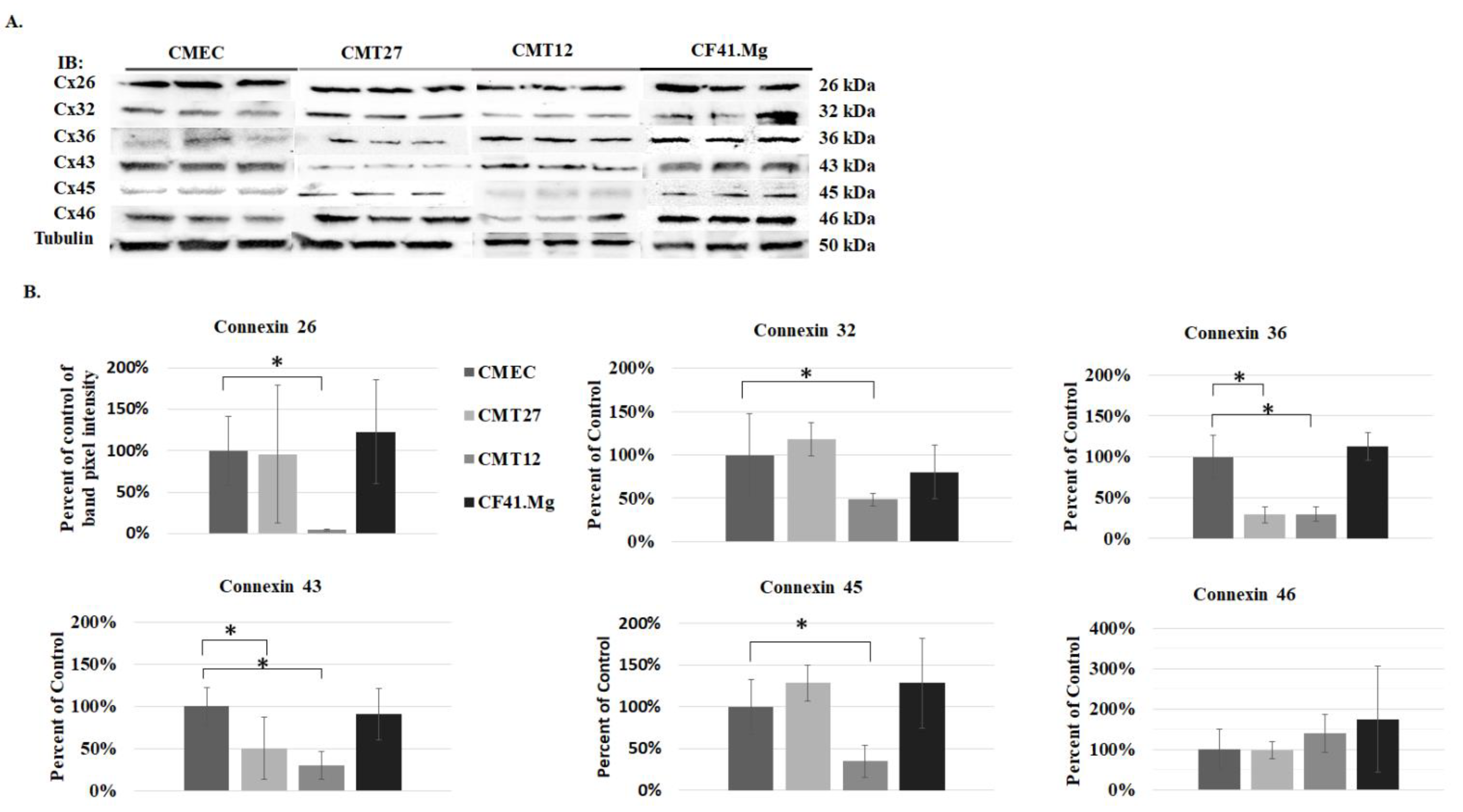
2.5. Western Blot Analysis
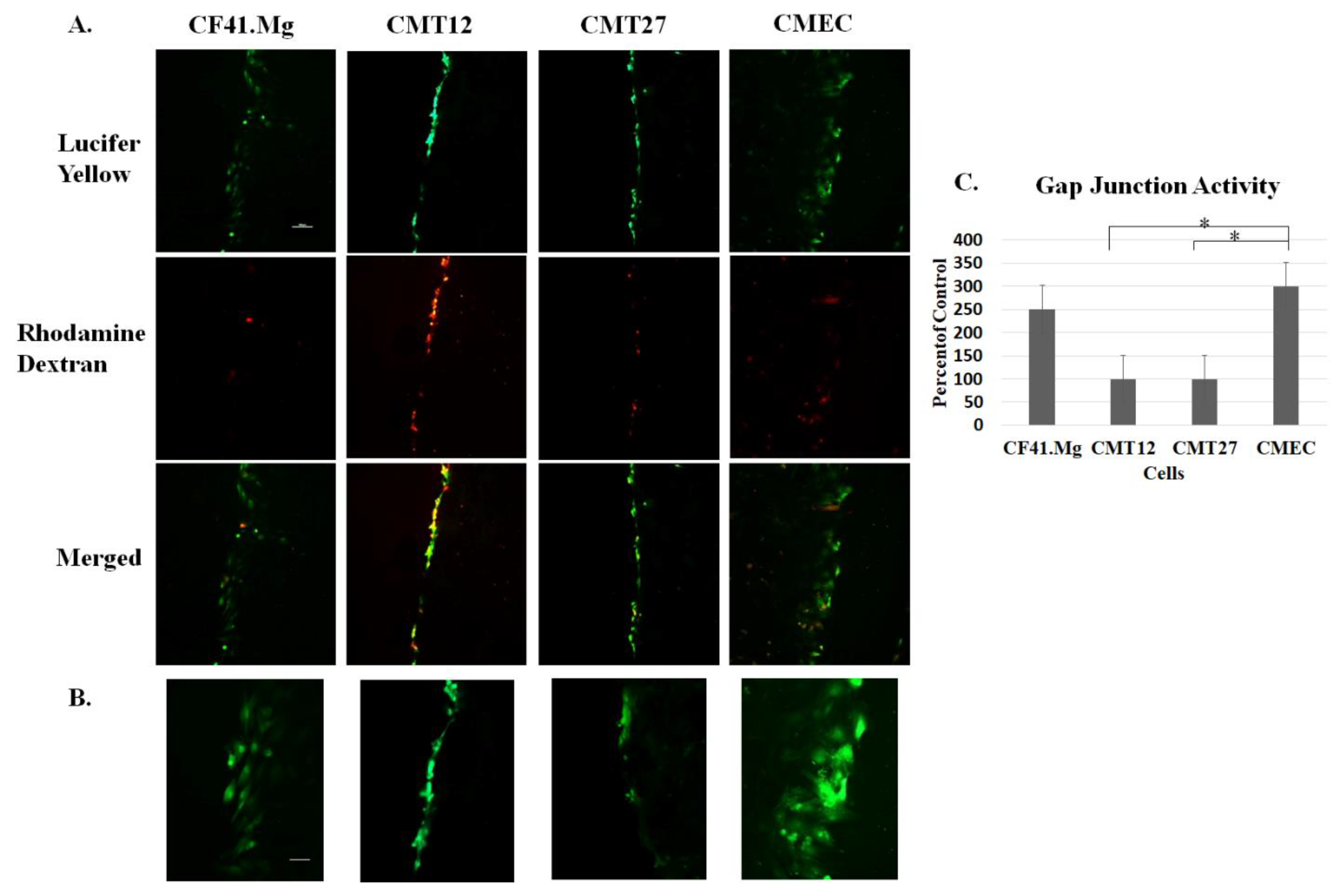
2.6. Scrape Load/Dye Transfer Assay
2.7. Immunohistochemistry
3. Results
3.1. Connexin Expression in CMEC and CMT Cells
3.2. Connexin Expression in Canine Mammary Cells Xenotransplanted to Nude Mice
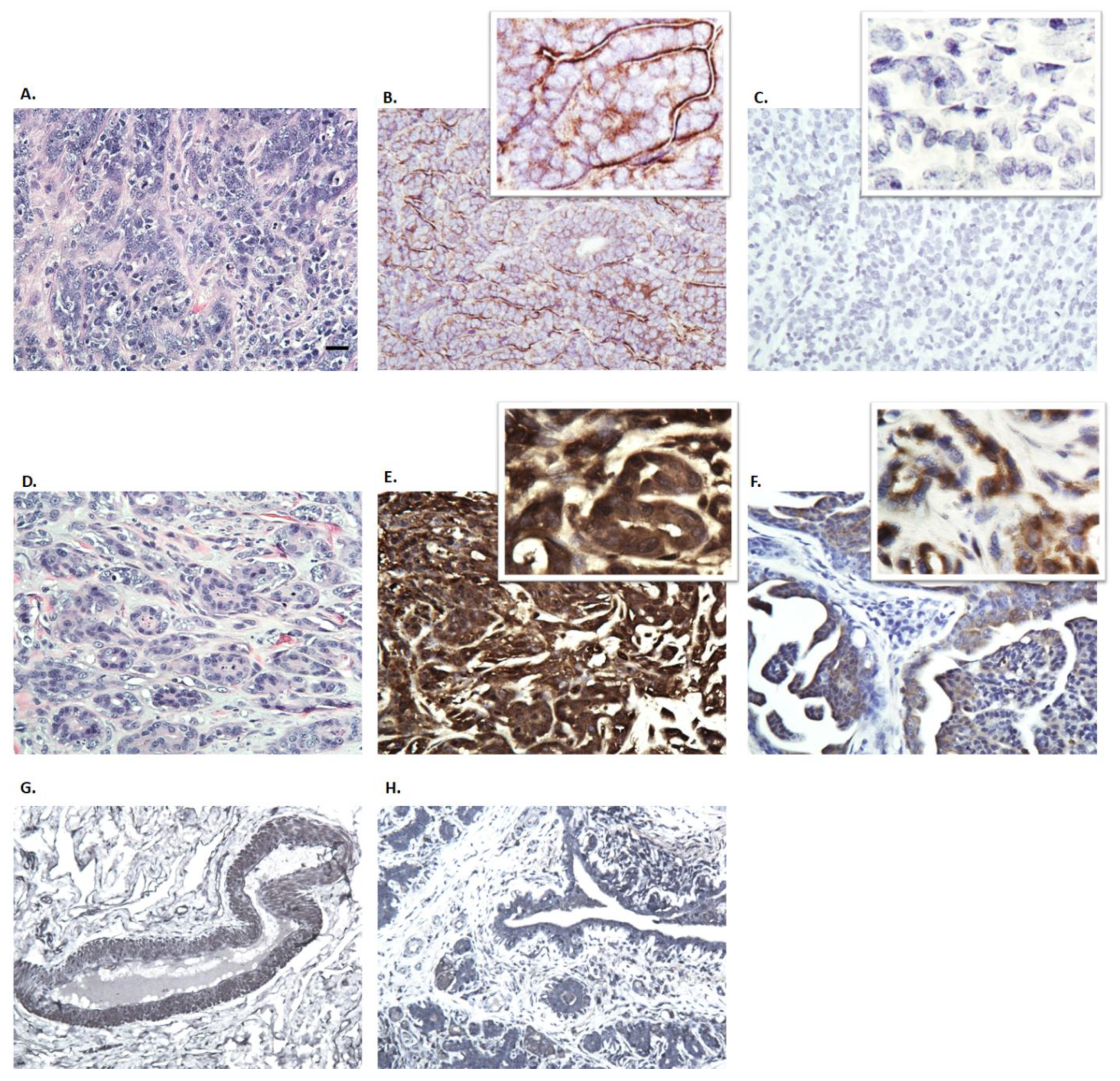
3.3. Immunohistochemistry of Canine Mammary Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C. Cancer Incidence in Pet Dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Alenza, M.D.P.; Pena, L.; Castillo, N.D.; Nieto, A.I. Factors influencing the incidence and prognosis of canine mammary tumours. J. Small Anim. Pract. 2000, 41, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and tissue growth. I. Cancerous growth. J. Cell Biol. 1967, 33, 225–234. [Google Scholar] [CrossRef]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Guldenagel, M. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef]
- Alexander, D.B.; Goldberg, G.S. Transfer of biologically important molecules between cells hrough gap junction channels. Curr. Med. Chem. 2003, 10, 2045–2058. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Xu, C.E.; Tsukamoto, T.; Sager, R. Gap junction genes Cx26 and Cx43 individually suppress the cancer phenotype of human mammary carcinoma cells and restore differentiation potential. Cell Growth Differ. 1996, 7, 861–870. [Google Scholar]
