Cellular Immunotherapy of Canine Cancer
Abstract
1. Introduction
2. Clinical Studies of Autologous Lymphocyte Infusions
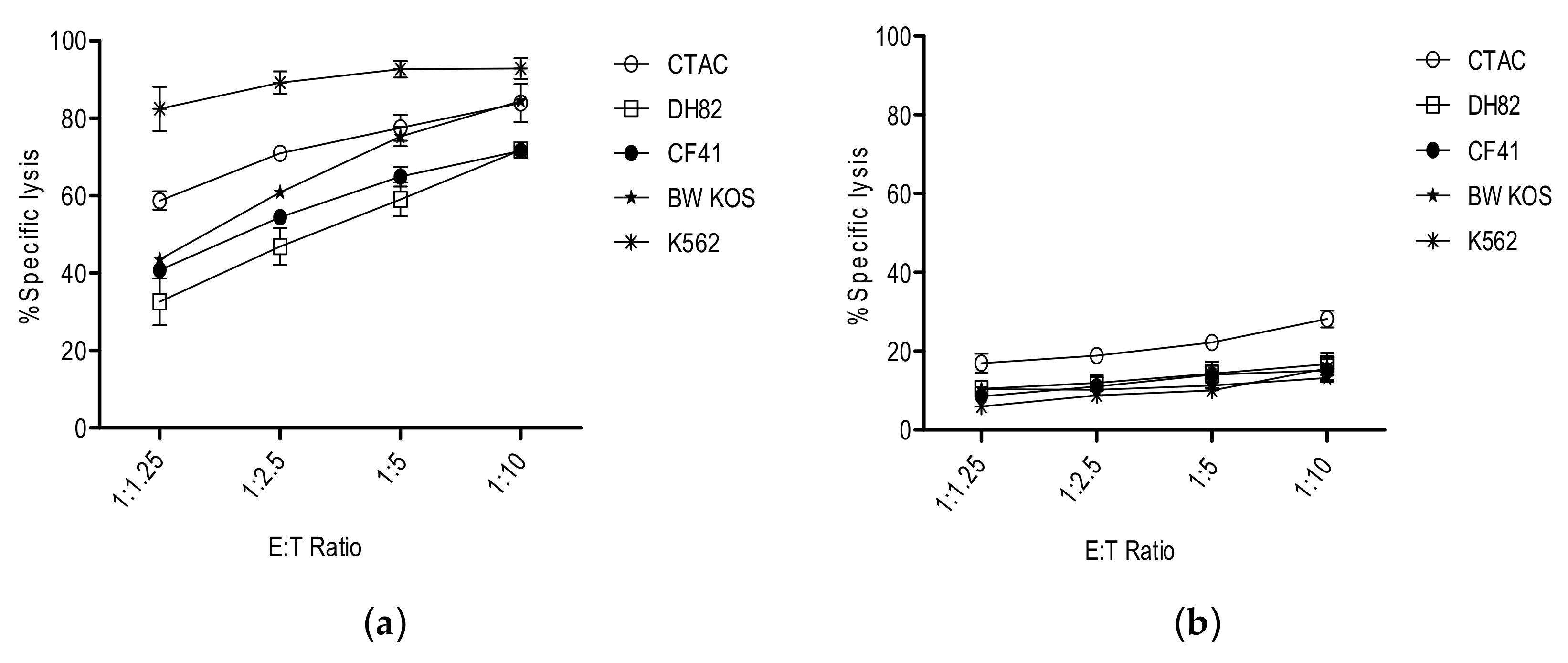
3. NK Cells
4. Clinical Studies with Xenogeneic Lymphocytes
5. Where to Go from Here?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.N.; Khanna, C. Defining the value of a comparative approach to cancer drug development. Clin. Cancer Res. 2016, 22, 2133–2138. [Google Scholar] [CrossRef]
- Gordon, I.; Paoloni, M.; Mazcko, C.; Khanna, C. The comparative oncology trials consortium: Using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009, 6, e1000161. [Google Scholar] [CrossRef]
- Regan, D.; Dow, S. Manipulation of innate immunotherapy for cancer therapy in dogs. Vet. Sci. 2015, 2, 423–439. [Google Scholar] [CrossRef]
- Rue, S.M.; Eckelman, B.P.; Efe, J.A.; Bloink, K.; Deveraux, Q.L.; Lowery, D.; Nasoff, M. Identification of a candidate therapeutic antibody for treatment of canine B-cell lymphoma. Vet. Immunol. Immunopathol. 2015, 164, 148–159. [Google Scholar] [CrossRef]
- Klingemann, H. Immunotherapy for dogs: Running behind humans. Front. Immunol. 2018, 9, 133. [Google Scholar] [CrossRef]
- Storb, R.; Epstein, R.B.; Rudolph, R.H.; Thomas, E.D. Allogeneic canine bone marrow transplantation following cyclophosphamide. Transplantation 1969, 7, 378–386. [Google Scholar] [CrossRef]
- Fefer, A.; Sullivan, K.M.; Weiden, P.; Buckner, C.D.; Schoch, G.; Storb, R.; Thomas, E.D. Graft versus leukemia effect in man: The relapse rate of acute leukemia is lower after allogeneic than after syngeneic marrow transplantation. Prog. Clin. Biol. Res. 1987, 244, 401–408. [Google Scholar]
- Konjevic, G.; Jurisic, V.; Jovic, V.; Vuletic, A.; Mirjacic Martinovic, K.; Radenkovic, S.; Spuzic, I. Investigation of NK cell function and their modulation in different malignancies. Immunol. Res. 2012, 52, 139–156. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015, 29, 281–290. [Google Scholar] [CrossRef]
- Hong, C.S.; Sharma, P.; Yernemi, S.S.; Simms, P.; Jackson, E.K.; Whiteside, T.L.; Boyiadzis, M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017, 7, 1468. [Google Scholar] [CrossRef]
- Geukes Foppen, M.H.; Donia, M.; Svane, I.M.; Haane, J.B. Tumor infiltrating lymphocytes for the treatment of metastatic cancer. Mol. Oncol. 2015, 9, 1918–1935. [Google Scholar] [CrossRef]
- Ohta, A. A metabolic immune checkpoint: Adenosine in tumor microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2018, in press. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Sheppard, S.; Hartline, C.A.; Huls, H.; Johnson, M.; Palla, S.L.; Maiti, S.; Ma, W.; Davis, R.E.; Craig, S.; et al. Adoptive T-cell therapy improves treatment of canine non–Hodgkin lymphoma post chemotherapy. Sci. Rep. 2012, 2, 249. [Google Scholar] [CrossRef]
- Flesner, B.K. Autologous activated T cell therapy for canine osteosarcoma. In Proceedings of the Veterinary Cancer Society Meeting, Louisville, KY, USA, 18 October 2018. [Google Scholar]
