Water-Soluble Vitamins (Riboflavin, Niacin, Pantothenic Acid) in Dogs with Chronic Liver Disease vs. Healthy Controls
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
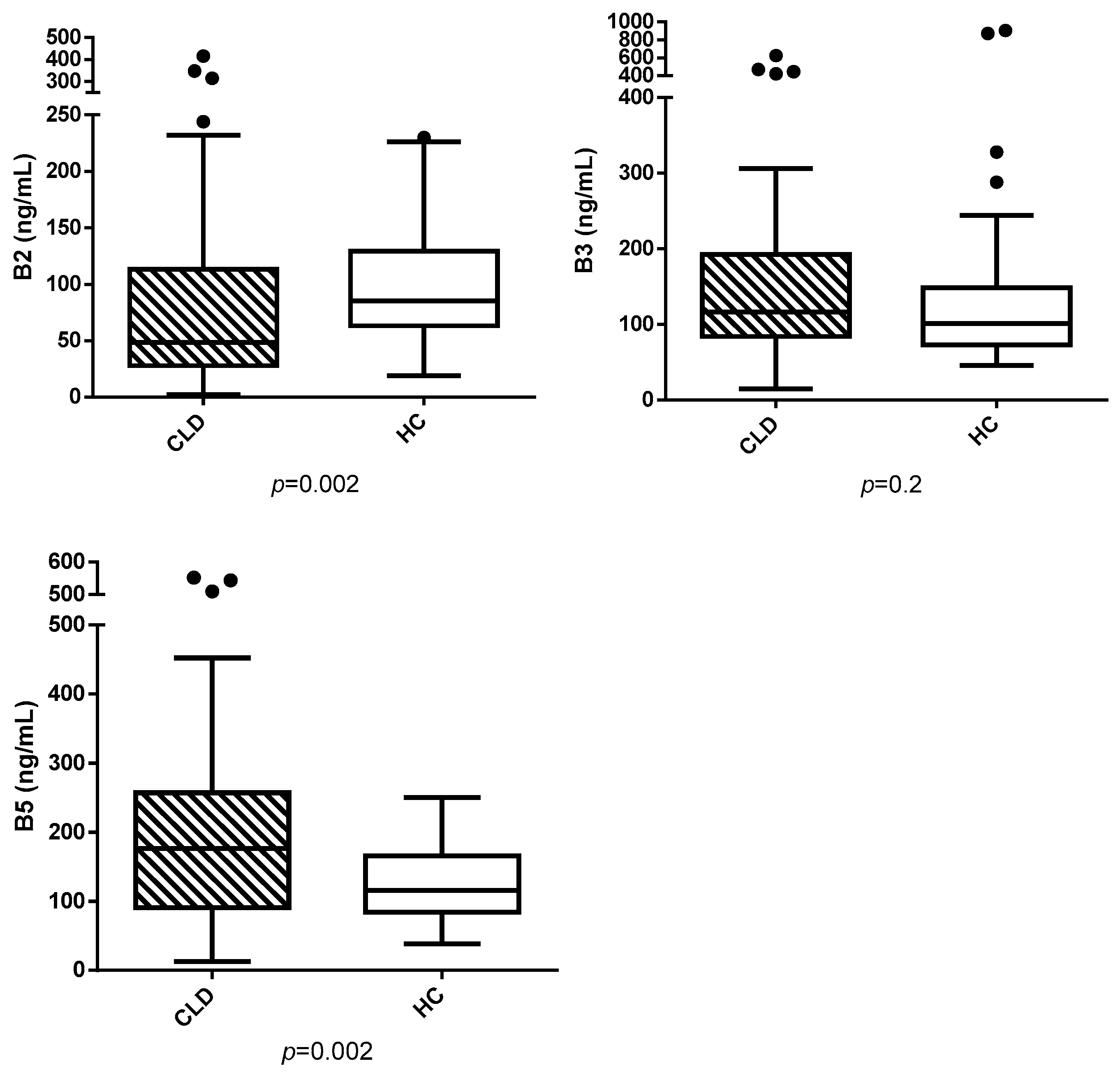
3. Results
3.1. Animals
3.2. Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| CLD | Chronic liver disease |
| ALP | Alkaline phosphatase |
| GGT | Gamma-glutamyl transferase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| HC | Healthy controls |
| BTD | Biliary tract disease |
| FAD | Flavin adenine dinucleotide |
| FMN | Flavin mononucleotide |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ROS | Reactive oxygen species |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NADP+ | Nicotinamide Adenine Dinucleotide Phosphate |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate—Reduced Form |
| HDL | High-density lipoprotein |
| CoA | Coenzyme A |
References
- Abe, R.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Mohammed, L. The Role of Vitamins in Non-Alcoholic Fatty Liver Disease: A Systematic Review. Cureus 2021, 13, e16855. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kessoku, T.; Ozaki, A.; Iwaki, M.; Honda, Y.; Ogawa, Y.; Imajo, K.; Yoneda, M.; Saito, S.; Nakajima, A. Vitamin B6 efficacy in the treatment of non-alcoholic fatty liver disease: An open-label, single-arm, single-center trial. J. Clin. Biochem. Nutr. 2021, 68, 181–186. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury: National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548176/ (accessed on 1 June 2020).
