Additional Progress in the Development and Application of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Antibodies
2.3. Rabies Virus Variant Comparison
2.4. Protocol
2.5. Overall Test Performance
2.6. U.S. National Inter-Laboratory Testing of Reference Brain Samples as A Surrogate for Reproducibility
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Expert Consultation on Rabies (Third Report); World Health Organization Technical Report Series 1012; World Health Organization: Geneva, Switzerland, 2018; pp. 1–195. [Google Scholar]
- OIE World Organisation for Animal Health Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: OIE—World Organisation for Animal Health. Rabies. 2017. Available online: http://www.oie.int/standard-setting/terrestrial-manual/access-online/ (accessed on 1 April 2018).
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primer 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Cliquet, F.; Fehlner-Gardiner, C.; Fooks, A.R.; Mueller, T.; Sabeta, C.; Slate, D. Progress in the development of a direct rapid immunohistochemical test for diagnosing rabies. Bull. OIE 2014, 3, 87–95. [Google Scholar]
- Middel, K.; Fehlner-Gardiner, C.; Pulham, N.; Buchanan, T. Incorporating Direct Rapid Immunohistochemical Testing into Large-Scale Wildlife Rabies Surveillance. Trop. Med. Infect. Dis. 2017, 2, 21. [Google Scholar] [CrossRef]
- Coetzer, A.; Anahory, I.; Dias, P.T.; Sabeta, C.T.; Scott, T.P.; Markotter, W.; Nel, L.H. Enhanced diagnosis of rabies and molecular evidence for the transboundary spread of the disease in Mozambique. J. S. Afr. Vet. Assoc. 2017, 88, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.N.; Isloor, S.; Veeresh, B.H.; Rathnamma, D.; Yathiraj, S.; Satyanarayana, M.L.; D’Souza, P.; Neelufer, M.S.; Sharada, R.; Rahman, S.A. Application of N-protein monoclonal antibody based direct fluorescent antibody assay (DFA) and direct rapid immunohistochemistry test (dRIT) for detection of rabies virus in brain samples of animals in India. Commonw. Vet. J. 2014, 30, 11–16. [Google Scholar]
