Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand
Abstract
1. Introduction
2. Materials and Methods
Study Area
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weeragidpanit, S.; Lekcharoensook, P. Rabies Situation in Thailand, Wildlife Rabies Workshop, Asia-Pacific Economic Cooperation. 13–17 November 2017. Available online: http://2017apecworkshop.nvri.gov.tw/ppt/session%203-7%20economy%20report-Thailand.pdf (assessed on 28 February 2018).
- Puanghat, A. Human rabies in Thailand. In Rabies Control in Asia; Dodet, E., Meslin, F.-X., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 252–253. [Google Scholar]
- Mitmoonpitak, C.; Tepsumethanon, V.; Wilde, H. Rabies in Thailand. Epidemiol. Infect. 1998, 120, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kingnate, D.; Sagarasaeranee, P.; Choomkasien, P. Thailand: Rabies control (human side). In Rabies Control in Asia; Dodet, E., Meslin, F.-X., Eds.; Elsevier: Paris, France, 1997; pp. 194–196. [Google Scholar]
- Sornnuwat, J. Animal rabies and animal rabies control in Thailand. In Rabies Control in Asia; Dodet, E., Meslin, F.-X., Eds.; John Libbey Eurotext: Paris, France, 2001; p. 254. [Google Scholar]
- Kasempimolporn, S.; Sichanasai, B.; Saengseesom, W.; Puempumpanich, S.; Sitprija, V. Stray dogs in Bangkok, Thailand: Rabies virus infection and rabies antibody prevalence. Dev. Biol. 2008, 131, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Kongkaew, W.; Coleman, P.; Pfeiffer, D.U.; Antarasena, C.; Thiptara, A. Vaccination coverage and epidemiological parameters of the owned-dog population in Thungsong District, Thailand. Prev. Vet. Med. 2004, 65, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Singhchai, C. Dog rabies control in Bangkok metropolitan area: Why has rabies not been eliminated from Bangkok? In Rabies Control in Asia; Dodet, E., Meslin, F.