- Shibata, K.; Fukuwatari, T.; Higashiyama, S.; Sugita, C.; Azumano, I.; Onda, M. Pantothenic acid refeeding diminishes the liver, perinephrical fats, and plasma fats accumulated by pantothenic acid deficiency and/or ethanol consumption. Nutrition 2013, 29, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská; Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; et al. On Behalf of The Oemonom. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Licata, A.; Zerbo, M.; Como, S.; Cammilleri, M.; Soresi, M.; Montalto, G.; Giannitrapani, L. The Role of Vitamin Deficiency in Liver Disease: To Supplement or Not Supplement? Nutrients 2021, 13, 4014. [Google Scholar] [CrossRef]
- Kather, S.; Sielski, L.; Dengler, F.; Jirasek, A.; Heilmann, R.M. Prevalence and clinical relevance of hypercobalaminaemia in dogs and cats. Vet. J. 2020, 265, 105547. [Google Scholar] [CrossRef]
- Devriendt, N.; Serrano, G.; Paepe, D.; Vandenabeele, S.; Stock, E.; De Rooster, H. Persistent hypercobalaminemia three months after successful gradual attenuation of extrahepatic shunts in dogs: A prospective cohort study. BMC Vet. Res. 2022, 18, 18. [Google Scholar] [CrossRef]
- Habermaass, V.; Bartoli, F.; Gori, E.; Dini, R.; Cogozzo, A.; Puccinelli, C.; Pierini, A.; Marchetti, V. Fecal Bile Acids in Canine Chronic Liver Disease: Results from 46 Dogs. Animals 2024, 14, 3051. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Pentieva, K.; Ward, M. Causes and Clinical Sequelae of Riboflavin Deficiency. Annu. Rev. Nutr. 2023, 43, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin deficiency-implications for general human health and inborn errors of metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hou, S.; Tang, J. Riboflavin Deficiency and Apoptosis: A Review. J. Nutr. 2025, 155, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, X.; Wan, M.; Dong, W.; Gao, W.; Yao, Z.; Guo, C. Effects of riboflavin deficiency and high dietary fat on hepatic lipid accumulation: A synergetic action in the development of non-alcoholic fatty liver disease. Nutr. Metab. 2024, 21, 1. [Google Scholar] [CrossRef]
- Sanches, S.C.; Ramalho, L.N.; Mendes-Braz, M.; Terra, V.A.; Cecchini, R.; Augusto, M.J.; Ramalho, F.S. Riboflavin (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem. Toxicol. 2014, 67, 65–71. [Google Scholar] [CrossRef]
- Tang, N.; Hong, F.; Hao, W.; Yu, T.T.; Wang, G.G.; Li, W. Riboflavin ameliorates mitochondrial dysfunction via the AMPK/PGC1α/HO-1 signaling pathway and attenuates carbon tetrachloride induced liver fibrosis in rats. Exp. Ther. Med. 2022, 24, 608. [Google Scholar] [CrossRef]
- Shen, X.; Shi, C.; Xu, J.; Zhi, F.; Luo, K.; Di, Y.; Li, W.; Ma, W.; Jiang, Y.; Sun, H. Intestinal microbiota homeostasis analysis in riboflavin-treated alcoholic liver disease. Commun. Biol. 2024, 7, 1030. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Thakur, K.; Feng, J.Y.; Zhang, J.G.; Hu, F.; Cespedes-Acuña, C.L.; Liao, C.; Wei, Z.J. Riboflavin Bioenriched Soymilk Alleviates Oxidative Stress Mediated Liver Injury, Intestinal Inflammation, and Gut Microbiota Modification in B2 Depletion-Repletion Mice. J. Agric. Food Chem. 2022, 70, 3818–3831. [Google Scholar] [CrossRef]
- Galler, A.; Tran, J.L.; Krammer-Lukas, S.; Höller, U.; Thalhammer, J.G.; Zentek, J.; Willmann, M. Blood vitamin levels in dogs with chronic kidney disease. Vet. J. 2012, 192, 226–231. [Google Scholar] [CrossRef]
