Abstract
A cross-sectional study aimed at assessing the seroprevalence and identifying the risk factors for Toxoplasma gondii infection in cattle, sheep, and goats in eight provinces located in two main Algerian agro-ecological zones was carried out from October 2015 to March 2018. Blood sera from 4074 animals of both sexes were tested for the presence of anti-T. gondii IgG antibodies, using the indirect, enzyme-linked, immunosorbent assay technique (ELISA). Moreover, to identify the potential risk factors of T. gondii infection, a survey through a breeders’ questionnaires was conducted. Nearly one-fourth of the total number of animals tested (1024/4074)—i.e., 25.1%—were seropositive. The seroprevalence in cattle, sheep, and goats was 28.7%, 25.6%, and 11.9%, respectively. The area, sex, age, and herd size were identified as risk factors for T. gondii infection. Higher seropositivity rates were recorded in cows and goats (odds ratio (OR) = 1.63 and 6.4), in old animals (cattle, OR = 2.1; sheep, OR = 1.9; and goat, OR = 3.9), and in small size herds (cattle, OR = 2.5; sheep, OR = 1.9; goat, OR = 2.2). In conclusion, there is widespread T. gondii infection in cattle, sheep, and goats in these two strategic agricultural areas. The identification of the risk factors determines the type of measures and strategies to be undertaken to reduce, control, and prevent T. gondii infection in domestic animals, and thereby reduce human infection.
1. Introduction
Toxoplasma gondii is an obligate intracellular protozoan that causes widespread infection in humans and many other warm-blooded animal species (mammals and birds); it has adverse effects on public health and animal production. While many animals serve as intermediate hosts, only domestic cats are definitive hosts. The parasite is transmitted to these hosts through contaminated meat, milk, and water. Such contamination can arise with oocyst-contaminated foodstuffs, which is an important route for the contamination of farm animals [1,2].
Ingestion of ecologically robust stages (sporozoites in oocysts), consumption of raw or undercooked meat, or meat products containing tachyzoites or bradyzoites is the main transmission route of Toxoplasma to humans [3,4]. Most infections in humans are asymptomatic, but serious complications may occur following congenital Toxoplasma infection, such as abortion, stillbirth, mortality, and hydrocephalus in newborns, or retinochoroidal lesions leading to chronic ocular disease and lymphadenopathy, retinitis, or encephalitis in immunocompromised individuals [5].
Toxoplasma infection has been reported in wild and domestic animals. In food-producing animals, however, sheep and goats are more often infected than cattle or chickens [3,6]. Sheep and goats have been reported as a major source of infection in several countries [1,6]. Recently, tachyzoites of T. gondii have also been detected in the milk of several intermediate hosts, including camels [2,7].
T. gondii infection in ruminants as a major cause of abortions and stillbirths [8], and brings about significant economic losses to the global sheep, goat, and cattle industry [9,10]. It gives rise to a wide variety of non-specific (fever and dyspnoea) and specific (fever, depression, lethargy, vomiting, diarrhea, chorioretinitis, and lymphadenopathy) symptoms [11,12].
The control of T. gondii infection in cattle, sheep, and goats is therefore important, not only for the efficient breeding of domestic animals, but also for public health.
In Algeria, although the prophylaxis of congenital toxoplasmosis is part of a national surveillance program of pregnant women that provides medical treatment of toxoplasmic seroconversions or active toxoplasmosis, the human seroprevalence reported is still quite high (51.6%) [13].
There are very few studies on toxoplasmosis prevalence in domestic animals, despite its omnipresence in Algeria. As a consequence, the status of the T. gondii infection is not yet established and is poorly understood. This is what motivated us to carry out a cross-sectional survey on cattle, sheep, and goats in several sensitive parts of Algeria, in order to assess the seroprevalence of T. gondii infection in food-producing domestic ruminants, as well as to identify the main risk factors that are associated with the infection.
2. Materials and Methods
2.1. Study Area
With an area of 2,381,741 square kilometres, Algeria is the largest country in Africa and the Mediterranean basin. Its southern part is mainly occupied by the Sahara. To the north, Atlas Tell forms with the Saharan Atlas; further south, two sets of parallel reliefs point east, between which are inserted vast plains and uplands. In fact, by type of livestock, it there are 26.88 million sheep, 4.90 million goats, and 1.90 million cattle. Sheep farming accounts for almost 80% of the total number of national herds.
The study area was chosen on purpose to represent two sensitive agroecological zones (high- and lowlands) of central Algeria. These two zones are distributed in 12 provinces called wilayates, in the highlands (Boumerdes, Tizi-Ouzou, Bouira, and Setif), midlands (Tiaret, M’sila, El-Bayadh, and Djelfa), and lowlands (Algiers, Medea, Saida, and Laghouat).
Livestock production is widely distributed in the region, and the number of herds is high. Semi-extensive production is predominant for cattle, sheep, and goats, and is characterized by housing in the winter months until early spring, at the time of delivery, and extensive pasture the rest of the year.
2.2. Animals and Samples
A cross-sectional study design was used. Different age and sex groups of cattle, sheep, and goats were included for this study. The study was conducted from October 2015 to March 2018. Serological investigation was used to detect anti-T. gondii antibodies from blood serum collected from animals in the districts under study. Since there was no previous expected prevalence in the area, sample size was calculated according to Thrusfield, using an expected prevalence of 50%, a desired precision of 5%, and with 95% level of confidence [14]. Hence, the sample size was 384 for each species. Due to the fact that the goat population at the study area was very limited in number, 478 goats, 2144 sheep, and 1452 blood samples of cattle sera were subjected to analysis. In total, 4074 samples were analyzed.