- Coetzer, A.; Sabeta, C.T.; Markotter, W.; Rupprecht, C.E.; Nel, L.H. Comparison of biotinylated monoclonal and polyclonal antibodies in an evaluation of a direct rapid immunohistochemical test for the routine diagnosis of rabies in southern Africa. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Ehimiyein, A.M.; Niesgoda, M.; Orciari, L.; Osinubi, M.O.V.; Ehimiyein, I.O.; Adawa, D.A.Y.; Abdullah, S.U.; Ogunkoya, A.B.; Rupprecht, C.E. Efficacy of a Direct Rapid Immunohistochemical Test (DRIT) For Rabies Detection in Nigeria. Afr. J. Biomed. Res. 2014, 17, 101–107. [Google Scholar]
- Madhusudana, S.N.; Subha, S.; Thankappan, U.; Ashwin, Y.B. Evaluation of a direct rapid immunohistochemical test (dRIT) for rapid diagnosis of rabies in animals and humans. Virol. Sin. 2012, 27, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Saturday, G.A.; King, R.; Fuhrmann, L. Validation and operational application of a rapid method for rabies antigen detection. US Army Med. Dep. J. 2009, 42–45. [Google Scholar]
- Tao, X.-Y.; Niezgoda, M.; Du, J.-L.; Li, H.; Wang, X.-G.; Huang, Y.; Jiao, Y.; Cao, L.; Tang, Q.; Liang, G.-D. The primary application of direct rapid immunohistochemical test to rabies diagnosis in China. Chin. J. Exp. Clin. Virol. 2008, 22, 168–170. [Google Scholar]
- Zhang, Y.-Z.; Fu, Z.F.; Wang, D.-M.; Zhou, J.-Z.; Wang, Z.-X.; Lv, T.-F.; Xiong, C.-L.; Zou, Y.; Yao, W.-R.; Li, M.-H.; et al. Investigation of the role of healthy dogs as potential carriers of rabies virus. Vector Borne Zoonotic Dis. 2008, 8, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Naïssengar, S.; Mindekem, R.; Diguimbye, C.; Niezgoda, M.; Kuzmin, I.; Rupprecht, C.E.; Zinsstag, J. Rabies diagnosis for developing countries. PLoS Negl. Trop. Dis. 2008, 2. [Google Scholar] [CrossRef] [PubMed]