-X., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 91–93. [Google Scholar]
- Panichabhongse, P. The Epidemiology of Rabies in Thailand. Master’s Thesis, Massey University, Palmerston North, New Zealand, 2001. [Google Scholar]
- Rosatte, R. Evolution of wildlife rabies control tactics. Adv. Virus Res. 2011, 79, 397–419. [Google Scholar] [PubMed]
- Müller, T.; Freuling, C.M.; Wysocki, P.; Roumiantzeff, M.; Freney, J.; Mettenleiter, T.C.; Vos, A. Terrestrial rabies control in the European Union: Historical achievements and challenges ahead. Vet. J. 2015, 203, 10–17. [Google Scholar]
- Vos, A.; Freuling, C.M.; Hundt, B.; Kaiser, C.; Nemitz, S.; Neubert, A.; Nolden, T.; Teifke, J.P.; Te Kamp, V.; Ulrich, R.; et al. Oral vaccination of wildlife against rabies: Differences among host species in vaccine uptake efficiency. Vaccine 2017, 35, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Report of WHO Consultation on Oral Immunization of Dogs against Rabies; WHO: Geneva, Switzerland, 1988. [Google Scholar]
- Sagaraaeranee, P.; Puanghat, A.; Kasempimolparn, S.; Khawplod, P. Efficacy of oral rabies vaccine in dogs in Thailand. In Rabies Control in Asia; Dodet, B., Meslin, F.-X., Eds.; John Libbey Eurotext: Paris, France, 2001. [Google Scholar]
- Bender, S.; Bergman, D.; Vos, A.; Martin, A.; Chipman, R. Field studies evaluating bait acceptance and handling by dogs in Navajo Nation, USA. Trop. Med. Infect. Dis. 2017, 2, 17. [Google Scholar] [CrossRef]