Three age groups were established: <2 years, 2–5 years, and ˃5 years. Individuals were randomly selected within herds, to a maximum of 30 sheep, 15 cattle, and 10 goats per herd. The herd is considered seropositive when at least one animal from the same herd had anti-T. gondii antibodies. Finally, 1452 cattle, 2144 sheep, and 478 goats from 95, 70, and 47 herds, respectively, were surveyed.
Additional data collected for each sampled animal included gender, breed, husbandry system (semi-intensive or extensive), and geographic origin, which is a well-known risk factor for T. gondii infection are retained for this study [15].
Blood samples were collected from the jugular or tail vein, depending on the animal species, in 10 mL vacutainer tubes with no anti-coagulants or preservatives, as approved by the National Consultative ethnical committee for life sciences and health. The samples, after sitting overnight at room temperature, were centrifuged at 3000 rpm for 10 min. The sera were stored at -20 °C in 1.5 mL Eppendorf tubes until analysis.
2.3. Serologic Testing
The sera were tested for the presence of anti-T. gondii antibodies using an Toxoplasmosis Indirect ELISA Multi-species kit (ID Screen, ID.VET. Innovative Diagnostics, Montpellier, France), according to manufacturer’s instructions. The sensitivity of this ELISA test reaches 100%, whereas specificity was determined to be of 96% (manufacturer’s data).
The results were expressed as optical density (OD); absorbance was read at 450 nm with an EL-800 ELISA Plate reader (Biotek Instruments Inc., USA). The 96-well plate was coated with P30 T. gondii antigens, and the antigen–antibody complex formed with the help of the peroxidase conjugate, which was added later. Positive and negative controls were provided by the manufacturer and were used to validate each test. The samples were considered positive if they had a value ≥50%, doubtful for values between 40% and 50%, and negative if ≤40%. This percentage was calculated as follows: percentage of positivity = 100 × OD of the sample/OD of the PC (OD: Optic Density; PC: Positive Control). Information about the sensitivity and specificity of this ELISA test (100% and 97.8%, respectively) was provided by the manufacturer.
2.4. Statistical Analysis
The data were recorded and coded using a Microsoft Excel spreadsheet, and analysed using the SPSS software (SPSS Inc., IBM Corporation, Version 22, Chicago, IL, USA). The seroprevalence was calculated by dividing the number of animals positive to anti-T. gondii antibodies by the total number of animals tested. The relationship of risk factors with the dependent variable was primarily assessed using cross tabulation. Univariable logistic regression analysis was performed, and the strength of the association between risk factors and T. gondii infection were evaluated with Chi-square tests. Multivariate logistic regression was used for all variables showing a moderate statistical significance (p ˂ 0.05) in the univariate analysis. The logistic regression model was developed in a stepwise forward approach, using a likelihood ratio test at each step (p < 0.05 to enter and p > 0.10 to exit). Model fit was assessed with the Hosmer and Lemeshow goodness-of-fit test. All statistical analyses were performed using the statistical software SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). In all analyses, two-tailed p values <0.05 were considered as statistically significant.
3. Results
The present seroepidemiological survey was based on field samples. It reflects the importance of studies on Toxoplasma on a regional basis from ruminant species in Algeria.
At least one seropositive animal was detected in 204 out of the 212 herds tested, which gave an estimated seroprevalence at the herd level of 96.2% (95% CI: 93.7%–98.8%).
Over the 204 farms that were tested (at least one animal), 93/95 (97.8%; 95% CI: 95%–100%), 70/70 (100%; 95% CI: 100%–100%), and 41/74 (87.2%; 95% CI: 77.7%–96.8%) concerned cattle, sheep, and goats, respectively (Table 1). We considered these farms as positive for T. gondii infection. It is important, though, to indicate that all the animals making up the herds were not blood-sampled.

Table 1.
Seroprevalence of Toxoplasma gondii infection in domestic cattle, sheep, and goats from Algeria.
The individual seroprevalence at the animal level in the study area (12 wilayates of central Algeria), adjusted for sampling sizes and for test sensitivity and specificity, was 25.1% (1024/4074, 95% CI: 23.8%–26.5%).
The T. gondii seropositivity rate was 28.7% (418/1452; 95% CI: 26.5%–31.1%) in cattle, 25.6% (549/2144; 95% CI: 23.8%–27.4%) in sheep, and 11.92% (57/478; 95% CI: 9%–14.8%) in goats (Table 1).
3.1. Cattle
Table 2 summarises the results of the univariate analysis of individual-level risk factors for T. gondii seroprevalence in cattle.

Table 2.
Analysis of risk factors related to T. gondii seroprevalence in cattle at the animal level.
Four factors were associated with seropositivity against T. gondii: gender (p ˂ 0.0000), age group (p ˂ 0.00001), herd size (p ˂ 0.00001), and geographical area and provinces (p ˂ 0.009). The individual seroprevalence was higher in females (32.9%) than in males (19.5%), in cattle more than 5 years old (52.4%) than those less than 2 years old (14.5%), in small (50.13%) than large herd sizes (20.8%), and in centrally located provinces (Table 2).
The cattle seroprevalence recorded in the 08 provinces involved ranged from 20.5% (Medea) to 37.3% (Boumerdes), as seen in Table 2 and Figure 1. The breed, rearing system, and water source were not significantly associated with seropositivity.