- Hinrichs, C.S.; Rosenberg, S.A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014, 257, 56–71. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef]
- Dotti, G.; Gottschalk, S.; Savoldo, B.; Brenner, M.K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014, 257, 107–126. [Google Scholar] [CrossRef]
- Teachey, D.T.; Bishop, M.R.; Maloney, D.G.; Grupp, S.A. Toxicity management after chimeric antigen receptor T cell therapy: One size does not fit ‘ALL’. Nat. Rev. Clin. Oncol. 2018, 15, 218. [Google Scholar] [CrossRef]
- Ruella, M.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; Nazimuddin, F.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Mata, M.; Vera, J.; Gerken, C.; Rooney, C.M.; Miller, T.; Pfent, C.; Wang, L.L.; Wilson-Robles, H.M.; Gottschalk, S. Toward immunotherapy with redirected T cells in a large animal model: Ex vivo activation, expansion, and genetic modification of canine T cells. J. Immunother. 2014, 37, 407–415. [Google Scholar] [CrossRef]
- Panjwani, M.K.; Smith, J.B.; Schutsky, K.; Gnanandarajah, J.; O’Connor, C.M.; Powell, D.J., Jr.; Mason, N.J. Feasibility and safety of RNA transfected CD20 specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol. Ther. 2016, 24, 1602–1614. [Google Scholar] [CrossRef]
- Osamu, S.; Nemoto, Y.; Mizuno, T. Generation of Canine CD20 Chimeric Antigen Receptor T cells and in vitro evaluation of cytotoxic effect on canine B cell lymphoma. In Proceedings of the Veterinary Cancer Society Meeting, Louisville, KY, USA, 18 October 2018. [Google Scholar]
- June, C. Paws for a Cure Symposium, Boston. Personal Communication, 13 December 2018. [Google Scholar]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Michael, H.; Ito, D.; McCullar, V.; Zhang, B.; Miller, J.S.; Modiano, J.F. Isolation and characterization of canine natural killer cells. Vet. Immunol. Immunopathol. 2013, 155, 211–217. [Google Scholar] [CrossRef]
- Foltz, J.A.; Somanchi, S.S.; Yang, Y.; Aquino-Lopez, A.; Bishop, E.E.; Lee, D.A. NCR1 expression identifies canine natural killer cell subsets with phenotypic similarity to human natural killer cells. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Canter, R.J.; Grossenbacher, S.K.; Foltz, J.A.; Sturgill, I.R.; Park, J.S.; Luna, J.I.; Kent, M.S.; Culp, W.T.N.; Chen, M.; Modiano, J.F.; et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J. Immunother. Cancer 2017, 5, 98. [Google Scholar] [CrossRef]
- Park, B.; Yee, C.; Lee, K.M. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 2014, 15, 927–943. [Google Scholar] [CrossRef]
- Raulet, D.E.; Gasser, S.; Gowen, B.J.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015, 6, 368–383. [Google Scholar] [CrossRef]
- Bergeron, L.M.; McCandless, E.E.; Dunham, S.; Dunkle, B.; Zhu, Y.; Shelly, J.; Lightle, S.; Gonzales, A.; Bainbridge, G. Comparative functional characterization of canine IgG subclasses. Vet. Immunol. Immunopathol. 2014, 157, 31–41. [Google Scholar] [CrossRef]
