Metabolite Profiles of Male and Female Humboldt Penguins
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Collection and Processing
2.2. Metabolite Profiling
2.3. Quantification
3. Results
3.1. GC/MS Identification of Metabolites
3.2. Gender Discrimination of Metabolite Concentrations
| Penguin Metabolite | CASRN/HMDB 1 | Example KEGG 2 (Map) and SMPDB 3 Pathways |
|---|---|---|
| 2-aminoadipic acid | 542-32-5 HMDB00510 | Lysine degradation (SMP00037) |
| 2-oxoglutarate | 328-50-7 HMDB00208 | 2-oxocarboxylic acid metabolism (map01210), citrate (TCA) cycle (map00020), Glyoxylate and dicarboxylate metabolism (map00630) |
| creatinine | 60-27-5 HMDB00562 | Arginine and proline metabolism (map00330), numerous metabolic pathways (map01100) |
| erythronic acid | 13752-84-6 HMDB00613 | a sugar component of aqueous humour (eye) (HMDB00613) |
| GABA (gamma-aminobutyric acid) | 56-12-2 HMDB00112 | Alanine, aspartate and glutamate metabolism (map00250), cAMP signaling pathway (map04024) |
| Glycerol 2-phosphate | 17181-54-3 HMDB02520 | glycerolipid metabolism (HMDB02520) |
| Glycerol 3-phosphate | 57-03-4 HMDB00126 | glycolysis metabolic pathway (HMDB00126), glycerol phosphate shuttle (SMP00124), mitochondrial electron transport chain (SMP00355), glycerolopid metabolism (SMP00039), phospholipid biosynthesis (SMP00025) |
| mannitol | 69-65-8 HMDB00765 | Fructose and mannose metabolism (map00051), Phosphotransferase system (PTS) (map02060) |
| O-phosphorylethanolamine | 1071-23-4 HMDB00224 | Arginine and Proline Metabolism (SMP00020), Lactose Synthesis (SMP00444), Tryptophan metabolism (SMP00063), Cysteine metabolism (SMP00013), Mitochondrial Beta-Oxidation of Long Chain Saturated Fatty Acids (SMP00482), Mitochondrial Beta-Oxidation of Short Chain Saturated Fatty Acids (SMP00480) |