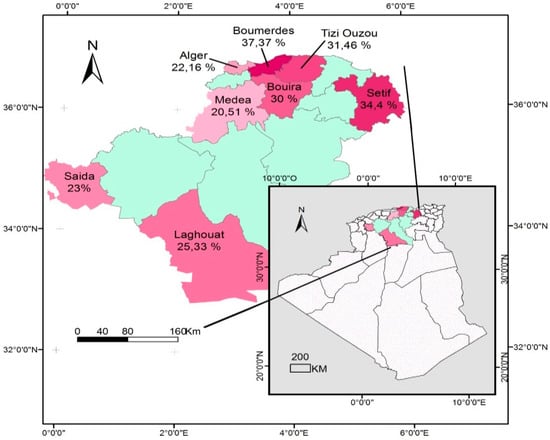
Figure 1.
Distribution of cattle seroprevalence of Toxoplasma gondii infection in Algeria.
The four risk factors investigated were simultaneously analysed via a logistic regression model to determine their relative contributions to T. gondii seropositivity. In the final model, the three following risk factors were found to be associated with T. gondii infection: farm location, age group, and herd size. A two-fold increased risk of infection was seen for cattle from small-herd-size farms (OR = 2.5; CI: 1.19–5.23; p = 0.004), and for the age group >5 years old (OR = 2.1; CI: 1.02–5.11; p = 0.024). Moreover, cattle reared in the highland areas of Algeria (Boumerdes, Tizi-Ouzou, Bouira, and Setif) had significantly (OR = 2.8; CI: 1.25–6.13; p = 0.014) higher risk of infection with T. gondii than those reared in the lowlands (Algiers, Medea, Saida, and Laghouat).
3.2. Sheep and Goats
The results of the univariate and multivariate analysis of the individual-level risk factors for T. gondii seroprevalence in sheep are summarised in Table 3, and those for goats are in Table 4.

Table 3.
Analysis of risk factors related to T. gondii seroprevalence in sheep at animal level (n = 2144).

Table 4.
Analysis of risk factors related to T. gondii seroprevalence in goats at animal level (n = 478).
In addition, the seroprevalence recorded in small ruminants in wilayates forming the study area ranged from 19% in Tiaret to 32.9% in El bayadh (Table 3 and Figure 2) in sheep, and from 8.8% in Tiaret to 18.4% in Laghouat (Table 4 and Figure 3) in goats.
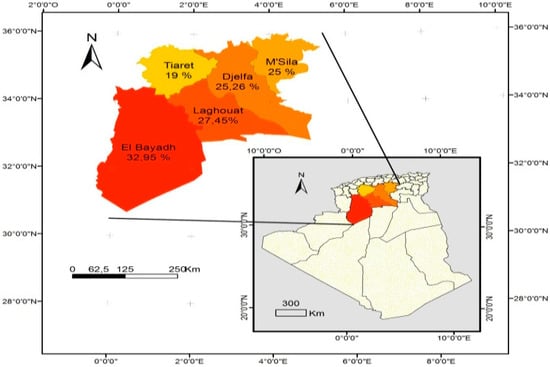
Figure 2.
Distribution of the sheep seroprevalence of Toxoplasma gondii infection in Algeria.
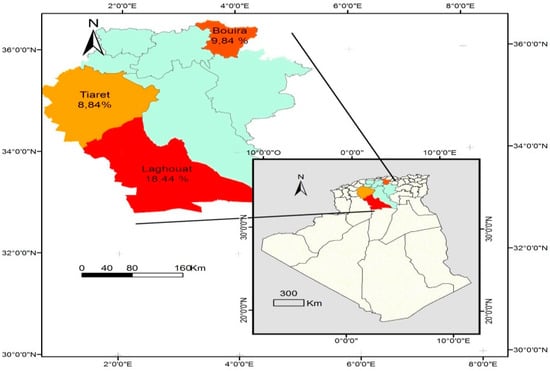
Figure 3.
Distribution of goat seroprevalence of Toxoplasma gondii infection in Algeria.
Factors associated with seropositivity in univariate analysis were age group (p ˂ 0.00001 for sheep and goats), female gender in goats (p ˂ 0.00001), herd size (p ˂ 0.00001 and 0.0016, respectively for sheep and goats), and region (p ˂ 0.0004 and 0.0169, respectively, for sheep and goats).
Results of multivariate logistic regression analysis indicates that the potential risk factors related to age group, gender, and herd size revealed that the likelihood of T. gondii infection was higher in adult sheep (OR = 1.93; CI: 1.12–3.36; p = 0.001) and in small herds (OR = 1.95; CI: 1.11–3.44; p = 0.002), when compared with young sheep and large herd sizes, respectively. The likelihood of T. gondii infection was two times higher in adult goats >5 years old (OR = 3.9; CI: 1.63–9.41; p = 0.015) and six times higher in female goats (OR = 6.08; CI: 2.2–14.6), with a two-fold increase from small compared to large farms (OR = 1.95; CI: 1.11–3.44; p = 0.002). However, there was no difference in the prevalence of infected animals from farms with an extensive rearing system and semi-intensive, or according to water source.
4. Discussion
Humans around the world are highly dependent on domestic animals for many reasons, including needs for meat, milk, and fat [16].
The present study provided a better understanding of Toxoplasma infection, and revealed widespread T. gondii infection in food-producing domestic ruminants in Algeria. The presence of anti-Toxoplasma antibodies has been confirmed in animal farms in all the provinces involved in the study, indicating extensive contamination by the parasite.
When considering the Algerian people’s habit of eating undercooked grilled beef, sheep, and goat meat, the risk of contracting toxoplasmosis is to be highly considered.
The prevalence of T. gondii infection in domestic ruminants has gained increasing interest in recent years, due to the animals’ role in the spread of the parasite, either through direct contact or via consumption of animal meat products [8,12,17,18].