- Singer, J.; Fazekas, J.; Wang, W.; Weichselbaumer, M.; Matz, M.; Mader, A.; Steinfellner, W.; Meitz, S.; Mechtcheriakova, D.; Sobanov, Y.; et al. Generation of a canine anti-EGFR (ErbB-1) antibody for passive immunotherapy in dog cancer patients. Mol. Cancer Ther. 2014, 13, 1777–1790. [Google Scholar] [CrossRef]
- Cesano, A.; Visonneau, S.; Jeglum, K.A.; Owen, J.; Wilkinson, K.; Carner, K.; Reese, L.; Santoli, D. Phase I clinical trial with a human major histocompatibility complex nonrestricted cytotoxic T-cell line (TALL-104) in dogs. Cancer Res. 1996, 56, 3021–3029. [Google Scholar]
- Visonneau, S.; Cesano, A.; Tran, T.; Jeglum, K.A.; Santoli, D. Successful treatment of canine malignant histiocytosis with the human major histocompatibility complex nonrestricted cytotoxic T-cell line TALL-104. Clin. Cancer Res. 1997, 3, 1789–1797. [Google Scholar]
- Visonneau, S.; Cesano, A.; Jeglum, K.A.; Santoli, D. Adoptive therapy of canine metastatic mammary carcinoma with the human MHC non-restricted cytotoxic T-cell line TALL-104. Oncol. Rep. 1999, 6, 1181–1188. [Google Scholar] [CrossRef]
- Suck, G.; Linn, Y.C.; Tonn, T. Natural killer cells for therapy of leukemia. Transfus. Med. Hemother. 2016, 43, 89–93. [Google Scholar] [CrossRef]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural killer cells for immunotherapy- advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Shin, D.J.; Park, J.Y.; Jang, Y.Y.; Lee, J.J.; Lee, Y.K.; Shin, M.G.; Jung, J.Y.; Carson, W.E., 3rd; Cho, D.; Kim, S.K. Ex vivo expansion of canine cytotoxic large granular lymphocytes exhibiting characteristics of natural killer cells. Vet. Immunol. Immunopathol. 2013, 153, 249–259. [Google Scholar] [CrossRef]
- Sherger, M.; Kisserberth, W.; London, C.; Olivio-Marston, S.; Papenfuss, T.L. Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs. BMC Vet. Res. 2012, 8, 209. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Thamm, D.H.; Biller, B.J. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J. Vet. Intern. Med. 2012, 26, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, N.; Konnai, S.; Takagi, S.; Kagawa, Y.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Deguchi, T.; Nakajima, C.; et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci. Rep. 2017, 7, 8951. [Google Scholar] [CrossRef] [PubMed]
- Boissel, L.; Klingemann, H.; Khan, J.; Soon-Shiong, P. Intra-tumor injection of CAR-engineered NK cells induces tumor regression and protection against tumor re-Challenge. Blood 2016, 128, 466. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addissie, S.; Klingemann, H. Cellular Immunotherapy of Canine Cancer. Vet. Sci. 2018, 5, 100. https://doi.org/10.3390/vetsci5040100
Addissie S, Klingemann H. Cellular Immunotherapy of Canine Cancer. Veterinary Sciences. 2018; 5(4):100. https://doi.org/10.3390/vetsci5040100
Chicago/Turabian StyleAddissie, Selamawit, and Hans Klingemann. 2018. "Cellular Immunotherapy of Canine Cancer" Veterinary Sciences 5, no. 4: 100. https://doi.org/10.3390/vetsci5040100
APA StyleAddissie, S., & Klingemann, H. (2018). Cellular Immunotherapy of Canine Cancer. Veterinary Sciences, 5(4), 100. https://doi.org/10.3390/vetsci5040100