| pantothenic acid | 79-83-4 HMDB00210 | Antidyslipidemic agents (map07052), Vitamin digestion and absorption (map04977) |
| sedoheptulose 7-phosphate | 2646-35-7 HMDB01068 | Lipopolysaccharide biosynthesis (map00540), Carbon metabolism (map01200), Biosynthesis of amino acids (map01230) |
| succinate | 110-15-6 HMDB00254 (succinic acid) | 2-Oxocarboxylic acid metabolism (map01210), Biosynthesis of secondary metabolites (map01110), Alanine, aspartate and glutamate metabolism (map00250), Glyoxylate and dicarboxylate metabolism (map00630), citrate (TCA) cycle (map00020) |
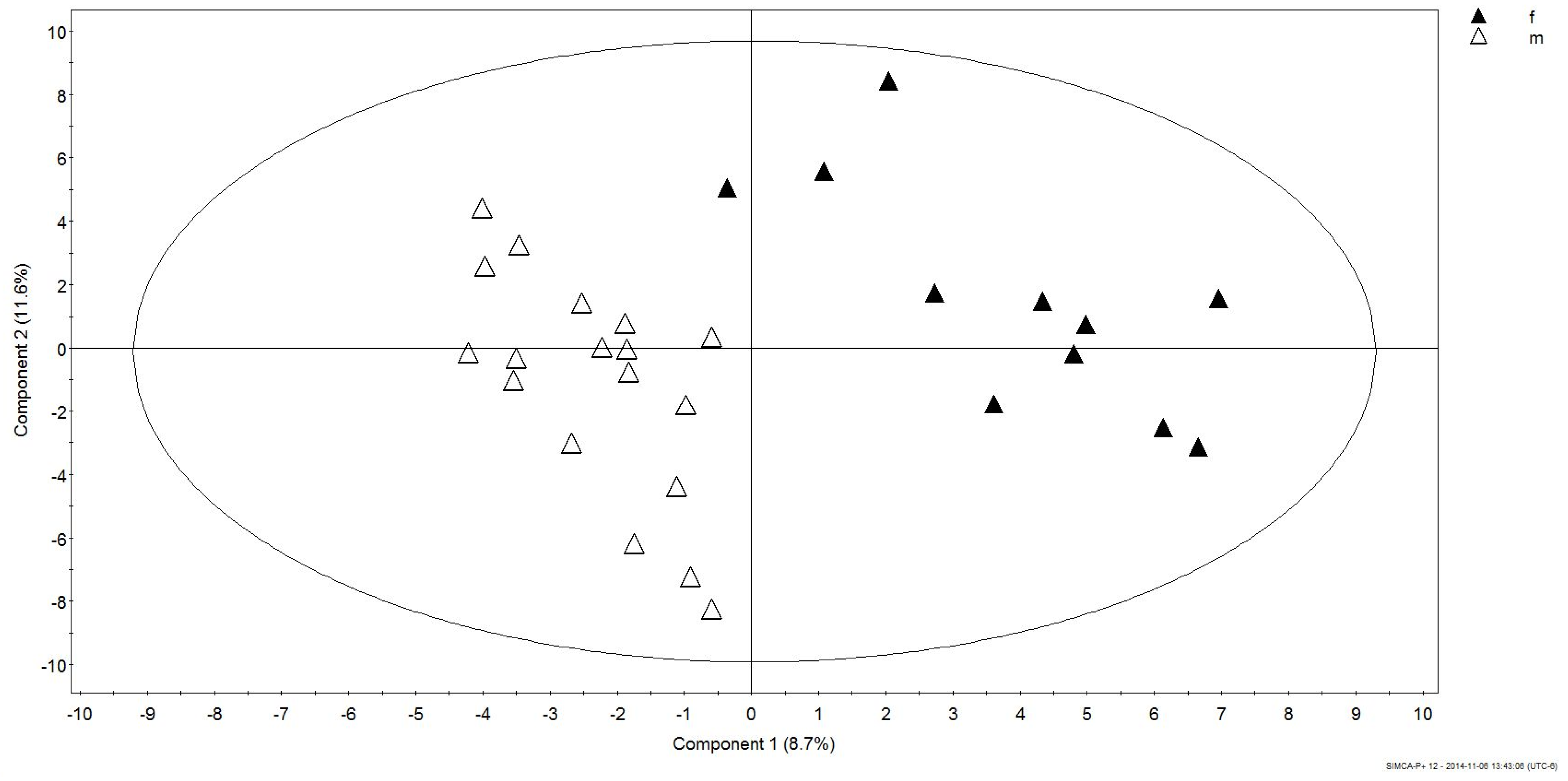
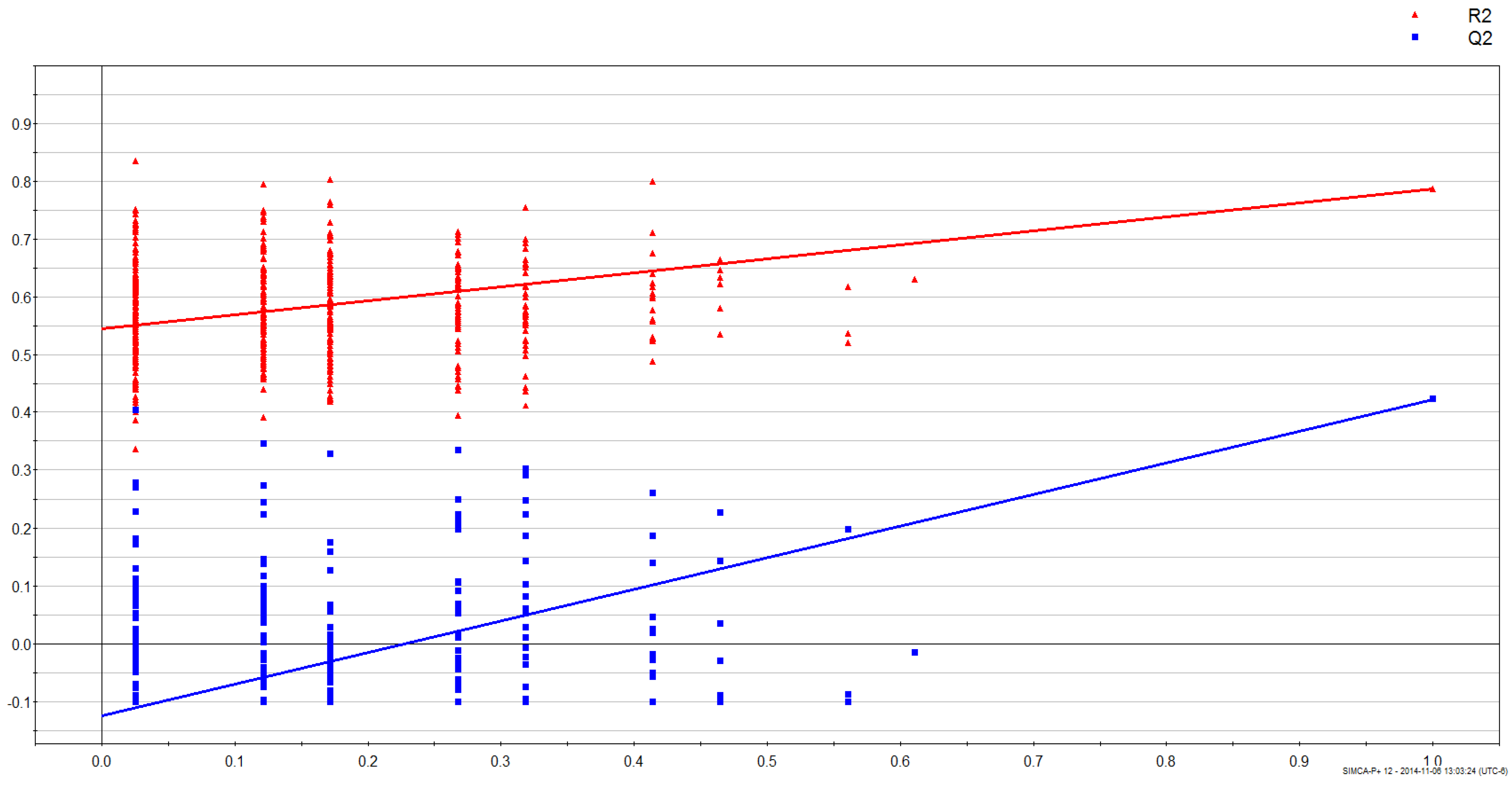
3.3. Gender Differences in Metabolites
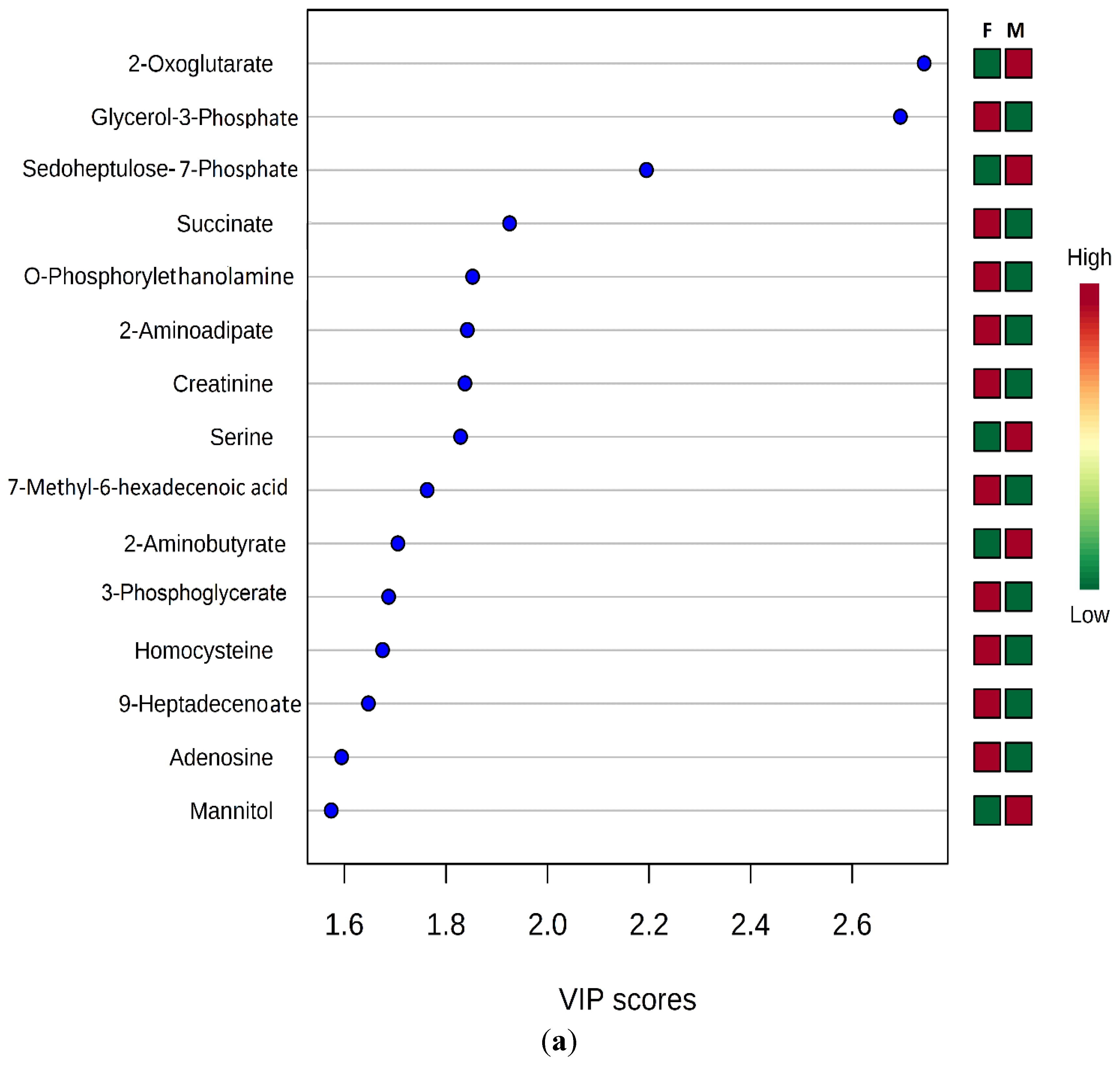
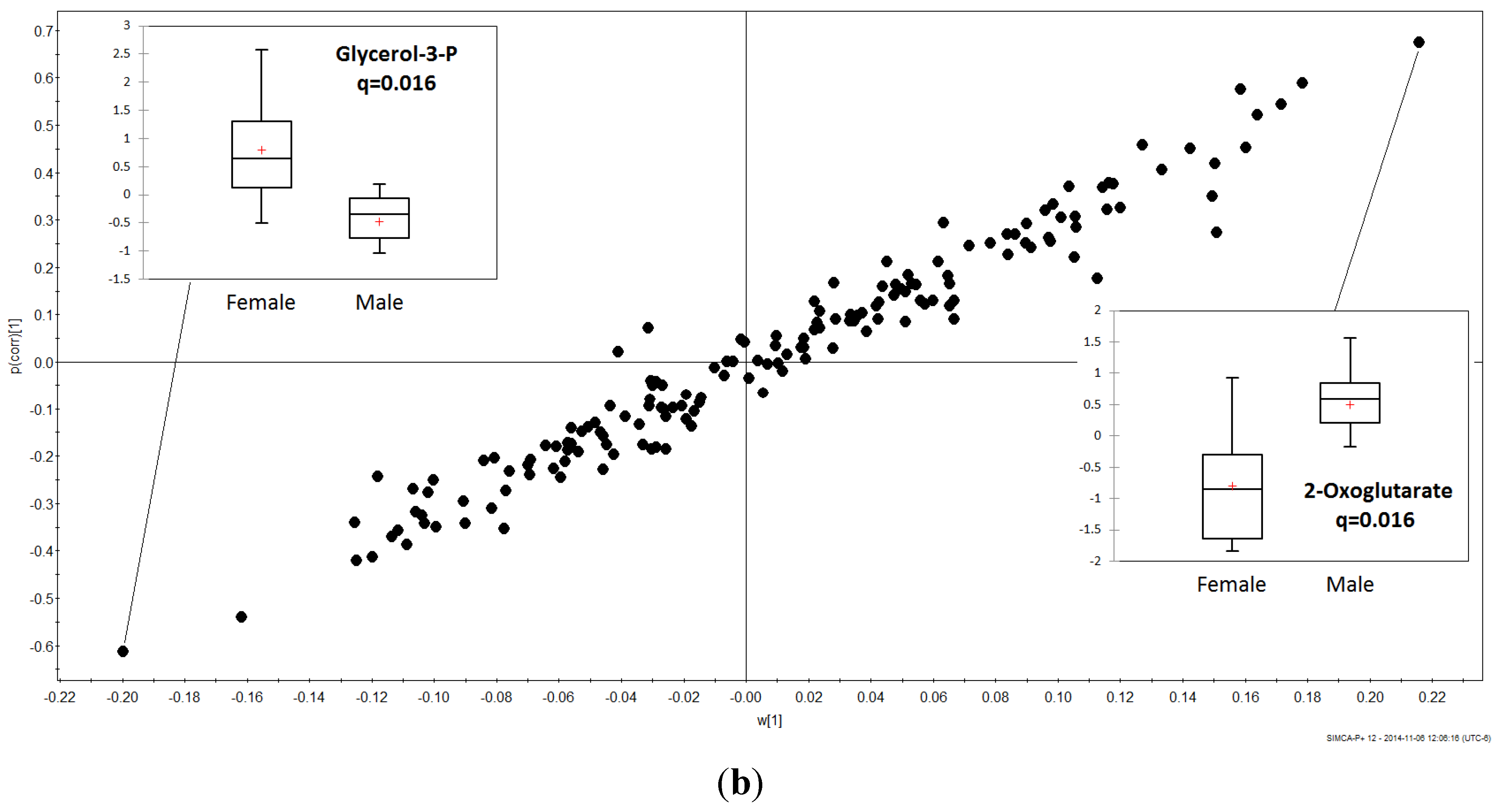
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De La Puente, S.; Bussalleu, A.; Cardena, M.; Valdes-Velaasquez, A.; Majluf, P. Humboldt Penguins (Spheniscus humboldti). In Penguins: Natural History and Conservation; Garcia-Borboroglu, P.G., Boersma, P.D., Eds.; University of Washington Press: Seattle, WA, USA, 2013; pp. 269–287. [Google Scholar]
- Paredes, R.; Zavalaga, C.B.; Boness, D.J. Patterns of egg laying and breeding success in Humboldt Penguins (Spheniscus humboldti) at Punta San Juan, Peru. Auk 2002, 119, 244–250. [Google Scholar] [CrossRef]
- Zavalaga, C.B.; Paredes, R. Sex determination of adult Humboldt Penguins using morphometric characters. J. Field Ornithol. 1997, 68, 102–112. [Google Scholar]
- Taylor, S.S.; Leonard, M.L.; Boness, D.J.; Majluf, P. Foraging by Humboldt Penguins (Spheniscus humboldti) during the chick-rearing period: General patterns, sex differences, and recommendations to reduce incidental catches in fishing nets. Can. J. Zool. 2002, 80, 700–707. [Google Scholar] [CrossRef]