- Harris, A.L.; Contreras, J.E. Motifs in the permeation pathway of connexin channels mediate voltage and Ca 2+ sensing. Front. Phys. 2014, 5, 113. [Google Scholar] [CrossRef]
- Yamasaki, H.; Naus, C. Role of connexin genes in growth control. Carcinogenesis 1996, 17, 1199–1213. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef]
- Warner, A. Gap junctions in development—A perspective. Semin. Cell Biol. 1992, 3, 81–91. [Google Scholar] [CrossRef]
- Lee, S.; Tomasetto, C.; Paul, D.; Keyomarsi, K.; Sager, R. Transcriptional down-regulation of gap-junction proteins blocks junctional communication in human mammary tumor cell lines. J. Cell Biol. 1992, 118, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Kanno, Y. Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature 1966, 209, 1248. [Google Scholar] [CrossRef] [PubMed]
- Wilgenbus, K.K.; Kirkpatrick, C.J.; Knuechel, R.; Willecke, K.; Traub, O. Expression of Cx26, Cx32 AND Cx43 gap junction proteins in normal and neoplastic human tissues. Int. J. Cancer 1992, 51, 522–529. [Google Scholar] [CrossRef]
- Monaghan, P.; Moss, D. Connexin expression and gap junctions in the mammary gland. Cell Biol. Int. 1996, 20, 121–125. [Google Scholar] [CrossRef]
- Gotoh, H.; Harada, K.; Suzuki, K.; Hashimoto, S.; Yamamura, H.; Sato, T. Expression patterns of connexin 26 and connexin 43 mRNA in canine benign and malignant mammary tumours. Vet. J. 2006, 172, 178–180. [Google Scholar] [CrossRef]