- Lembo, T.; Niezgoda, M.; Velasco-Villa, A.; Cleaveland, S.; Ernest, E.; Rupprecht, C.E. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg. Infect. Dis. 2006, 12, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Hamir, A.N.; Moser, G.; Wampler, T.; Hattel, A.; Dietzschold, B.; Rupprecht, C.E. Use of a single anti-nucleocapsid monoclonal antibody to detect rabies antigen in formalin-fixed, paraffin-embedded tissues. Vet. Rec. 1996, 138, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Dietzschold, B.; Wunner, W.H.; Koprowski, H. Antigenic Relationships of Lyssa viruses. In The Natural History of Rabies, 2nd ed.; Baer, G.M., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 69–102. ISBN 9780849367601. [Google Scholar]
- Ma, X.; Monroe, B.P.; Cleaton, J.M.; Orciari, L.A.; Yager, P.; Li, Y.; Kirby, J.D.; Blanton, J.D.; Petersen, B.W.; Wallace, R.M. Rabies surveillance in the United States during 2016. J. Am. Vet. Med. Assoc. 2018, 252, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.D.; Chipman, R.B.; Nelson, K.M.; Rupprecht, C.E.; Blanton, J.D.; Algeo, T.P.; Slate, D. Enhanced Rabies Surveillance to Support Effective Oral Rabies Vaccination of Raccoons in the Eastern United States. Trop. Med. Infect. Dis. 2017, 2, 34. [Google Scholar] [CrossRef]
- Mayes, B.; Rupprecht, C.E. Direct Fluorescent Antibody Test for Rabies Diagnosis. In Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention; Acad Press: Waltham, MA, USA, 2015; Volume 2, pp. 83–92. ISBN 9780128019191. [Google Scholar]
- Rupprecht, C.E.; Niezgoda, M. Standard operating procedure for the direct rapid immunohistochemistry test (DRIT) for the detection of rabies virus antigens. US Cent. Dis. Control 2006, 1–16. [Google Scholar]
- Guesdon, J.L.; Ternynck, T.; Avrameas, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1979, 27, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Peränen, J. Rapid affinity-purification and biotinylation of antibodies. Biotechniques 1992, 13, 546–549. [Google Scholar] [PubMed]