- Rey, A.R.; Pütz, K.; Simeone, A.; Hiriart-Bertrand, L.; Reyes-Arriagada, R.; Riquelme, V.; Luthi, B. Comparative foraging behaviour of sympatric Humboldt and Magellanic Penguins reveals species-specific and sex-specific strategies. EMU 2013, 113, 145–153. [Google Scholar] [CrossRef]
- Otsuka, R.; Aoki, K.; Hori, H.; Wada, M. Changes in circulating LH, sex steroid hormones, thyroid hormones and corticosterone in relation to breeding and molting in captive Humboldt Penguins (Spheniscus humboldti) kept in an outdoor open display. Zool. Sci. 1998, 15, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Guglielmo, C.G.; Egler, O.; Martyniuk, C.J. Plasma lipid metabolites provide information on mass change over several days in captive Western Sandpipers. Auk 1990, 116, 994–1000. [Google Scholar]
- Anteau, M.J.; Afton, A.D. Lipid reserves of lesser scaup (Aythya affinis) migrating across a large landscape are consistent with the “spring condition” hypothesis. Auk 2009, 126, 873–993. [Google Scholar] [CrossRef]
- Cherel, Y.; Robin, J.P.; Walch, O.; Karmann, H.; Netchitailo, P.; Le Maho, Y. Fasting in king penguin. I. Hormonal and metabolic changes during breeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988, 254, 170–177. [Google Scholar]
- Cortright, R.N.; Koves, T.R. Sex differences in substrate metabolism and energy homeostasis. Can. J. Appl. Physiol. 2000, 25, 288–311. [Google Scholar] [PubMed]
- Tate, C.A.; Holtz, R.W. Gender and fat metabolism during exercise: A review. Can. J. Appl. Physiol. 1998, 23, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Lucidi, P.; Bolli, G.B.; Santeusanio, F.; De Feo, P. Gender differences in basal protein kinetics in young adults. J. Clin. Endocr. Metab. 1998, 83, 4363–4367. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Roca, P. Caloric restriction and gender: Which is the stronger sex? Agro Food Ind. Hi-Tech. 2007, 18, 13–15. [Google Scholar]