- Schuster, P.; Gülsen, N.; Neubert, A.; Vos, A. Field Trials Evaluating Bait Uptake by an Urban Dog Population in Turkey. J. Etlik Vet. Microbiol. 1998, 9, 73–81. [Google Scholar]
- Frontini, M.G.; Fishbein, D.B.; Garza Ramos, J.; Flores Collins, E.; Balderas Torres, J.M.; Quiroz Huerta, G.; Gamez Rodriguez, J.J.; Belotto, A.J.; Dobbins, J.G.; Linhart, S.B.; et al. A Field Evaluation in Mexico of Four Baits for Oral Rabies Vaccination of Dogs. Am. J. Trop. Med. Hyg. 1992, 47, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kharmachi, H.; Haddad, N.; Matter, H. Tests of Four Baits for Oral Vaccination of Dogs against Rabies in Tunisia. Vet. Rec. 1992, 130, 494. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.C.; Kharmachi, H.; Haddad, N.; Youssef, S.B.; Sghaier, C.; Khelifa, R.B.; Jemli, J.; Mrabet, L.; Meslin, F.X.; Wandeler, A.I. Test of Three Bait Types for Oral Immunization of Dogs against Rabies in Tunisia. Am. J. Trop. Med. Hyg. 1995, 52, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Vos, A.; De Leon, R. Acceptability of Local-Made Baits for Oral Vaccination of Dogs against Rabies in the Philippines. BMC Infect. Dis. 2001, 1, 19. [Google Scholar] [CrossRef]
- Corn, J.L.; Méndez, J.R.; Catalán, E.E. Evaluation of Baits for Delivery of Oral Rabies Vaccine to Dogs in Guatemala. Am. J. Trop. Med. Hyg. 2003, 69, 155–158. [Google Scholar] [PubMed]
- Berentsen, A.R.; Bender, S.; Bender, P.; Bergman, D.; Hausig, K.; VerCauteren, K.C. Preference among 7 Bait Flavors Delivered to Domestic Dogs in Arizona: Implications for Oral Rabies Vaccination on the Navajo Nation. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 169–171. [Google Scholar] [CrossRef]