- Torres, L.N.; Matera, J.M.; Vasconcellos, C.H.; Avanzo, J.L.; Hernandez-Blazquez, F.J.; Dagli, M.L.Z. Expression of connexins 26 and 43 in canine hyperplastic and neoplastic mammary glands. Vet. Pathol. 2005, 42, 633–641. [Google Scholar] [CrossRef]
- Chen, S.C.; Pelletier, D.B.; Ao, P.; Boynton, A.L. Connexin43 reverses the phenotype of transformed cells and alters their expression of cyclin/cyclin-dependent kinases. Cell Growth Differ. 1995, 6, 681–690. [Google Scholar]
- Wolfe, L.G.; Smith, B.B.; Toivio-Kinnucan, M.A.; Sartin, E.A.; Kwapien, R.P.; Henderson, R.A. Biologic properties of cell lines derived from canine mammary carcinomas. J. Natl. Cancer Inst. 1986, 77, 783–792. [Google Scholar] [CrossRef]
- Rash, J.; Davidson, K.; Kamasawa, N.; Yasumura, T.; Kamasawa, M.; Zhang, C. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J. Neurocytol. 2005, 34, 307–341. [Google Scholar] [CrossRef]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Krol, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Mitra, S.; Katoch, P.; Kelsey, L.S.; Johnson, K.R.; Mehta, P.P. Phosphorylation on Ser-279 and Ser-282 of connexin43 regulates endocytosis and gap junction assembly in pancreatic cancer cells. Mol. Biol. Cell. 2013, 24, 715. [Google Scholar] [CrossRef] [PubMed]