- Merimee, T.J.; Misbin, R.I.; Pulkkinen, A.J. Sex variations in free fatty acids and ketones during fasting: Evidence for a role of glucagon. J. Clin. Endocr. Metab. 1978, 46, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Lamont, L.S. Gender differences in amino acid use during endurance exercise. Nut. Rev. 2005, 63, 419–422. [Google Scholar] [CrossRef]
- Colom, B.; Alcolea, M.P.; Valle, A.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell. Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [PubMed]
- Floridi, A.; Blotta, I.; Marcante, M.L. Metabolic evaluation of sexual dimorphism. IV. Metabolic differences related to the oxidative metabolism. Experientia 1973, 29, 1228–1229. [Google Scholar] [PubMed]
- Bundy, J.G.; Davey, M.P.; Viant, M.R. Environmental metabolomics: A critical review and future perspectives. Metabolomics 2009, 35, 3–21. [Google Scholar] [CrossRef]
- Wallace, R.S.; Dubach, J.; Michaels, M.G.; Keuler, N.S.; Diebold, E.D.; Grzybowski, K.; Teare, J.A.; Willis, M.J. Morphometric determination of gender in adult Humboldt Penguins (Spheniscus humboldti). Waterbirds 2008, 31, 448–453. [Google Scholar]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Prot. 2011, 6, 1060–1083. [Google Scholar]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Jiye, A.; Trygg, J.; Gullberg, J.; Johansson, A.I.; Jonsson, P.; Antti, H.; Marklund, S.L.; Moritz, T. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar]
- Ulanov, A.; Widholm, J.M. Effect of the expression of cyanamide hydratase on metabolites in cyanamide treated soybean plants kept in the light or dark. J. Exp. Bot. 2007, 58, 4319–4332. [Google Scholar] [CrossRef]