- Nokireki, T.; Nevalainen, M.; Sihvonen, L.; Gadd, T. Adverse Reactions from Consumption of Oral Rabies Vaccine Baits in Dogs in Finland. Acta Vet. Scand. 2016, 58. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Millien, M.; Vos, A.; Fracciterne, F.A.; Crowdis, K.; Chirodea, C.; Medley, A.; Chipman, R.; Qin, Y.; Blanton, J.; et al. Evaluation of immune responses in dogs to oral rabies vaccine under field conditions. Vaccine 2017. [Google Scholar] [CrossRef] [PubMed]
- Gräßer, N. Beitrag Zur Entwicklung Eines Köders Für Wölfe (Canis Lupus) Zur Oralen Vakzination Gegen Tollwut. Ph.D. Thesis, University of Veterinary Medicine, Hannover, Germany, 2016. [Google Scholar]
- Harischindra, P.A.L. Dog Vaccination Coverage and Oral Rabies Vaccination in Sri Lanka, an Update. In Rabies Control in Asia; Dodet, E., Meslin, F.-X., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 97–100. [Google Scholar]
- World Health Organization (WHO). Oral Vaccination of Dogs: Guidance for Research on Oral Rabies Vaccine and Field Application of Oral Vaccination of Dogs against Rabies; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Vos, A.; Aylan, O.; Estrada, R. Oral vaccination campaigns of dogs against rabies. In Proceedings of the Seventh Southern and Eastern African Rabies Group/World Health Organization Meeting, Ezulwini, Swaziland, 12–15 May 2003; Editions Fondation Marcel Merieux: Lyon, France, 2003; pp. 125–130. [Google Scholar]