The high seroprevalence in cattle (97.89%), sheep (100%), and goats (87.23%) recorded in the present study could reflect the high level of environmental contamination and widespread distribution of the parasite.
These seroprevalences recorded in domestic ruminants are higher than those reported previously: 58.89% and 84.61% in Algeria [19], 45.17% in Tanzania [20], and 70.48% in Ethiopia [21]. This seroprevalence could reflect the heavy agricultural soil contamination with oocysts, as has been stated in many studies [17,22]. These agreements can be attributed to grazing systems where many herds graze daily. As a result, the risk of contact with contaminated feed and pasture during the grazing season was high in herds of animals.
The results of this first region-wide survey on T. gondii infection in cattle, sheep, and goats determined high seroprevalences in domestic food-producing ruminants (28.7%, 25.6%, and 11.9% in cattle, sheep, and goats, respectively).
The seroprevalences obtained for cattle (28.7%) is higher than those reported previously, which were 3.9% [19], 4.4% [23], and 15.2% [24] in Algeria; this shows that T. gondii infection has increased considerably. However, some of the discrepancy between the reported results could be due to (1) the small sample size of animals, which may impair the representativity and objectivity of data; (2) the wide geographic area concerned or covered; (3) management practices (traditional, semi-intensive, and extensive); and (4) the serological methods used for detecting the infection. In this study, and in another recently published paper [25], the ELISA technique was used, whereas the results reported earlier in Algeria were obtained through an indirect immunofluorescence test (IFAT) [19] and microscopic agglutination test (MAT) [23]. This difference may be due to the sensitivity and specificity of the different serological methods applied [6,12].
Throughout the world, data on T. gondii seroprevalence in cattle are considerably different. They vary from 0% to 99% [15], including from 2% to 92% in Europe [3] and from 10% to 37% in North Africa [12]. The seroprevalence of T. gondii infection in cattle reported in the present study is more or less similar to that reported (27.4%) in Libya [26], but it is higher than that reported (2.68%) in Brazil [27], in Central Ethiopia (6.6%) [28], in Indonesia (9%) [29], in East Ethiopia (10.4%) [30], in Vietnam (10.5%) [31], in Tanzania (13%) [32], in Iran (15.77%) [33], and in Thailand (22.3%) [34]. Other studies have reported a lower value: 30% in the Netherlands [35], 32% in Sudan [36], and 51.96% in Brazil [37]. Such data should be analyzed with caution, since geographical variations occur not only between different countries, but also within countries. In addition, the comparison of data reported in different countries requires the use of standardized tests, procedures, and appropriate size sampling. These data are not directly comparable, because of the variability in the sampling strategy, the type of testing methods used, and the protocol of analysis adopted.
Although some studies have reported a high seropositivity in cattle, Toxoplasma is considered to be far less infective to cattle. In this species, the clinical signs are usually not observed in naturally infected animals, and the parasite has very rarely been detected in tissues of an adult cows [38] or in aborted fetuses [39].
In sheep, data on T. gondii infection vary widely. The observed value in the present study (25.6%) is quite similar with those previously reported in Morocco (27.6%) [40] and in Libya (26.2%) [26], but it is slightly higher than those recorded in many other countries, such as central Ethiopia (22.9%) [27], Morocco (20.8%) [41], Tunisia (19%) [42], Pakistan (11.1%) [43], Algeria (11.6% and 8.3%) [19,44], and northeastern China (3.0%) [45]. Lower seroprevalence values have, however, been observed in other parts of the world, such as in Brazil (29.41% and 32.9%) [46,47], Ethiopia (31.5% and 33.7%) [21,30], Italy (33.3%) [48], Tunisia (40.2%) [49], and Egypt (52.7%) [50], as well as in Nazareth, Ethiopia (56%) [51].
In goats, the T. gondii infection seroprevalence that we observed was close to that recorded (11.2%) in Pakistan [43], Central Ethiopia (11.6%) [28], northern America (12.1%) [52], and Algeria (13.2%) [19], but was higher than that observed in Morocco (8.5%) [41], South Africa (4.3%) [53], and India (3.8%) [54]. Other studies reported higher seroprevalences than ours, such as those reported in Ethiopia (25.9%) [55], Thailand (27.9%) [56], Tunisia (34%) [49], Pakistan (41.8%) [57], Egypt (41.7% and 44.3%) [58,59], Libya (50%) [26], and Southern Ethiopia (55.18%) [60].
The variations in the overall seroprevalence observed in the current study, as well as those mentioned above, could be due to the access level of the small ruminants to contaminated feed and water, climatic variation, and the diagnostic techniques used [61,62].
The logistic regression indicated that, in addition to farm location, small herd size, female gender, and adulthood are the main risk factors for cattle infection.
Moreover, cattle raised in highland areas had significantly higher risk of T. gondii infection than those raised in lowland ones. This observation was also made in almost all studies throughout the world [21,43,57,60,63].
This discrepancy between authors could be due to the variation in temperature and humidity in the areas of studies, since it has been reported that environment influences the epidemiology of toxoplasmosis [3,61,64]. Algerian highlands have a very high moisture content, which increases the chance of oocyst survival in the environment and the contact probability with contaminated sources, thus leasing to higher seroprevalences [2,30,65]. On the contrary, a dry climate has a negative impact on the survival and epidemiological distribution of the parasite [2,60].