- Sarup, P.; Pedersen, S.M.M.; Nielsen, N.C.; Malmendal, A.; Loeschcke, V. The metabolic profile of long-lived Drosophila melanogaster. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Sellitto, M.; Bai, G.; Serena, G.; Fricke, W.F.; Sturgeon, C.; Gajer, P.; White, J.R.; Koenig, S.S.K.; Sakamoto, J.; Boothe, D.; et al. Proof of concept of microbiome -metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Hazard, D.; Fernandez, X.; Pinguet, J.; Chambon, C.; Letisse, F.; Portais, J.C.; Wadih-Moussa, Z.; Remignon, H.; Molette, C. Functional genomics of the muscle response to restraint and transport in chickens. J. Anim. Sci. 2011, 89, 2717–2730. [Google Scholar] [PubMed]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [PubMed]
- Desbrosses, G.; Steinhauser, D.; Kopka, J.; Udvardi, M. Metabolome Analysis Using GC-MS. In Lotus japonicus Handbook; Márquez, A.J., Ed.; Springer: Dordrecht, NL, USA, 2005; pp. 165–174. [Google Scholar]
- Storey, J.D. A direct approach to false discovery rates. J. Royal Stat. Soc. Ser. B: Stat. Method 2002, 64, 479–498. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chem. 2003, 17, 6–33. [Google Scholar]
- Van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, S.; Johansson, E.; Sjöström, M.L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar]
- Elarabany, N.F. Sex-related variation in metabolic profile of two wagtail species. J. Exp. Zool. Part A: Ecol. Gen. Physiol. 2015, 323, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Arizmendi-Mejia, R.; Militao, T.; Viscor, G.; Gonzales-Solis, J. Pre-breeding ecophysiology of a long-distance migratory seabird. J. Exp. Mar. Biol. Ecol. 2013, 443, 162–168. [Google Scholar] [CrossRef]