- Matter, H.C.; Schumacher, C.L.; Kharmachi, H.; Hammami, S.; Tlatli, A.; Jemli, J.; Mrabet, L.; Meslin, F.X.; Aubert, M.F.; Neuenschwander, B.E.; et al. Field evaluation of two bait delivery systems for the oral immunization of dogs against rabies in Tunisia. Vaccine 1998, 16, 657–665. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Blass, L.; Smith, K.; Orciari, L.A.; Niezgoda, M.; Whitfield, S.G.; Gibbons, R.V.; Guerra, M.; Hanlon, C.A. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N. Engl. J. Med. 2001, 345, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Vos, A.; De Leon, R.; Mueller, T. Field trial with oral vaccination of dogs against rabies in the Philippines. BMC Infect. Dis. 2001, 1, 23. [Google Scholar] [CrossRef]
- Hemachudha, T. Rabies and dog population control in Thailand: Success or failure? J. Med. Assoc. Thail. 2005, 88, 120–123. [Google Scholar]
- Kasempimolporn, S.; Jitapunkul, S.; Sitprija, V. Moving towards the elimination of rabies in Thailand. J. Med. Assoc. Thail. 2008, 91, 433–437. [Google Scholar]
- Sriaroon, C.; Sriaroon, P.; Daviratanasilpa, S.; Khawplod, P.; Wilde, H. Retrospective: Animal attacks and rabies exposures in Thai children. Travel Med. Infect. Dis. 2006, 4, 270–274. [Google Scholar] [CrossRef] [PubMed]
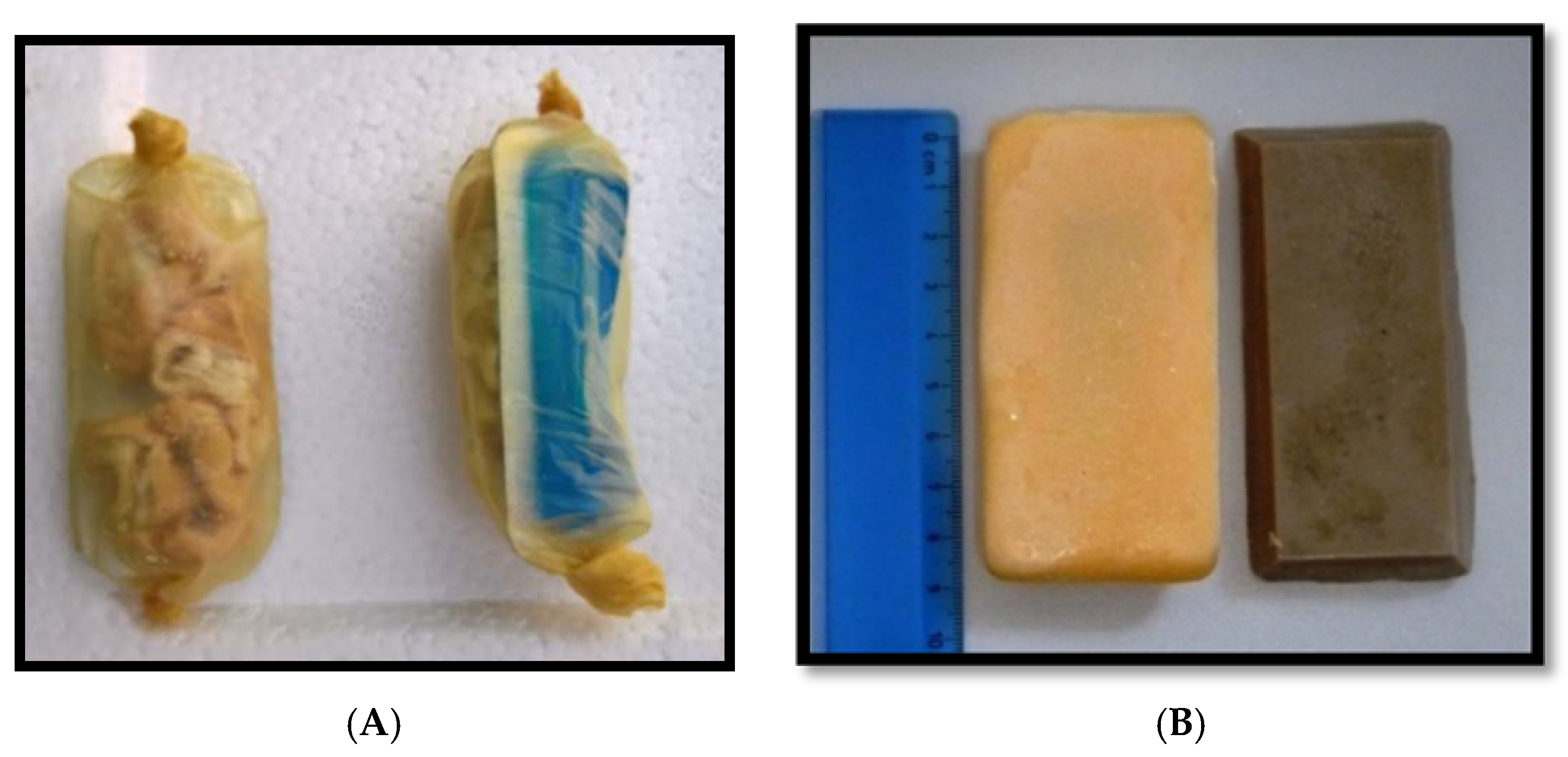
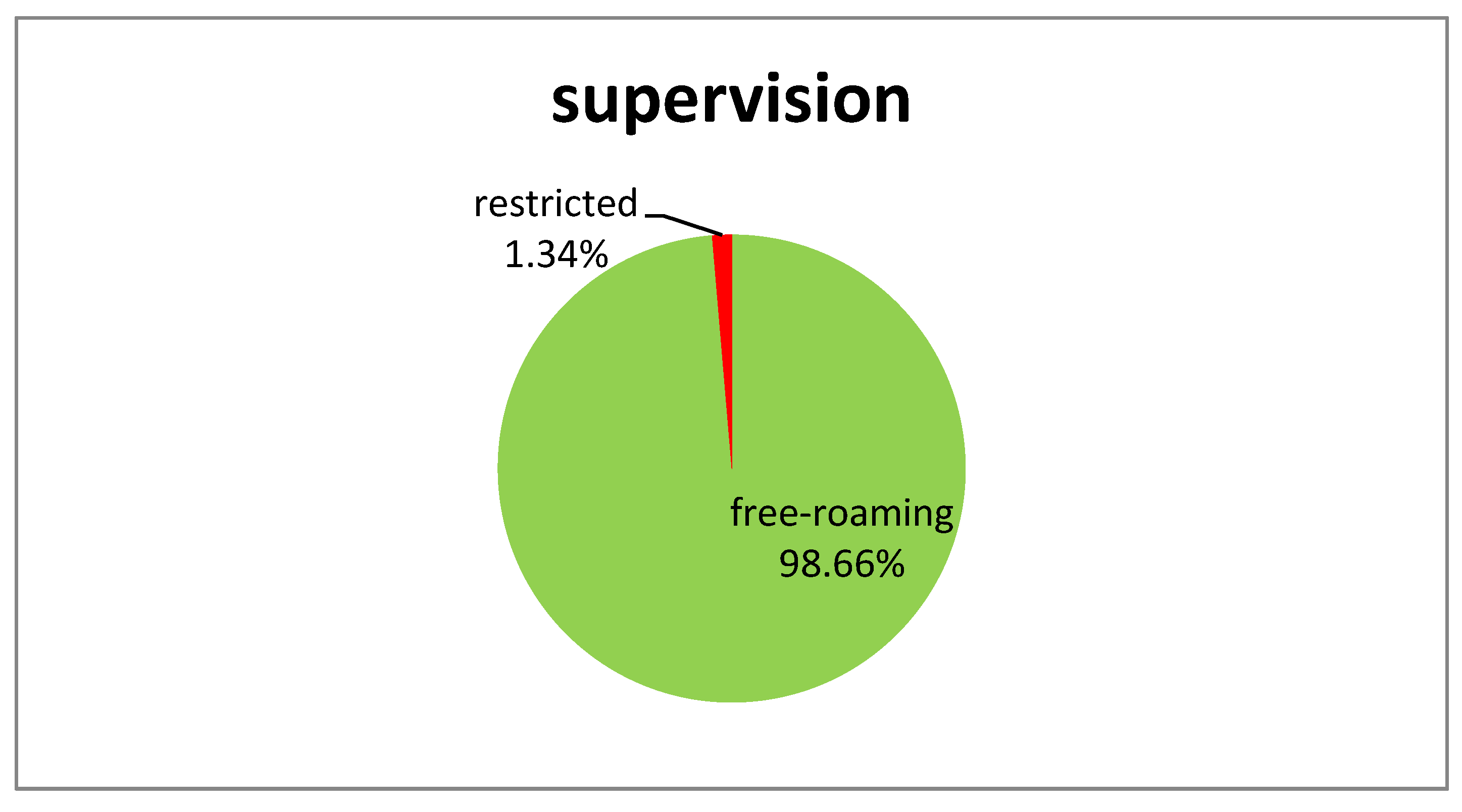
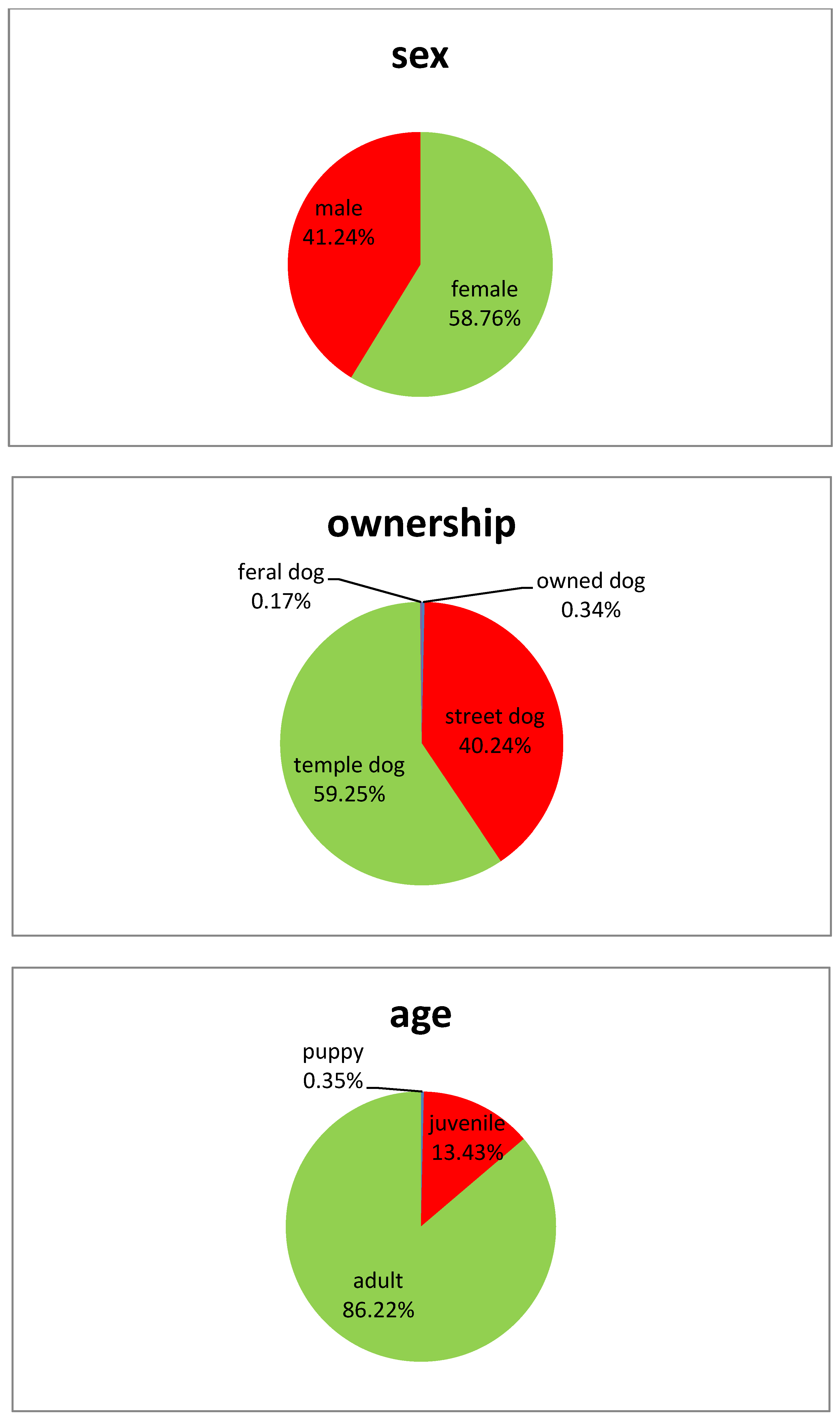
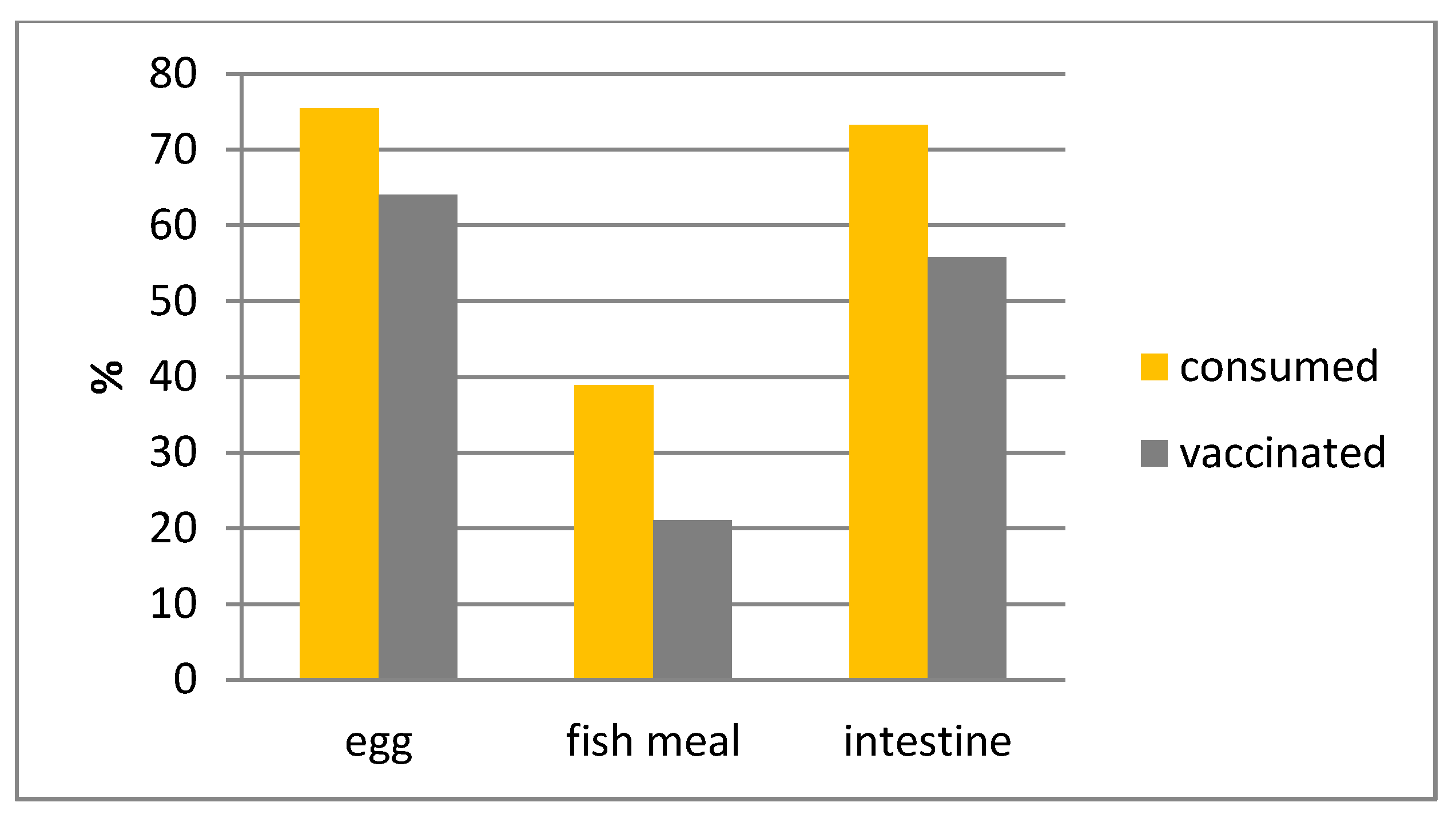

| Material | Size (cm) | Weight (gr) | |
|---|---|---|---|
| Bait | |||
| Intestine | Collagen casing filled with pieces of boiled local pork intestine | 7–10 cm long | 15–25 |
| Fishmeal | vegetable fats + fishmeal | 8.5 × 4.0 × 1.2 | 43 |
| Egg | gelatin + egg powder | 8.5 × 4.0 × 1.2 | 43 |
| Bait-Type | Bait Accepted | Bait Consumed | Capsule Discarded | Capsule Perforated | “Vaccinated” | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | n/N | % | n/N | % | |