The size of the herd, and notably when it is small, is considered as the major risk factor in the present study, no matter the species considered. In Algeria, and particularly in the areas of study, the type of farm management adopted could be at the origin of such an observation. In Algeria, small herds are the ones managed traditionally, because (1) the livestock’s aliment is widely accessible to cats; (2) the animals’ grazing is frequent, and the transition from intensive to extensive and vice versa was done daily; and (3) the lack of zoo-hygienic measures, including feeding organizing, cleaning, etc. These were extensively reviewed by Tenter et al. [3], Klun et al. [66], and Dubey et al. [2].
The relationship between the age of the 1452 cattle, 2144 sheep, and 478 goats sampled and T. gondii seroprevalence was studied. This latter was significantly higher in the adults (52.43% cattle, 31.5% sheep, and 31.48% goats) than in the young animals (14.51% cattle, 20.66% sheep, and 9.12% goats). The multivariable logistic regression analysis showed that the likelihood of acquiring infection was higher in the adults (cattle: OR = 2.1, CI: 1.02–5.11, p = 0.024; sheep: OR = 1.93, CI: 0.89–3.33, p = 0.001; goats: OR = 3.9, CI: 1.81–6.32; p = 0.002) than in the young animals. These findings are similar to those of Jittapalapong et al. [56]; Teshale et al. [51], and Tilahun et al. [30], who reported a low prevalence in young animals and a high one in adults. Seroprevalence of the T. gondii antibody has been found to increase with age, no matter the animal species. This could be due to a longer exposure of the adults to T. gondii infection [2]. Animals that have lived longer are more likely to be T. gondii parasite sources [12,64,67].
With regard to the sex risk factor, the study showed that the seroprevalence of anti-T. gondii antibody is higher in cattle females (33%) than in cattle males (19.5%). The multivariable logistic regression analysis revealed that the likelihood of acquiring infection was higher in females (OR = 1.63, CI: 1.01–3.16, p = 0.018) than in males of this species. This is in agreement with what was reported by Clementino et al. [47] and Zewdu et al. [21], but not by Silva et al. [37] and Lashari and Tasawar [36], who observed the opposite—a higher seroprevalence in males than in females. Other authors, however, have reported no significant differences between the two genders [64]. The increased sensitivity of females may be associated with a lower immunological resistance during certain periods of their lives [68], such as the stress of lactation and pregnancy, which causes an immunosuppression that renders them more liable to T. gondii infection [30,69].
In our study, the presence of cats was not investigated as a risk factor, since it is partly ubiquitous in almost all farms studied or those located nearby. The presence of resident or stray cats may explain the high prevalence of T. gondii-specific antibodies observed in this study, due to oocyst clearance and environmental contamination. The significant of cats as a reservoir of T. gondii infection is confirmed by the finding of a high infection rate in domestic ruminants, which strongly supports the high seroprevalence of toxoplasmosis in women observed in Algeria [13,70].
5. Conclusions
T. gondii infection has been found to be widespread among cattle, sheep, and goats reared in the studied areas. The risk factors identified are area, sex, age, and herd size; these are important to set the control and prophylactic measures and strategies to reduce T. gondii infection in domestic animals, and thereby in humans. Thus, the higher seroprevalence encountered in these animal species used as a food source reveals the potential risk of T. gondii infection presented to people through consumption of their meat. Therefore, the population should be sensitized through education on the modes of transmission and prevention of T. gondii infection, and further study should be conducted to explore the impact of the disease on food animal production
Author Contributions
M.-C.A. and A.-O.K. conceived the study design, took part in the coordination and management as well as field studies. M.-C.A., B.K., and A.S. carried out laboratory work, and A.-O.K., M.K., K.R., and K.D. participated in data analysis and interpretation. M.-C.A. and A.-O.K. drafted the manuscript. M.-C.A., A.-O.K. and M.K. critically revised the manuscript. M.-C.A. and A.-O.K. revised the manuscript according to the reviewers’ helpful suggestions. All authors read and approved the final manuscript.
Funding
This study was supported by the Ministry of Higher Education and Scientific Research in Algeria, and was carried out within the High National Veterinary School of Algiers.
Acknowledgments
The authors thank the Algerian Ministry of Higher Teaching and Scientific Research for its contribution to my PhD training.
Conflicts of Interest
We declare that we have no conflicts of interest related to this work.
References
- Tenter, A.M. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz 2009, 104, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Toxoplasmosis of Animals and Humans. Available online: https://www.crcpress.com/Toxoplasmosis-of-Animals-and-Humans/Dubey/p/book/9781420092363 (accessed on 22 May 2019).
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in sheep—The last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 2002, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii prevalence in farm animals in the United States. Int. J. Parasitol. 2013, 43, 107–113. [Google Scholar] [CrossRef]
- Saad, N.M.; Hussein, A.A.A.; Ewida, R.M. Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in Upper Egypt. Vet. World 2018, 11, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Cenci-Goga, B.T.; Rossitto, P.V.; Sechi, P.; McCrindle, C.M.E.; Cullor, J.S. Toxoplasma in Animals, Food, and Humans: An Old Parasite of New Concern. Foodborne Pathog. Dis. 2011, 8, 751–762. [Google Scholar] [CrossRef]
- Kortbeek, L.M.; De Melker, H.E.; Veldhuijzen, I.K.; Conyn-van Spaendonck, M.A. Population-based Toxoplasma seroprevalence study in The Netherlands. Epidemiol. Infect. 2004, 138, 839–845. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Sounders: London, UK, 2006; pp. 1518–1522. [Google Scholar]
- Center for Disease Control and Prevention (CDC), United States. Available online: http://www.cdc.gov/ncidod/dpd/parasites/toxoplasmosis/default.htm (accessed on 14 November 2018).