- Villegas, A.; Masero, J.A.; Corbacho, C.; Gutierrez, J.S.; Albano, N.; Sanchez-Guzman, J.M. Sex-specific vulnerability to breeding conditions in chicks of the sexually monomorphic Gull-billed Tern. J. Ornithol. 2013, 154, 431–439. [Google Scholar] [CrossRef]
- Ghebremeskel, K.; Williams, T.D.; Williams, G.; Gardner, D.A.; Crawford, M.A. Plasma metabolites in Macaroni Penguins (Eudyptes chrysolophus) arriving on land for breeding and moulting. Comp. Biochem. Physiol. A 1991, 99, 245–250. [Google Scholar] [CrossRef]
- Ghebremeskel, K.; Williams, T.D.; Williams, G.; Gardner, D.A.; Crawford, M.A. Dynamics of plasma nutrients and metabolites in molting macaroni (Eudyptes chrysolophus) and gentoo (Pygoscelis papua) penguins. Comp. Biochem. Physiol. A 1992, 101, 301–307. [Google Scholar] [CrossRef]
- Ji, B.; Ernest, B.; Gooding, J.R.; Das, S.; Saxton, A.M.; Simon, J.; Dupont, J.; Metayer-Coustard, S.; Campagna, S.R.; Voy, B.H. Transcriptomic and metabolomic profiling of chicken adipose tissue in response to insulin neutralization and fasting. BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Work, T.M. Weights, hematology, and serum chemistry of seven species of free-ranging tropical Pelagic seabirds. J. Wildl. Dis. 1996, 32, 643–657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bourgeon, S.; Leat, E.H.K.; Magnusdóttir, E.; Fisk, A.T.; Furness, R.W.; Strom, H.; Hansson, S.A.; Petersen, A.; Olafsdottir, K.; Borga, K.; et al. Individual variation in biomarkers of health: Influence of persistent organic pollutants in Great skuas (Stercorarius skua) breeding at different geographical locations. Environ. Res. 2012, 118, 31–39. [Google Scholar] [PubMed]
- Cherel, Y.; Leloup, J.; Maho, Y.L. Fasting in king penguin. II. Hormonal and metabolic changes during molt. Am. J. Physiol. 1988, 254, R178–R184. [Google Scholar] [PubMed]
