Sensitive SYBR Green—Real Time PCR for the Detection and Quantitation of Avian Rotavirus A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. RNA Extraction
2.3. Primer Design
2.4. Reverse-Transcription Reaction
2.5. Reverse-Transcription PCR (RT-PCR)
2.6. Reverse Transcription qPCR (RT-qPCR)
2.7. Standard Curve Construction
2.8. Analytical Sensitivity and Specificity
2.9. DNA Sequencing and Data Analysis
3. Results
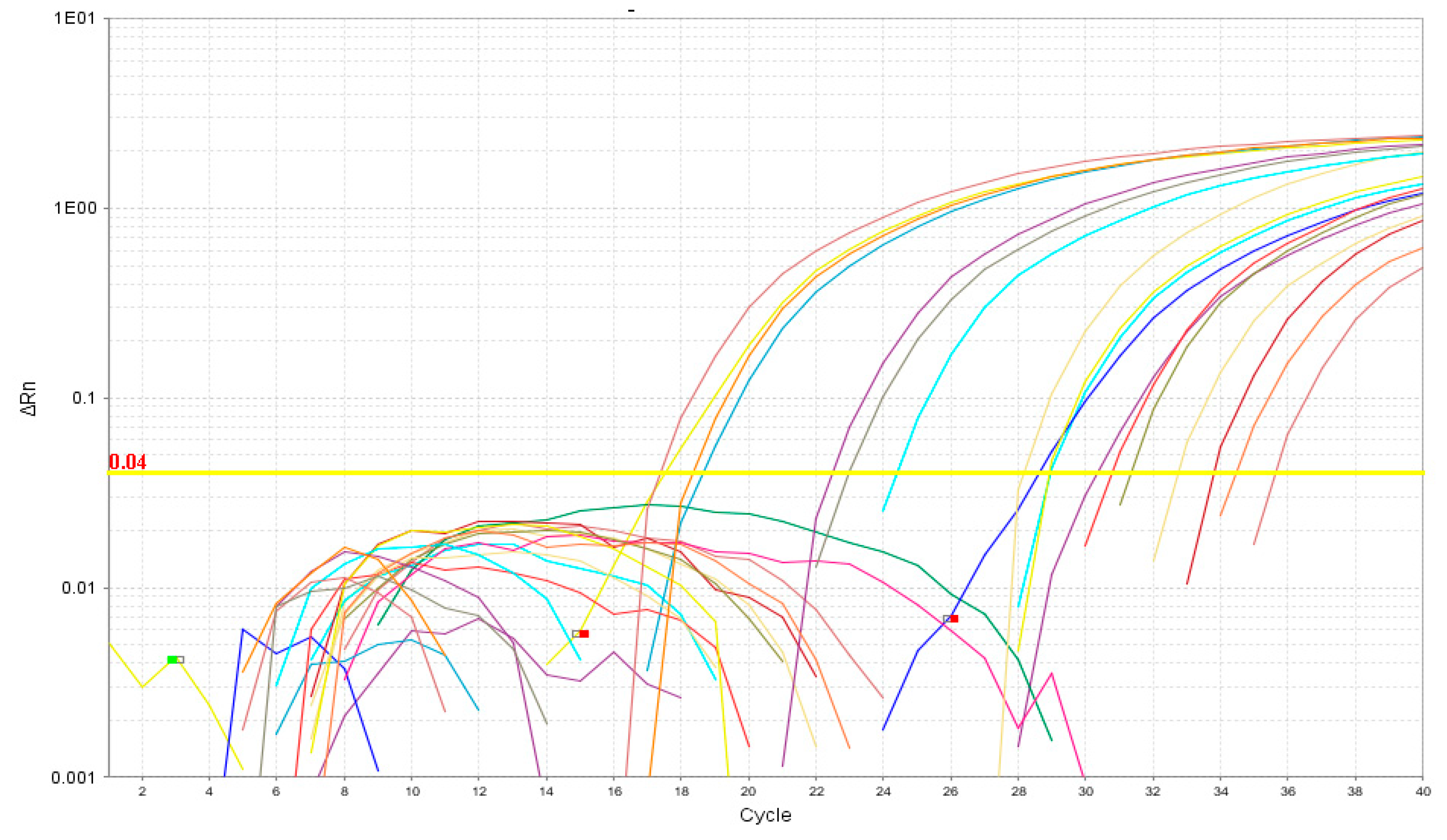
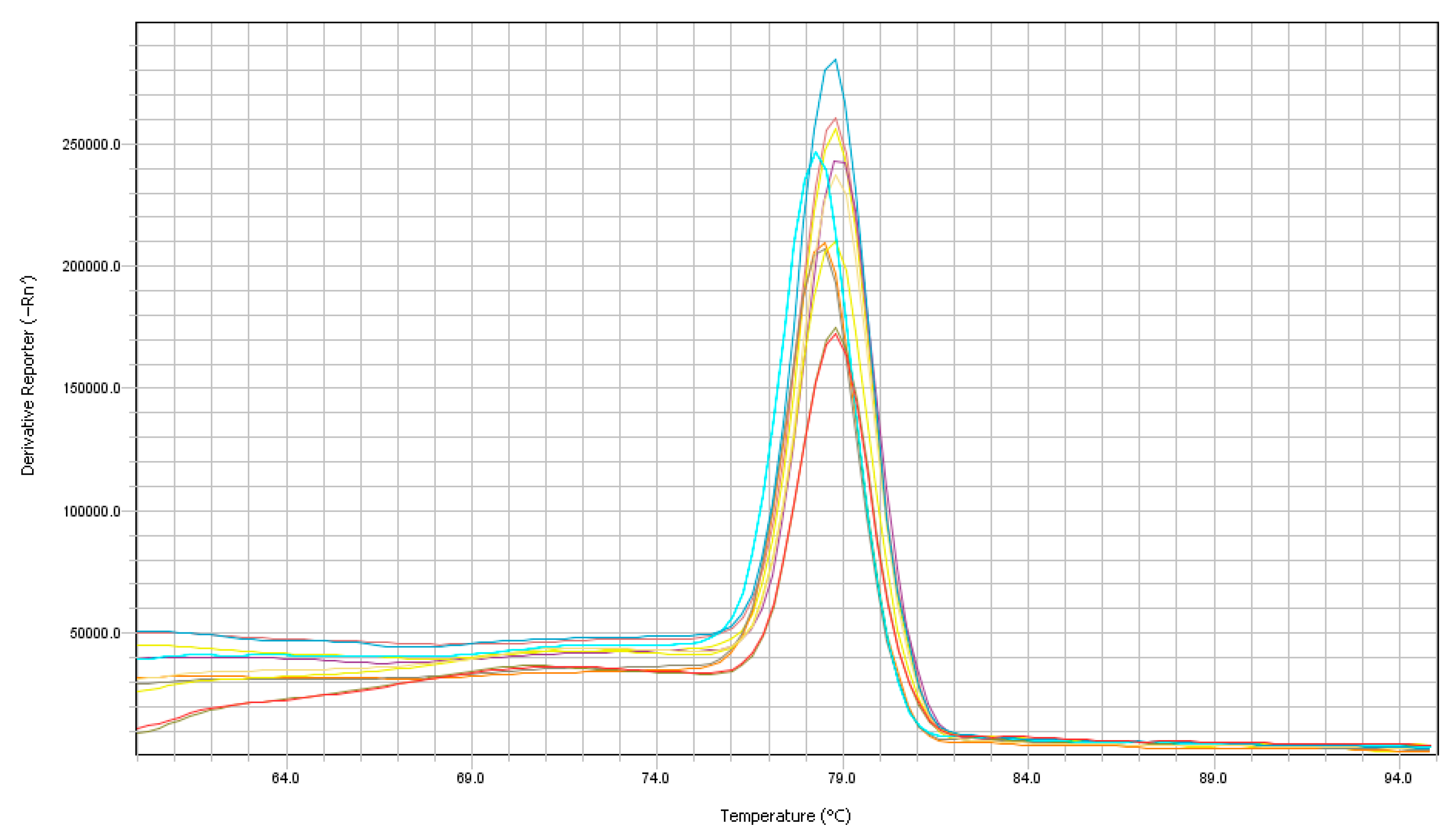
3.1. RT-qPCR
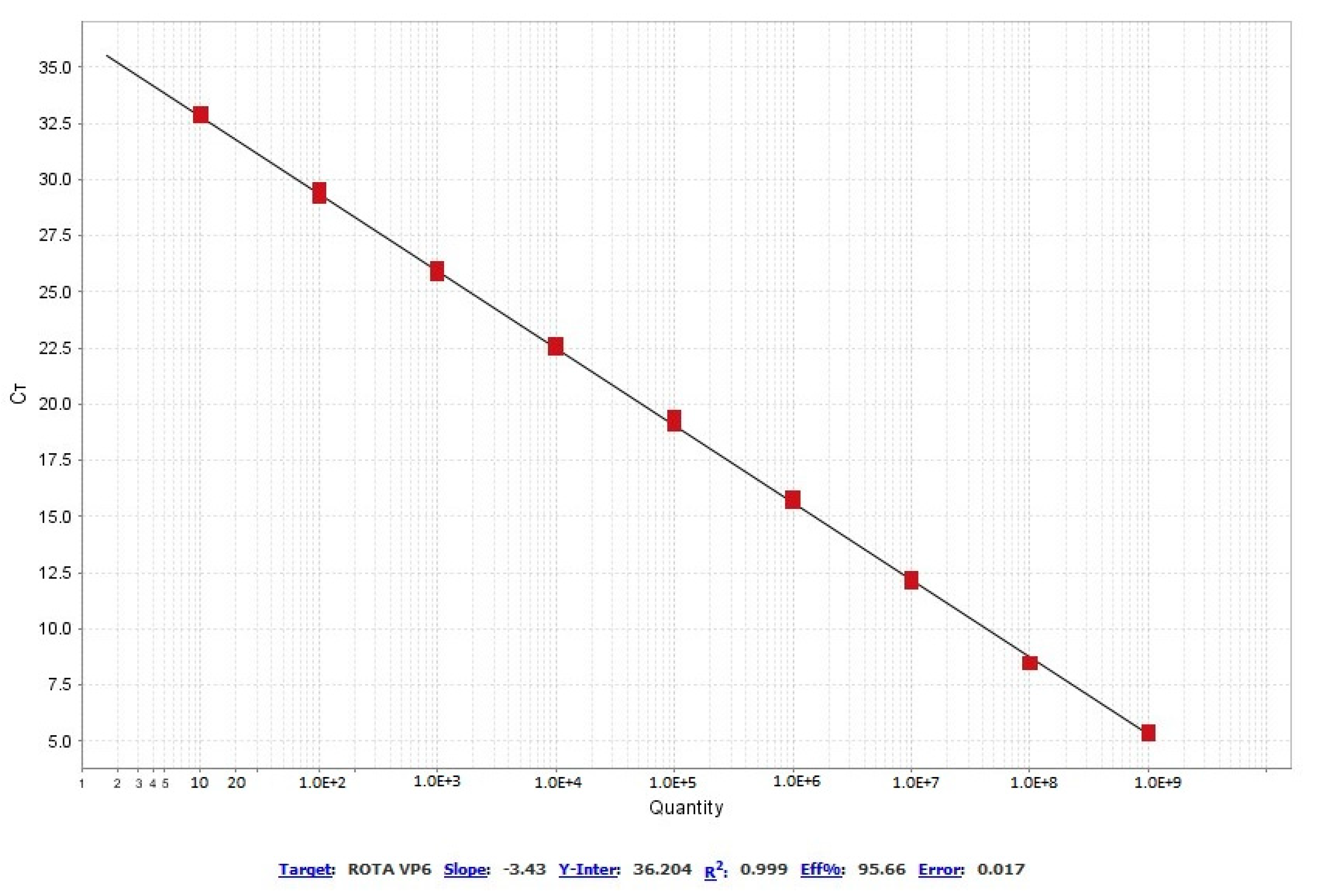
3.2. Standard Curve
3.3. Analytical Sensitivity and Specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spackman, E.; Day, J.M.; Pantin-Jackwood, M.J. Astrovirus, reovirus, and rotavirus concomitant infection causes decreased weight gain in broad-breasted white poults. Avian Dis. 2010, 54, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Esona, M.D.; Gautam, R. Rotavirus. Clin. Lab. Med. 2015, 35, 363–391. [Google Scholar] [CrossRef] [PubMed]
- Lobani, A.M.; Gharaibeh, S.M.; Al-Majali, A.M. Relationship between different enteric viral infections and the occurrence of diarrhea in broiler flocks in Jordan. Poult. Sci. 2016, 95, 1257–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, P.H.; Ahmed, M.U.; Hotzel, H.; Machnowska, P.; Reetz, J.; Roth, B.; Trojnar, E.; Johne, R. Detection of avian rotaviruses of groups A, D, F and G in diseased chickens and turkeys from Europe and Bangladesh. Vet. Microbiol. 