- Leithe, E.; Kjenseth, A.; Sirnes, S.; Stenmark, H.; Brech, A.; Rivedal, E. Ubiquitylation of the gap junction protein connexin-43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg101. J. Cell Sci. 2009, 122, 3883. [Google Scholar] [CrossRef] [PubMed]
- Leonel, C.; Borin, T.; Ferreira, L.; Moschetta, M.; Bajgelman, M.; Viloria-Petit, A. Inhibition of epithelial-mesenchymal transition and metastasis by combined TGFbeta knockdown and metformin treatment in a canine mammary cancer xenograft model. J. Mammary Gland Biol. Neoplasia 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Gelaleti, G.B.; Borin, T.F.; Maschio-Signorini, L.B.; Moschetta, M.G.; Hellmén, E.; Viloria-Petit, A.M. Melatonin and IL-25 modulate apoptosis and angiogenesis mediators in metastatic (CF-41) and non-metastatic (CMT-U229) canine mammary tumour cells. Vet. Comp. Oncol. 2017, 15, 1572–1584. [Google Scholar] [CrossRef]
| Connexin | Organ/Tissue Distribution |
|---|---|
| Cx26 | Liver, mammary gland, pancreas, skin, lung, brain, and endometrium |
| Cx32 | Liver, brain, uterus, thyroid, kidney, and pancreas |
| Cx36 | Neuronal cells of the central nervous system |
| Cx43 | Epithelia, heart, lens, bone, skin, pancreas, uterus, gonads, bone, connective tissue, and brain |
| Cx45 | Heart, smooth muscle, and neurons |
| Cx46 | Heart, lens, kidney, lung, bone, and testes |
| Summary of Immunohistochemistry | ||||