- Rouatbi, M.; Amairia, S.; Amdouni, Y.; Boussaadoun, M.A.; Ayadi, O.; Al-Hosary, A.A.T.; Rekik, M.; Ben Abdallah, R.; Aoun, K.; Darghouth, M.A.; et al. Toxoplasma gondii infection and toxoplasmosis in North Africa: A review. Parasite 2019, 26, 6. [Google Scholar] [CrossRef]
- Messerer, L.; Bouzbid, S.; Gourbdji, E.; Mansouri, R.; Bachi, F. Séroprévalence de la toxoplasmose chez les femmes enceintes dans la wilaya d’Annaba, Algérie. Rev. Épidémiologie Santé Publique 2014, 62, 160–165. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: London, UK, 2005; pp. 227–247. [Google Scholar]
- Hall, S.; Ryan, M.; Buxton, M. The epidemiology of toxoplasma infection. In Toxoplasmosis: A Comprehensive Clinical Guide; Joynson, D.H.M., Wreghitt, T.G., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 58–124. [Google Scholar]
- Jones, J.L.; Dubey, J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Lunney, J.K.; Shen, S.K.; Kwok, O.C.; Ashford, D.A.; Thulliez, P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 1996, 82, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef] [PubMed]
- Dechicha, A.S.; Bachi, F.; Gharbi, I.; Gourbdji, E.; Baazize-Ammi, D.; Brahim-Errahmani, O.; Guetarni, D. Sero-epidemiological survey on toxoplasmosis in cattle, sheep and goats in Algeria. AJAR 2015, 10, 2113–2119. [Google Scholar]
- Swai, E.S.; Kaaya, J.E. A survey of Toxoplasma gondii antibodies by latex agglutination assay in dairy goats in Northern Tanzania. Trop. Anim. Health Prod. 2012, 45, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zewdu, E.; Agonafir, A.; Tessema, T.S.; Tilahun, G.; Medhin, G.; Vitale, M.; Di Marco, V.; Cox, E.; Vercruysse, J.; Dorny, P. Seroepidemiological study of caprine toxoplasmosis in East and West Shewa Zones, Oromia Regional State, Central Ethiopia. Res. Vet. Sci. 2013, 94, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Frenkel, J.K. Toxoplasma Gondii in Costa Rican Cats. Am. J. Trop. Med. Hyg. 1980, 29, 1150–1160. [Google Scholar] [CrossRef]
- Khames, M.; Yekkour, F.; Fernández-Rubio, C.; Aubert, D.; Nguewa, P.; Villena, I. Serological survey of cattle toxoplasmosis in Medea, Algeria. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 89–90. [Google Scholar] [CrossRef]
- Abdelhadi, F.Z.; Abdelhadi, S.A.; Niar, A.; Benallou, B.; Meliani, S.; Smail, N.L.; Mahmoud, D. Abortions in cattle on the level of Tiaret Area (Algeria). Glob. Vet. 2015, 14, 638–645. [Google Scholar]
- Benlakehal, A.; Miroud, K.; Djeghim, H.; Kaidi, R. Serological survey for anti-Toxoplasma gondii antibodies in sheep of northeastern Algeria. Trop. Anim. Health Prod. 2019. [Google Scholar] [CrossRef]
- Azwai, S.M.; El-Gammoudi, F.T.; Gameel, S. A serological survey of toxoplasmosis in some animal species in Libya. Alex. J. Vet. Sci. 1993, 9, 133–135. [Google Scholar]
- Fajardo, H.V.; D’ávila, S.; Bastos, R.R.; Cyrino, C.D.; de Lima Detoni, M.; Garcia, J.L.; das Neves, L.B.; Nicolau, J.L.; Amendoeira, M.R.R. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, Southern Brazil. Parasites Vectors 2013, 6, 191. [Google Scholar] [CrossRef]
- Bekele, T.; Kasali, O.B. Toxoplasmosis in sheep, goats and cattle in central Ethiopia. Vet. Res. Commun. 1989, 13, 371–375. [Google Scholar] [CrossRef]
- Matsuo, K.; Husin, D. A survey of Toxoplasma gondii antibodies in goats and cattle in Lampung province, Indonesia. Southeast Asian J. Trop. Med. Public Health 1996, 27, 554–555. [Google Scholar]
- Tilahun, B.; Tolossa, Y.H.; Tilahun, G.; Ashenafi, H.; Shimelis, S. Seroprevalence and Risk Factors of Toxoplasma Gondii Infection among Domestic Ruminants in East Hararghe Zone of Oromia Region, Ethiopia. Available online: https://www.hindawi.com/journals/vmi/2018/4263470/abs/ (accessed on 22 May 2019).