| Egg | 183/192 | 95.81 | 141/179 | 78.77 | 133/135 | 98.52 | 123/132 | 93.19 | 109/129 | 84.50 |
| Fish | 174/206 | 84.47 | 86/171 | 50.29 | 80/82 | 97.56 | 62/83 | 74.70 | 40/74 | 54.85 |
| Intestine | 179/192 | 93.23 | 137/173 | 79.19 | 130/132 | 98.48 | 119/132 | 90.15 | 95/124 | 76.61 |
| Total | 536/590 | 90.85 | 364/523 | 69.60 | 343/349 | 98.28 | 304/347 | 87.61 | 244/327 | 74.62 |
| Bait-Type | Street Dogs | Temple Dogs | Comparison (Chi2-Test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | 95% CI | n | N | % | 95% CI | ||||
| Egg | 55 | 75 | 73.33 | 61.86 | 82.89 | 82 | 100 | 82.00 | 71.36 | 87.59 | n.s., p = 0.17 |
| Fish | 26 | 66 | 39.39 | 27.58 | 52.19 | 55 | 100 | 55.00 | 44.73 | 64.97 | sign., p = 0.05 |
| Intestine | 46 | 67 | 68.66 | 56.16 | 79.44 | 87 | 99 | 87.88 | 79.78 | 93.58 | sign., p = 0.002 |
| Total | 127 | 208 | 61.06 | 54.07 | 67.72 | 224 | 299 | 74.92 | 69.60 | 79.73 | sign., p < 0.001 |
| Bait-Type | Female | Male | Comparison (Chi²-Test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | 95% CI | n | N | % | 95% CI | ||||
| Egg | 80 | 99 | 80.81 | 71.66 | 88.03 | 57 | 76 | 75.00 | 63.74 | 84.23 | n.s., p = 0.36 |
| Fish | 57 | 101 | 56.44 | 46.21 | 66.28 | 28 | 66 | 42.42 | 30.34 | 55.21 | n.s., p = 0.08 |
| Intestine | 88 | 104 | 84.62 | 76.22 | 90.94 | 49 | 67 | 73.13 | 60.90 | 83.24 | n.s., p = 0.07 |
| Total | 225 | 304 | 74.01 | 68.70 | 78.85 | 134 | 209 | 64.12 | 57.21 | 70.62 | sign., p = 0.02 |
| Bait-Type | Small-Sized | Medium-Sized | Comparison (Chi²-Test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | 95% CI | n | N | % | 95%CI | ||||