- Hirsch, J.D.; Haugland, R.P. Conjugation of antibodies to biotin. Methods Mol. Biol. 2005, 295, 135–154. [Google Scholar] [PubMed]
- Robardet, E.; Andrieu, S.; Rasmussen, T.B.; Dobrostana, M.; Horton, D.L.; Hostnik, P.; Jaceviciene, I.; Juhasz, T.; Müller, T.; Mutinelli, F.; et al. Comparative assay of fluorescent antibody test results among twelve European National Reference Laboratories using various anti-rabies conjugates. J. Virol. Methods 2013, 191, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.L.; Niezgoda, M.; Orciari, L.A.; Yager, P.A.; Ellison, J.A.; Rupprecht, C.E. Evaluation of an indirect rapid immunohistochemistry test for the differentiation of rabies virus variants. J. Virol. Methods 2013, 190, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.N.; Isloor, S.; Veeresh, B.H.; Rathnamma, D.; Sharada, R.; Das, L.J.; Satyanarayana, M.L.; Hegde, N.R.; Rahman, S.A. Application and Comparative Evaluation of Fluorescent Antibody, Immunohistochemistry and Reverse Transcription Polymerase Chain Reaction Tests for the Detection of Rabies Virus Antigen or Nucleic Acid in Brain Samples of Animals Suspected of Rabies in India. Vet. Sci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, M.; Liu, T.; Zhang, Y.; Tu, Z.; Guo, H.; Zhang, C.; Zhu, R.; Ren, W.; Sun, L.; et al. Evaluation of monoclonal antibody-based direct, rapid immunohistochemical test for rabies diagnosis. J. Virol. Methods 2018, 256, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.J.C.; Llenaresas-Manalo, D.; Jarilla, B.; Tuason, L.; Avenido, E.; Demetria, C.; Medina, P.; Quiambao, B.; Acosta, L.; Inoue, S.; et al. Evaluation of Direct Rapid Immunohistochemical Test (DRIT) of canis lupus familiaris hippocampal touch impression smears using a monospecific polyclonal antibody for rabies virus detection. Acta Med. Philipp. 2016, 50, 51–55. [Google Scholar]