2012, 156, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alvarez, J.; Chacon, J.V.; Scanavini, L.S.; Nuñez, L.F.; Astolfi-Ferreira, C.S.; Jones, R.C.; Piantino Ferreira, A.J. Enteric viruses in Brazilian turkey flocks: Single and multiple virus infection frequency according to age and clinical signs of intestinal disease. Poult. Sci. 2013, 92, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, N.; Patnayak, D.P.; Chander, Y.; Ziegler, A.F.; Goyal, S.M. Detection and molecular characterization of enteric viruses in breeder turkeys. Avian Pathol. 2010, 39, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Roussan, D.A.; Shaheen, I.A.; Khawaldeh, G.Y.; Totanji, W.S.; Al-Rifai, R.H. Simultaneous detection of astrovirus, rotavirus, reovirus and adenovirus type I in broiler chicken flocks. Pol. J. Vet. Sci. 2012, 15, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Mettifogo, E.; Nuñez, L.F.; Chacón, J.L.; Santander Parra, S.H.; Astolfi-Ferreira, C.S.; Jerez, J.A.; Jones, R.C.; Piantino Ferreira, A.J. Emergence of enteric viruses in production chickens is a concern for avian health. Sci. World J. 2014, 22, 450423. [Google Scholar] [CrossRef]