| Egg | 20 | 22 | 90.91 | 70.84 | 98.88 | 113 | 148 | 76.35 | 68.68 | 82.94 | n.s., p = 0.12 |
| Fish | 17 | 23 | 73.91 | 51.60 | 98.77 | 65 | 140 | 46.43 | 37.97 | 55.05 | sign., p = 0.01 |
| Intestine | 24 | 25 | 96.00 | 76.95 | 99.90 | 107 | 140 | 76.43 | 68.52 | 83.19 | sign., p = 0.03 |
| Total | 61 | 70 | 87.14 | 76.99 | 93.95 | 285 | 428 | 66.59 | 61.90 | 71.05 | sign., p < 0.001 |
| Bait-Type | Juvenile | Adult | Comparison (Chi²-test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | N | % | 95% CI | n | N | % | 95% CI | ||||
| Egg | 21 | 26 | 80.77 | 60.65 | 93.45 | 112 | 141 | 79.43 | 71.82 | 85.77 | n.s., p = 0.87 |
| Fish | 8 | 11 | 72.73 | 39.03 | 93.98 | 73 | 150 | 48.67 | 40.43 | 56.96 | n.s., p = 0.12 |
| Intestine | 28 | 30 | 93.33 | 77.93 | 99.18 | 101 | 131 | 77.10 | 68.95 | 83.98 | sign., p = 0.04 |
| total | 57 | 67 | 85.07 | 74.26 | 92.60 | 286 | 422 | 67.77 | 63.08 | 72.21 | sign., p = 0.004 |
| Time- | Acceptance | Consumption | ||||||
|---|---|---|---|---|---|---|---|---|
| Period * | n/N | % | 95%CI | n/N | % | 95%CI | ||
| 6:00 –7:59 | 12/14 | 85.71 | 57.19 | 98.22 | 8/13 | 61.54 | 31.58 | 86.14 |
| 8:00–9:59 | 145/156 | 92.95 | 87.73 | 96.43 | 103/156 | 66.03 | 58.02 | 73.41 |
| 10:00–11:59 | 138/156 | 88.46 | 82.38 | 93.02 | 103/154 | 66.88 | 58.85 | 74.25 |
| 12:00–13:59 | 30/32 | 93.75 | 79.19 | 99.23 | 22/30 | 73.33 | 54.11 | 87.72 |
| 14:00–15:59 | 52/61 | 85.25 | 73.81 | 93.03 | 38/64 | 59.38 | 46.37 | 71.49 |
| 16:00–17:59 | 148/158 | 93.67 | 88.67 | 96.92 | 89/156 | 57.05 | 48.89 | 64.94 |
| 18:00–19:59 | 12/13 | 92.31 | 63.97 | 99.81 | 4/13 | 30.77 | 9.09 | 61.43 |