- Rahmadane, I.; Certoma, A.F.; Peck, G.R.; Fitria, Y.; Payne, J.; Colling, A.; Shiell, B.J.; Beddome, G.; Wilson, S.; Yu, M.; et al. Development and validation of an immunoperoxidase antigen detection test for improved diagnosis of rabies in Indonesia. PLoS Negl. Trop. Dis. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.; Hsu, C.L.; Lee, M.S.; Chang, J.C.; Wu, C.H. Lyssavirus in Japanese Pipistrelle, Taiwan. Emerg. Infect. Dis. 2018, 24, 782–785. [Google Scholar] [CrossRef] [PubMed]
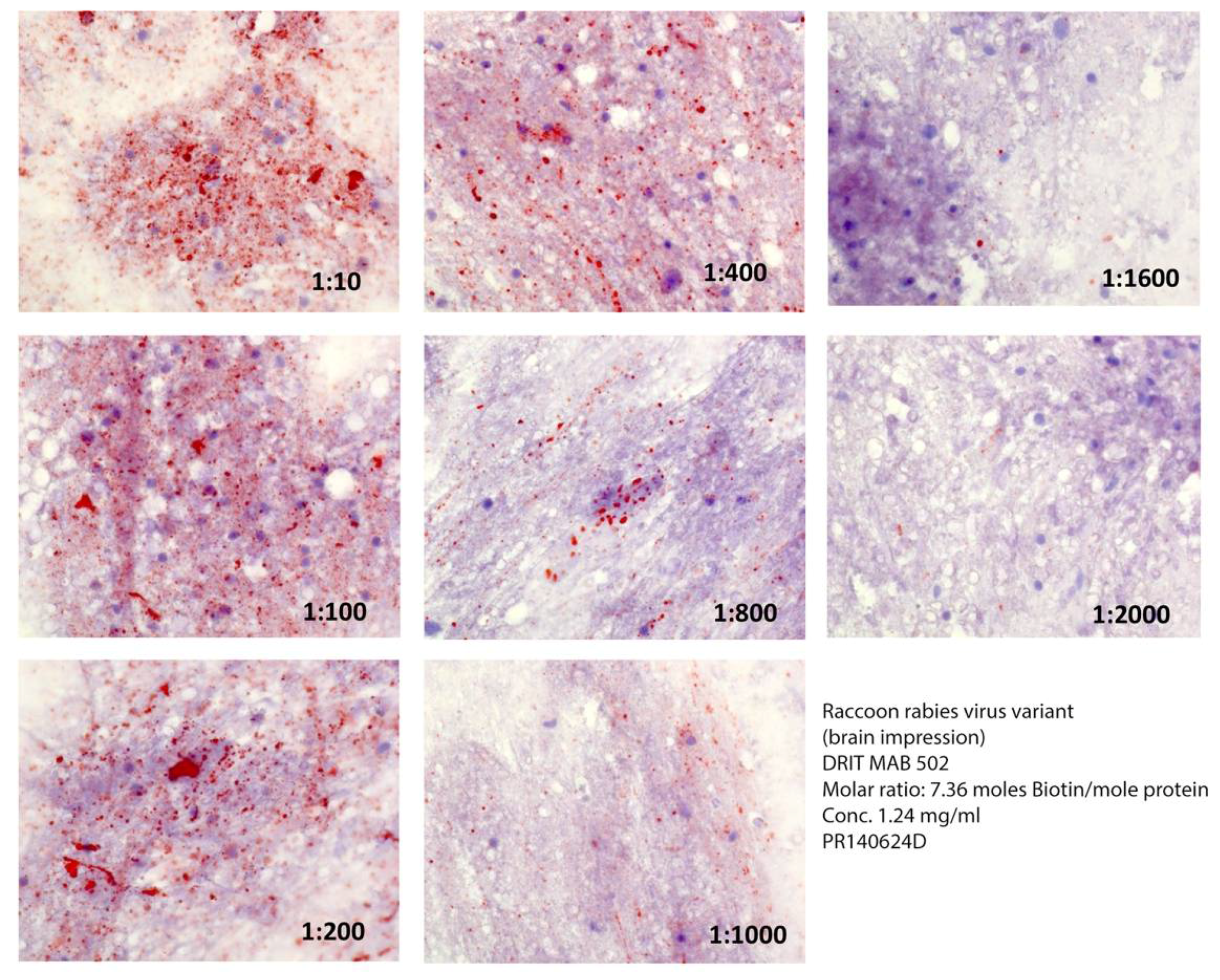
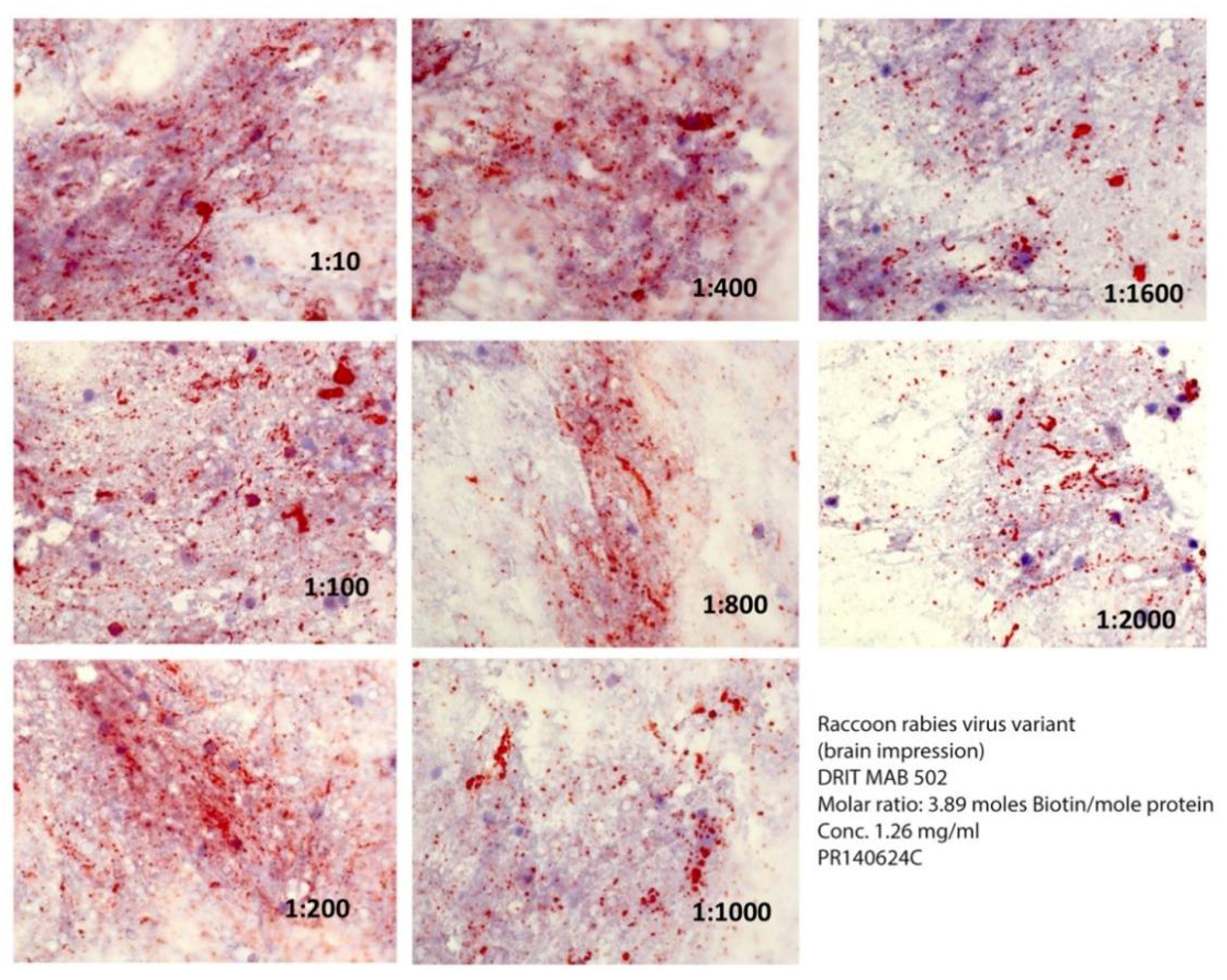
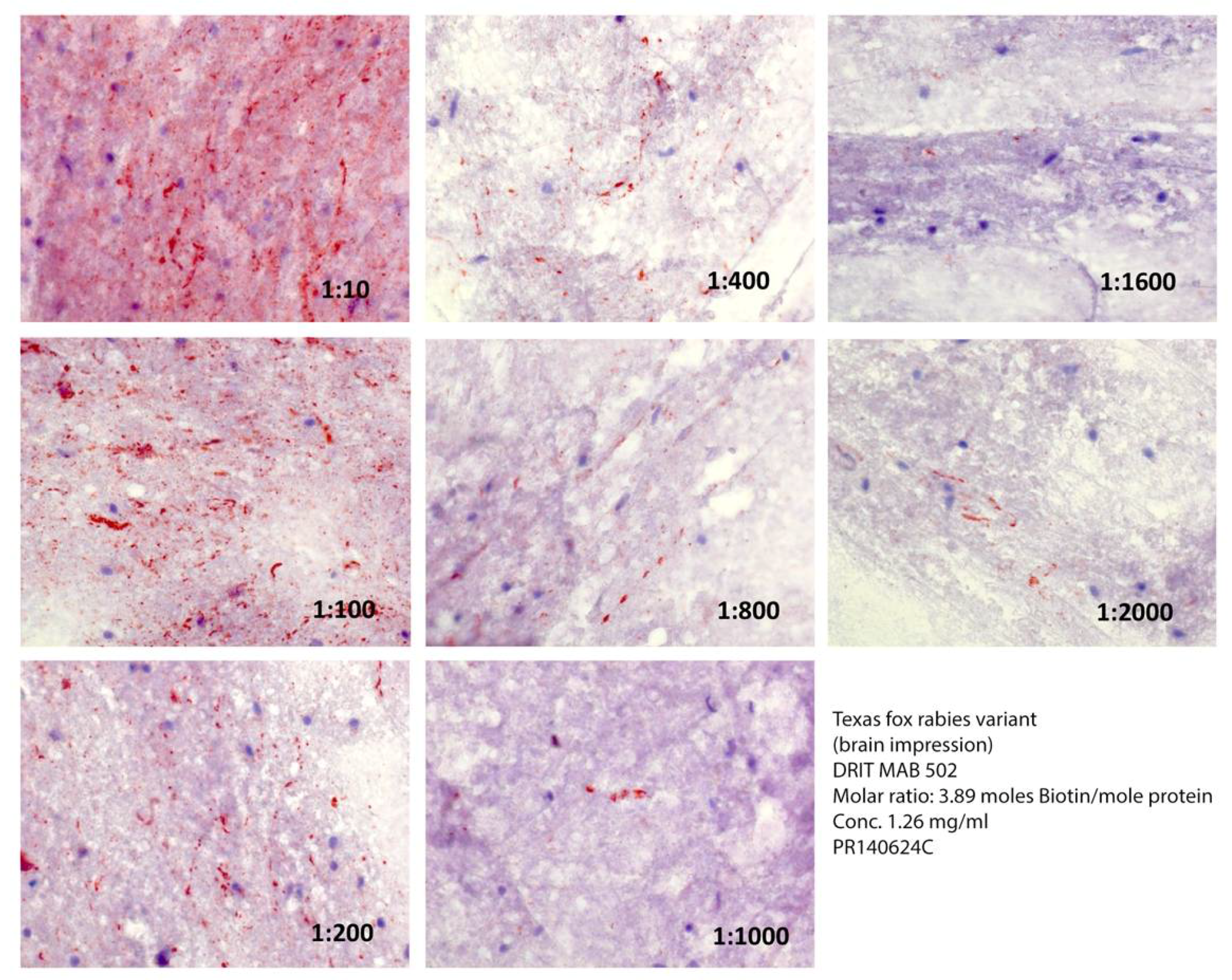
| Animal | Total Number | Number of Rabid Animals (Positive FAT) |
|---|---|---|
| Raccoon | 495 | 105 |
| Skunk (4 taxa) | 153 | 96 |
| Bat (8 taxa) | 49 | 10 |
| Red fox | 32 | 23 * |
| Dog | 25 | 12 ** |
| Cat | 17 | 1 |
| Coyote | 13 | 0 |
| Cattle | 8 | 7 |
| Gray fox | 8 | 0 |
| Javelina | 3 | 0 |
| Wolf | 2 | 1 |
| Otter | 2 | 0 |
| Marten | 2 | 0 |
| Bobcat | 2 | 0 |
| Deer | 1 | 1 |
| Stone Marten | 1 | 0 |
| Badger | 1 | 0 |
| Woodchuck | 1 | 0 |
| Grey squirrel | 1 | 0 |
| FAT Positive | FAT Negative | Total | |
|---|---|---|---|
| DRIT Positive | 252 | 30 | 282 |
| DRIT Negative | 4 | 530 | 534 |
| 256 | 560 | ||
| Value | 95% CI | ||
| Sensitivity | 98% | 96% to 100% | |
| Specificity | 95% | 92% to 96% | |
| Positive Likelihood Ratio | 18 | 13 to 26 | |
| Negative Likelihood Ratio | 0.02 | 0.01 to 0.04 | |
| Disease prevalence | 31% | 28% to 35% | |
| Positive Predictive Value | 89% | 85% to 93% | |
| Negative Predictive Value | 99% | 98% to 100% |
| Sample | Animal | History/Signs | FAT Status | DRIT PT Findings | Specificity | Sensitivity |
|---|---|---|---|---|---|---|
| RA-B301 | Raccoon | Killed by a vaccinated dog | Negative | 1 positive, 14 negative | 93% | NA |
| RA-B312 | Dog | Vaccinated; bit member of its owner’s family | Negative | 15 negative | 100% | NA |