- Nuñez, L.F.N.; Parra, S.H.S.; Astolfi-Ferreira, C.S.; Carranza, C.; La Torre, D.I.D.D.; Pedroso, A.C.; Ferreira, A.J.P. Detection of enteric viruses in pancreas and spleen of broilers with runting-stunting syndrome (RSS). Pesqui. Vet. Bras. 2016, 36, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Devaney, R.; Trudgett, J.; Trudgett, A.; Meharg, C.; Smyth, V. A metagenomic comparison of endemic viruses from broiler chickens with runting-stunting syndrome and from normal birds. Avian Pathol. 2016, 45, 616–629. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Rahman, M.; Attoui, H.; Bányai, K.; Estes, M.K.; Gentsch, J.R.; Iturriza-Gómara, M.; Kirkwood, C.D.; Martella, V.; et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 2008, 153, 1621–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindler, E.; Trojnar, E.; Heckel, G.; Otto, P.H.; Johne, R. Analysis of rotavirus species diversity and evolution including the newly determined full-length genome sequences of rotavirus F and G. Infect. Genet. Evol. 2013, 14, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Falcone, E.; Busi, C.; Lavazza, A.; Monini, M.; Bertoletti, M.; Canelli, E.; Vignolo, E.; Ruggeri, F.M.; Boniotti, M.B. Molecular characterization of avian rotaviruses circulating in Italian poultry flocks. Avian Pathol. 2015, 44, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Mascarenhas, J.D.; Bezerra, D.A.; Silva, R.R.; Silva, M.J.; Júnior, E.C.; Soares, L.S. Detection of the VP6 gene of group F and G rotaviruses in broiler chicken fecal samples from the Amazon region of Brazil. Arch. Virol. 