- Huong, L.T.T.; Ljungström, B.L.; Uggla, A.; Björkman, C. Prevalence of antibodies to Neospora caninum and Toxoplasma gondii in cattle and water buffaloes in southern Vietnam. Vet. Parasitol. 1998, 75, 53–57. [Google Scholar] [CrossRef]
- Schoonman, L.B.; Wilsmore, T.; Swai, E.S. Sero-epidemiological investigation of bovine toxoplasmosis in traditional and smallholder cattle production systems of Tanga Region, Tanzania. Trop. Anim. Health Prod. 2010, 42, 579–587. [Google Scholar] [CrossRef]
- Hamidinejat, H.; Ghorbanpour, M.; Nabavi, L.; Haji Hajikolaie, M.R.; Razi Jalali, M.H. Occurrence of anti-Toxoplasma gondii antibodies in female cattle in south-west of Iran. Trop. Anim. Health Prod. 2010, 42, 899–903. [Google Scholar] [CrossRef]
- Jittapalapong, S.; Sangwaranond, A.; Inpankaew, T.; Phasuk, C.; Pinyopanuwat, N.; Chimnoi, W.; Kengradomkij, C.; Arunwipat, P.; Maruyama, S. Seroprevalence of Toxoplasma gondii Infection in dairy cows in Northestern Thailand. Southeast Asian J. Trop. Med. Public Health 2008, 39, 5. [Google Scholar]
- Opsteegh, M.; Teunis, P.; Züchner, L.; Koets, A.; Langelaar, M.; van der Giessen, J. Low predictive value of seroprevalence of Toxoplasma gondii in cattle for detection of parasite DNA. Int. J. Parasitol. 2011, 41, 343–354. [Google Scholar] [CrossRef]
- Khalil, K.M.; Elrayah, I.E. Seroprevalence of Toxoplasma Gondii Antibodies in Farm Animals (Camels, Cattle, and Sheep) in Sudan. J. Med. Anim. Heal. 2011, 3, 36–39. [Google Scholar]
- Da Silva, A.V.; Cunha, E.L.P.; Meireles, L.R.; Gottschalk, S.; Mota, R.A.; Langoni, H. Toxoplasmose em ovinos e caprinos: Estudo soroepidemiológico em duas regiões do Estado de Pernambuco, Brasil. Ciência Rural 2003, 33, 115–119. [Google Scholar] [CrossRef]
- Dubey, J.P. Isolation of Toxoplasma gondii from a naturally infected beef cow. J. Parasitol. 1992, 78, 151–153. [Google Scholar] [CrossRef]
- Canada, N.; Meireles, C.S.; Rocha, A.; Correia da Costa, J.M.; Erickson, M.W.; Dubey, J.P. Isolation of viable Toxoplasma gondii from naturally infected aborted bovine fetuses. J. Parasitol. 2002, 88, 1247–1248. [Google Scholar] [CrossRef]
- Sawadogo, P.; Hafid, J.; Bellete, B.; Sung, R.T.M.; Chakdi, M.; Flori, P.; Raberin, H.; Hamouni, I.B.; Chait, A.; Dalal, A. Seroprevalence of T. gondii in sheep from Marrakech, Morocco. Vet. Parasitol. 2005, 130, 89–92. [Google Scholar] [CrossRef]
- Benkirane, A.; Essamkaoui, S.; El Idrissi, A.; Lucchese, L.; Natale, A. A sero-survey of major infectious causes of abortion in small ruminants in Morocco. Vet. Ital. 2015, 51, 25–30. [Google Scholar]
- Gharbi, M.; Zribi, L.; Jedidi, M.; Chakkhari, H.; Hamdi, S.; R’hayem, S.; Zribi, N.; Souli, M.; Darghouth, M.A. Prévalence d’infection des ovins par Toxoplasma gondii en Tunisie. Bull. Soc. Pathol. Exot. 2013, 106, 184–187. [Google Scholar] [CrossRef]
- Ramzan, M.; Akhtar, M.; Muhammad, F.; Hussain, I.; Hiszczyńska-Sawicka, E.; Haq, A.U.; Mahmood, M.S.; Hafeez, M.A. Seroprevalence of Toxoplasma gondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Trop. Anim. Health Prod. 2009, 41, 1225. [Google Scholar] [CrossRef]
- Dahmani, A.; Harhoura, K.; Aissi, M.; Zenia, S.; Hamriouri, B.; Guechi, N.; Athmane, M.A.; Kadour, R. The zoonotic protozoan of sheep carcasses in the north of Algeria: A case of ovine toxoplasmosis. J. Hell. Vet. Med. Soc. 2018, 69, 1004–1012. [Google Scholar] [CrossRef][Green Version]
- Wang, C.R.; Qiu, J.H.; Gao, J.F.; Liu, L.M.; Wang, C.; Liu, Q.; Yan, C.; Zhu, X.Q. Seroprevalence of Toxoplasma gondii infection in sheep and goats in northeastern China. Small Rumin. Res. 2011, 97, 130–133. [Google Scholar] [CrossRef]
- Pinheiro, J.W.; Mota, R.A.; da Fonseca Oliveira, A.A.; Faria, E.B.; Gondim, L.F.P.; da Silva, A.V.; Anderlini, G.A. Prevalence and risk factors associated to infection by Toxoplasma gondii in ovine in the State of Alagoas, Brazil. Parasitol. Res. 2009, 105, 709. [Google Scholar] [CrossRef]
- Clementino, M.M.; Souza, M.F.; Neto, V.F.A. Seroprevalence and Toxoplasma gondii-IgG avidity in sheep from Lajes, Brazil. Vet. Parasitol. 2007, 146, 199–203. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Ciampelli, A.; Sechi, P.; Veronesi, F.; Moretta, I.; Cambiotti, V.; Thompson, P.N. Seroprevalence and risk factors for Toxoplasma gondii in sheep in Grosseto district, Tuscany, Italy. BMC Vet. Res. 2013, 9, 25. [Google Scholar] [CrossRef]