| RA-B316 | Dog | Vaccinated; bit its owner | Negative | 1 indeterminate, 14 negative | 100% | NA |
| RA-B319 | Cow | Paralysis; unusual vocalizations | Positive | 15 positive | NA | 100% |
| RA-B305 | Cat | Ataxia; disorientation | Negative | 1 positive, 14 negative | 93% | |
| RA-B306 | Elk | Farm-raised; neurological signs | Positive | 13 positive, 1 indeterminate, 1 negative | NA | 93% |
| RA-B308 | Fox | Attacked a dog accompanied by a human | Positive | 14 positive, 1 indeterminate | NA | 100% |
| RA-B310 | Dog | Unknown vaccination status; bit its owner | Negative | 1 positive, 14 negative | 93% | NA |
| RA-B311 | Dog | Vaccinated; bit its owner | Negative | 1 positive, 14 negative | 93% | NA |
| RA-B300 | Cat | Unknown vaccination status; bit its owner | Negative | 1 indeterminate, 16 negative | 100% | NA |
| RA-B303 | Raccoon | Bit a person | Negative | 1 positive, 16 negative | 94% | NA |
| RA-B304 | Horse | Incoordination; restlessness; self-mutilation | Positive | 14 positive, 3 negative | NA | 82% |
| RA-B315 | Goat | Clinical signs for 10 days prior to euthanasia | Positive | 10 positive, 2 indeterminate, 3 negative | NA | 77% |
| RA-B317 | Dog | Unknown vaccination status; bit a person | Negative | 1 indeterminate, 14 negative | 100% | NA |
| RA-B318 | Fox | Killed by a vaccinated dog | Negative | 14 negative | 100% | NA |
| RA-B322 | Dog | Vaccinated; bit its owner | Negative | 1 indeterminate, 14 negative | 100% | NA |
| RA-B327 | Deer | Apparently blind, with swollen eyes | Positive | 15 positive | NA | 100% |
| RA-B328 | Dog | Questionable vaccination status; bit a person | Negative | 1 indeterminate, 11 negative | 100% | NA |
| RA-B330 | Cat | Vaccinated; bit a person | Negative | 1 indeterminate, 12 negative | 100% | NA |
| RA-B332 | Dog | Unknown vaccination status; bit a person | Negative | 1 indeterminate, 12 negative | 100% | NA |