2016, 161, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Day, J.M.; Spackman, E.; Pantin-Jackwood, M. A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus. Avian Dis. 2007, 51, 681–684. [Google Scholar] [CrossRef]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of phenol-chloroform RNA extraction. MethodsX 2018, 5, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.S.; Lee, H.R.; Jeon, E.O.; Jang, H.S.; Han, M.S.; Mo, I.P. An unusual case of concomitant infection with chicken astrovirus and group A avian rotavirus in broilers with a history of severe clinical signs. J. Vet. Sci. 2013, 14, 231–233. [Google Scholar] [CrossRef]
- Kutsuzawa, T.; Konno, T.; Suzuki, H.; Kapikian, A.Z.; Ebina, T.; Ishida, N. Isolation of human rotavirus subgroups 1 and 2 in cell culture. J. Clin. Microbiol. 1982, 16, 727–730. [Google Scholar] [PubMed]
- Gouvea, V.; Santos, N.; do C Timenetsky, M. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994, 32, 1338–1340. [Google Scholar] [PubMed]
- Schumann, T.; Hotzel, H.; Otto, P.; Johne, R. Evidence of interspecies transmission and reassortment among avian group A rotaviruses. Virology 2009, 386, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.L.; Lee, B.; Boroumand, N.; Leblanc, B.; Preiksaitis, J.K.; Yu Ip, C.C. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J. Med. Virol. 2004, 72, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, C.M.M.; Nara, T.C.G.B.; Laila, A.R.B.; Fernanda, D.F.S.; Fabio, G. Development of a Real-time PCR test for porcine group A rotavirus diagnosis. Pesqui. Vet. Bras. 2015, 35, 39–43. [Google Scholar] [Green Version]
- Mo, Q.H.; Wang, H.B.; Tan, H.; Wu, B.M.; Feng, Z.L.; Wang, Q.; Lin, J.C.; Yang, Z. Comparative detection of rotavirus RNA by conventional RT-PCR, TaqMan RT-PCR and real-time nucleic acid sequence-based amplification. J. Virol. Methods 2015, 213, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Gouvea, V.; Santos, N.; do C Timenetsky, M. VP4 typing of bovine and porcine group A rotaviruses by PCR. J. Clin. Microbiol. 1994, 32, 1333–1337. [Google Scholar] [PubMed]
- Beserra, L.A.; Barbosa, B.R.; Bernardes, N.T.; Brandão, P.E.; Gregori, F. Occurrence and characterization of rotavirus A in broilers, layers, and broiler breeders from Brazilian poultry farms. Avian Dis. 2014, 58, 153–157. [Google Scholar] [CrossRef] [PubMed]
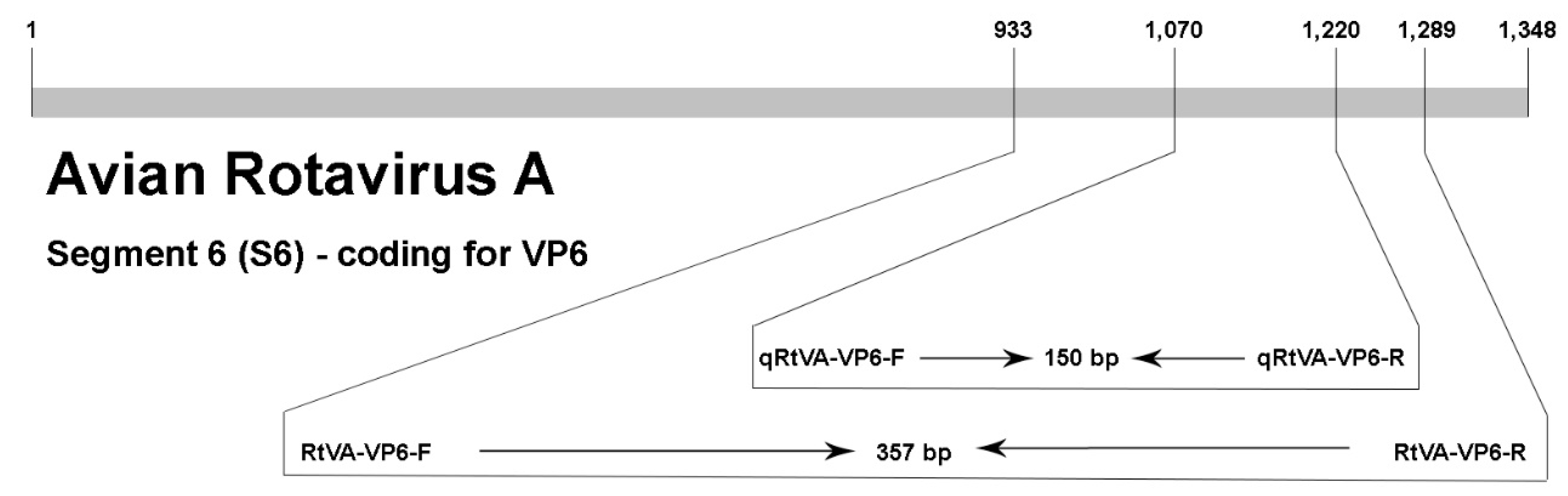
| Name | Gene Target | Assay | Sequence | Nt Position | Reference |
|---|---|---|---|---|---|
| RtVA-VP6-F | VP6 | RT-PCR | 5′-GTAGCAGCACTTTTCCCAGT-3′ | 933-953 | This study |
| RtVA-VP6-R | 5′-GTCCGCTACGGACTATTCG-3′ | 1289-1271 | |||