- Arwa Lachkhem, I.L.; Wahiba Sakly, D.S. Prevalence of Toxoplasmosis in Sheep, Goats and Cattle in Southern Tunisia. J. Bacteriol. Parasitol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohamed, A.H.; El-Sharaawy, A.A.; El-Shqanqery, H.E. Molecular and serological prevalence of Toxoplasma gondii in pregnant women and sheep in Egypt. Asian Pac. J. Trop. Med. 2017, 10, 996–1001. [Google Scholar] [CrossRef]
- Teshale, S.A.; Dumetre, M.L.; Darde, B.; Merger, D. Study on Toxoplasmosis in sheep and goats in Debre Birhan and surrounding areas in Ethiopia. Bull. Anim. Health Prod. Afr. 2007, 50, 138–147. [Google Scholar]
- Dubey, J.P.; Foreyt, W.J. Seroprevalence of Toxoplasma gondii in Rocky Mountain Bighorn Sheep (Ovis canadensis). J. Parasitol. 2000, 86, 622–623. [Google Scholar] [CrossRef]
- Samraa, N.A.; McCrindle, C.M.E.; Penzhorn, B.L.; Cenci-Goga, B. Seroprevalence of toxoplasmosis in sheep in South Africa. J. S. Afr. Vet. Assoc. 2007, 78, 116–120. [Google Scholar] [CrossRef]
- Sharma, S.; Sandhu, K.S.; Bal, M.S.; Kumar, H.; Verma, S.; Dubey, J.P. Serological Survey of Antibodies to Toxoplasma gondii in Sheep, Cattle, and Buffaloes in Punjab, India. J. Parasitol. 2008, 94, 1174–1175. [Google Scholar] [CrossRef]
- Negash, T.; Tilahun, G.; Patton, S.; Prévot, F.; Dorchies, P. Serological survey on Toxoplasmosis in sheep and goats in Nazareth, Ethiopia. Rev. Méd. Vétérinaire 2004, 155, 486–487. [Google Scholar]
- Jittapalapong, S.; Sangvaranond, A.; Pinyopanuwat, N.; Chimnoi, W.; Khachaeram, W.; Koizumi, S.; Maruyama, S. Seroprevalence of Toxoplasma gondii infection in domestic goats in Satun Province, Thailand. Vet. Parasitol. 2005, 127, 17–22. [Google Scholar] [CrossRef]
- Ahmed, H.; Malik, A.; Arshad, M.; Mustafa, I.; Khan, M.R.; Afzal, M.S.; Ali, S.; Mobeen, M.; Simsek, S. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in North-Eastern Region of Pakistan. Korean J. Parasitol. 2016, 54, 439. [Google Scholar] [CrossRef]
- Ghoneim, N.H.; Shalaby, S.I.; Hassanain, N.A.; Zeedan, G.S.; Soliman, Y.A.; Abdalhamed, A.M. Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathog. Dis. 2010, 7, 17–22. [Google Scholar] [CrossRef]
- Shaapan, R.M.; Hassanain, M.A.; Khalil, F.A.M. Modified Agglutination Test for Serologic Survey of Toxoplasma gondii Infection in Goats and Water Buffaloes in Egypt. Res. J. Parasitol. 2010, 5, 13–17. [Google Scholar]
- Tegegne, D.; kelifa, A.; Abdurahaman, M.; Yohannes, M. Seroepidemiology and associated risk factors of Toxoplasma gondii in sheep and goats in Southwestern Ethiopia. BMC Vet. Res. 2016, 12, 280. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis—A waterborne zoonosis. Vet. Parasitol. 2004, 126, 57–72. [Google Scholar] [CrossRef]
- Innes, E.A.; Bartley, P.M.; Buxton, D.; Katzer, F. Ovine toxoplasmosis. Parasitology 2009, 136, 1887–1894. [Google Scholar] [CrossRef]
- Gebremedhin, E.Z.; Abdurahaman, M.; Hadush, T.; Tessema, T.S. Seroprevalence and risk factors of Toxoplasma gondii infection in sheep and goats slaughtered for human consumption in Central Ethiopia. BMC Res. Notes 2014, 7, 696. [Google Scholar] [CrossRef]
- Gebremedhin, E.Z.; Agonafir, A.; Tessema, T.S.; Tilahun, G.; Medhin, G.; Vitale, M.; Di Marco, V.; Cox, E.; Vercruysse, J.; Dorny, P. Seroepidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet. Res. 2013, 9, 117. [Google Scholar] [CrossRef]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef]
- Klun, I.; Djurković-Djaković, O.; Katić-Radivojević, S.; Nikolić, A. Cross-sectional survey on Toxoplasma gondii infection in cattle, sheep and pigs in Serbia: Seroprevalence and risk factors. Vet. Parasitol. 2006, 135, 121–131. [Google Scholar] [CrossRef]
- De Pereira, M.F.; de Peixoto, R.M.; Langoni, H.; Greca Junior, H.; de Azevedo, S.S.; Porto, W.J.N.; de Medeiros, E.S.; Mota, R.A. Risk factors for Toxoplasma gondii infection in sheep and goats in Pernambuco, Brazil. Pesqui. Veterinária Bras. 2012, 32, 140–146. [Google Scholar]
- Guimarães, L.A.; Bezerra, R.A.; de Rocha, D.S.; Albuquerque, G.R. Prevalence and risk factors associated with anti-Toxoplasma gondii antibodies in sheep from Bahia state, Brazil. Rev. Bras. Parasitol. Veterinária 2013, 22, 220–224. [Google Scholar]
- Dubey, J.P.; Romand, S.; Hilali, M.; Kwok, O.C.H.; Thulliez, P. Seroprevalence of antibodies to Neospora caniuum and Toxoplasma gondii in water buffaloes (Bubalus bubalis) from Egypt. Int. J. Parasitol. 1998, 28, 527–529. [Google Scholar] [CrossRef]
- Berredjem, H.; Aouras, H.; Benlaifa, M.; Becheker, I.; Djebar, M.R. Contribution of IgG avidity and PCR for the early diagnosis of toxoplasmosis in pregnant women from the North-Eastern region of Algeria. Afr. Health Sci. 2017, 17, 647–656. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).