| RA-B334 | Sheep | Unvaccinated; compatible signs of encephalitis | Positive | 13 positive | NA | 100% |
| RA-B335 | Raccoon | Attacked a dog; bit a person | Negative | 1 indeterminate, 12 negative | 100% | NA |
| RA-B333 | Cat | Questionable vaccination status; bit and scratched a person | Negative | 1 positive, 2 indeterminate, 11 negative | 92% | NA |
| RA-B339 | Dog | Vaccinated; injured; bit owner | Negative | 1 positive, 1 indeterminate, 12 negative | 92% | NA |
| RA-B342 | Dog | Unknown vaccination status; chased and bit a person | Negative | 1 indeterminate, 13 negative | 100% | NA |
| RA-B347 | Dog | Died after a skunk exposure | Negative | 2 positive, 12 negative | 86% | NA |
| RA-B349 | Cow | Bellowing; hypersalivation; unable to stand | Positive | 14 positive | NA | 100% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupprecht, C.E.; Xiang, Z.; Servat, A.; Franka, R.; Kirby, J.; Ertl, H.C.J. Additional Progress in the Development and Application of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis. Vet. Sci. 2018, 5, 59. https://doi.org/10.3390/vetsci5020059
Rupprecht CE, Xiang Z, Servat A, Franka R, Kirby J, Ertl HCJ. Additional Progress in the Development and Application of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis. Veterinary Sciences. 2018; 5(2):59. https://doi.org/10.3390/vetsci5020059
Chicago/Turabian StyleRupprecht, Charles E., Zhiquan Xiang, Alexandre Servat, Richard Franka, Jordona Kirby, and Hildegund C. J. Ertl. 2018. "Additional Progress in the Development and Application of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis" Veterinary Sciences 5, no. 2: 59. https://doi.org/10.3390/vetsci5020059
APA StyleRupprecht, C. E., Xiang, Z., Servat, A., Franka, R., Kirby, J., & Ertl, H. C. J. (2018). Additional Progress in the Development and Application of a Direct, Rapid Immunohistochemical Test for Rabies Diagnosis. Veterinary Sciences, 5(2), 59. https://doi.org/10.3390/vetsci5020059