| qRtVA-VP6-F | VP6 | RT-qPCR | 5′-TTGGACCAGTATTTCCTGCTG-3′ | 1070-1091 | This study |
| qRtVA-VP6-R | 5′-TGGTATGAGCTGTTACCCTCAA-3′ | 1220-1198 |
| Concentration of the RT-PCR Product (DNA copies/µL) | Ct Mean | Ct SD | %CV |
|---|---|---|---|
| 1.0 × 101 | 32.62 | 0.51 | 1.57% |
| 1.0 × 102 | 29.35 | 0.16 | 0.55% |
| 1.0 × 103 | 25.90 | 0.15 | 0.59% |
| 1.0 × 104 | 22.57 | 0.13 | 0.56% |
| 1.0 × 105 | 19.23 | 0.17 | 0.90% |
| 1.0 × 106 | 15.74 | 0.10 | 0.64% |
| 1.0 × 107 | 12.21 | 0.11 | 0.93% |
| 1.0 × 108 | 8.47 | 0.03 | 0.30% |
| 1.0 × 109 | 5.37 | 0.09 | 1.69% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Torre, D.; Astolfi-Ferreira, C.S.; Chacon, R.D.; Piantino Ferreira, A.J. Sensitive SYBR Green—Real Time PCR for the Detection and Quantitation of Avian Rotavirus A. Vet. Sci. 2019, 6, 2. https://doi.org/10.3390/vetsci6010002
De la Torre D, Astolfi-Ferreira CS, Chacon RD, Piantino Ferreira AJ. Sensitive SYBR Green—Real Time PCR for the Detection and Quantitation of Avian Rotavirus A. Veterinary Sciences. 2019; 6(1):2. https://doi.org/10.3390/vetsci6010002
Chicago/Turabian StyleDe la Torre, David, Claudete S. Astolfi-Ferreira, Ruy D. Chacon, and Antonio J. Piantino Ferreira. 2019. "Sensitive SYBR Green—Real Time PCR for the Detection and Quantitation of Avian Rotavirus A" Veterinary Sciences 6, no. 1: 2. https://doi.org/10.3390/vetsci6010002