| total | 537/590 | 91.02 | 88.42 | 93.2 | 367/586 | 62.63 | 58.57 | 66.56 |
| Bait Handling | Single (n = 88) | Together (n = 265) | ||||
|---|---|---|---|---|---|---|
| (s) | % | 95% CI | % | 95% CI | ||
| <10 | 3.41 | 0.71 | 9.64 | 6.04 | 3.49 | 9.62 |
| 10–30 | 9.09 | 4.01 | 17.13 | 21.89 | 17.06 | 27.36 |
| 30–60 | 15.91 | 8.98 | 25.25 | 21.13 | 16.38 | 26.55 |
| >60 | 71.59 | 60.98 | 80.70 | 57.11 | 44.76 | 57.11 |
| Bait Type | n/N | % | 95%CI | |
|---|---|---|---|---|
| Egg | 93/114 | 81.58 | 73.23 | 88.22 |
| Fish | 51/72 | 70.83 | 58.93 | 80.96 |
| Intestine | 106/115 | 92.17 | 85.66 | 96.36 |
| total | 250/301 | 83.06 | 78.33 | 87.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasemsuwan, S.; Chanachai, K.; Pinyopummintr, T.; Leelalapongsathon, K.; Sujit, K.; Vos, A. Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand. Vet. Sci. 2018, 5, 47. https://doi.org/10.3390/vetsci5020047
Kasemsuwan S, Chanachai K, Pinyopummintr T, Leelalapongsathon K, Sujit K, Vos A. Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand. Veterinary Sciences. 2018; 5(2):47. https://doi.org/10.3390/vetsci5020047
Chicago/Turabian StyleKasemsuwan, Suwicha, Karoon Chanachai, Tanu Pinyopummintr, Kansuda Leelalapongsathon, Kitipat Sujit, and Ad Vos. 2018. "Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand" Veterinary Sciences 5, no. 2: 47. https://doi.org/10.3390/vetsci5020047
APA StyleKasemsuwan, S., Chanachai, K., Pinyopummintr, T., Leelalapongsathon, K., Sujit, K., & Vos, A. (2018). Field Studies Evaluating Bait Acceptance and Handling by Free-Roaming Dogs in Thailand. Veterinary Sciences, 5(2), 47. https://doi.org/10.3390/vetsci5020047