|---|---|---|---|---|
| Dog | Breed | Spayed/Intact | Cx26 | Cx43 |
| 1 | English setter | Spayed | + | − |
| 2 | German shepherd | Spayed | + | − |
| 3 | Unknown | Intact | + | + |
| 4 | Rat terrier | Spayed | + | − |
| 5 | Miniature pinscher | Spayed | + | − |
| 6 | Great Dane | Spayed | + | − |
| 7 | German shorthaired pointer | Spayed | + | − |
| 8 | Mixed breed | Intact | + | Weak + |
| 9 | Schnauzer | Spayed | + | + |
| 10 | Labrador retriever | Spayed | + | − |
| 11 | Shih Tzu | Spayed | + | − |
| 12 | Beagle | Spayed | Weak + | − |
| Overall positivity | 100% | 25% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu, S.; Bell, C.; Schneider, S.; Nguyen, T.A. Connexin 26 and Connexin 43 in Canine Mammary Carcinoma. Vet. Sci. 2019, 6, 101. https://doi.org/10.3390/vetsci6040101
Luu S, Bell C, Schneider S, Nguyen TA. Connexin 26 and Connexin 43 in Canine Mammary Carcinoma. Veterinary Sciences. 2019; 6(4):101. https://doi.org/10.3390/vetsci6040101
Chicago/Turabian StyleLuu, Savannah, Cynthia Bell, Sarah Schneider, and Thu Annelise Nguyen. 2019. "Connexin 26 and Connexin 43 in Canine Mammary Carcinoma" Veterinary Sciences 6, no. 4: 101. https://doi.org/10.3390/vetsci6040101
APA StyleLuu, S., Bell, C., Schneider, S., & Nguyen, T. A. (2019). Connexin 26 and Connexin 43 in Canine Mammary Carcinoma. Veterinary Sciences, 6(4), 101. https://doi.org/10.3390/vetsci6040101