- Clarke, J.; Manly, B.; Kerry, K.; Gardner, H.; Franchi, E.; Corsolini, S.; Focardi, S. Sex differences in Adélie penguin foraging strategies. Polar Biol. 1998, 20, 248–258. [Google Scholar] [CrossRef]
- Chappell, M.A.; Shoemaker, V.H.; Janes, D.N.; Maloney, S.K.; Bucher, T.L. Energetics of foraging in breeding Adélie Penguins. Ecology 1993, 74, 2450–2461. [Google Scholar] [CrossRef]
- Groscola, R. Changes in body mass, body temperature, and plasma fuel levels during the natural breeding fast in male and female Emperor Penguins Aptenodytes forsteri. J. Comp. Physiol. B 1986, 156, 521–527. [Google Scholar] [CrossRef]
- Hays, C. Effects of the 1982–1983 El Niño on Humboldt Penguin colonies in Peru. Biol. Conserv. 1986, 36, 169–180. [Google Scholar] [CrossRef]
- Gonzalez-Solis, J.; Becker, P.H.; Wendeln, H.; Wink, M. Hatchling sex ratio and sex specific mortality in common Terns Sterna hirundo. J. Ornithol. 2005, 146, 235–243. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levengood, J.M.; Schaeffer, D.J.; Ulanov, A.V. Metabolite Profiles of Male and Female Humboldt Penguins. Vet. Sci. 2015, 2, 349-362. https://doi.org/10.3390/vetsci2040349
Levengood JM, Schaeffer DJ, Ulanov AV. Metabolite Profiles of Male and Female Humboldt Penguins. Veterinary Sciences. 2015; 2(4):349-362. https://doi.org/10.3390/vetsci2040349
Chicago/Turabian StyleLevengood, Jeffrey M., David J. Schaeffer, and Alexander V. Ulanov. 2015. "Metabolite Profiles of Male and Female Humboldt Penguins" Veterinary Sciences 2, no. 4: 349-362. https://doi.org/10.3390/vetsci2040349
APA StyleLevengood, J. M., Schaeffer, D. J., & Ulanov, A. V. (2015). Metabolite Profiles of Male and Female Humboldt Penguins. Veterinary Sciences, 2(4), 349-362. https://doi.org/10.3390/vetsci2040349