- Zhong, O.; Wang, J.; Tan, Y.; Lei, X.; Tang, Z. Effects of NAD+ precursor supplementation on glucose and lipid metabolism in humans: A meta-analysis. Nutr. Metab. 2022, 19, 20. [Google Scholar] [CrossRef]
- Verdin, E. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. (BBA)-Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar]
- Makarov, M.V.; Trammell, S.A.J.; Migaud, M.E. The chemistry of the vitamin B3 metabolome. Biochem. Soc. Trans. 2019, 47, 131–147. [Google Scholar] [PubMed]
- Sauve, A.A. NAD+ and vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. [Google Scholar] [CrossRef]
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef]
- Shibata, K. Nutritional Aspects of Tryptophan Metabolism. In Targeting the Broadly Pathogenic Kynurenine Pathway; Mittal, S., Ed.; Springer: Cham, Switzerland, 2015; pp. 31–43. [Google Scholar]
- Pan, J.; Hu, Y.; Pang, N.; Yang, L. Association between Dietary Niacin Intake and Nonalcoholic Fatty Liver Disease. Nutrents 2023, 15, 4128. [Google Scholar]
- Kashyap, M.L.; Ganji, S.; Nakra, N.K.; Kamanna, V.S. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): Novel use for an old drug? J. Clin. Lipidol. 2019, 13, 873–879. [Google Scholar] [CrossRef]
- Ganji, S.H.; Kukes, G.D.; Lambrecht, N.; Kashyap, M.L.; Kamanna, V.S. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G320–G327. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kashyap, M.L.; Kamanna, V.S. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: Impact on non-alcoholic fatty liver disease. Metabolism 2015, 64, 982–990. [Google Scholar] [CrossRef]
- Zhou, J.; Han, J. Association of niacin intake and metabolic dysfunction-associated steatotic liver disease: Findings from National Health and Nutrition Examination Survey. BMC Public Health 2024, 24, 2742. [Google Scholar] [CrossRef]
- Le Bloc’h, J.; Leray, V.; Nazih, H.; Gauthier, O.; Serisier, S.; Magot, T.; Krempf, M.; Nguyen, P.; Ouguerram, K. Nicotinic Acid Accelerates HDL Cholesteryl Ester Turnover in Obese Insulin-Resistant Dogs. PLoS ONE 2015, 10, e0136934. [Google Scholar] [CrossRef]
- Mitu, O.; Cirneala, I.A.; Lupsan, A.I.; Iurciuc, M.; Mitu, I.; Dimitriu, D.C.; Costache, A.D.; Petris, A.O.; Costache, I.I. The effect of vitamin supplementation on subclinical atherosclerosis in patients without manifest cardiovascular diseases: Never-ending hope or underestimated effect? Molecules 2020, 25, 1717. [Google Scholar] [CrossRef] [PubMed]
- Nitto, T.; Onodera, K. Linkage between coenzyme a metabolism and inflammation: Roles of pantetheinase. J. Pharmacol. Sci. 2013, 123, 1–8. [Google Scholar] [CrossRef]
- Lim, L.O.; Hu, Y.F.; Wang, L.; Mitchell, M.; Berger, A.; Coleman, R.A. Early hepatic insulin resistance in mice: A metabolomics analysis. Mol. Endocrinol. 2010, 24, 657–666. [Google Scholar] [CrossRef]
- Gaylord, L.; Remillard, R.; Saker, K. Risk of nutritional deficiencies for dogs on a weight loss plan. J. Small Anim. Pract. 2018, 59, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Kruger, L.; Jewell, M.L.; Michelotti, G.A.; Pereira, A.; Xie, G.; Moylan, C.A.; Diehl, A.M. Vitamin B5 and N-Acetylcysteine in Nonalcoholic Steatohepatitis: A Preclinical Study in a Dietary Mouse Model. Dig. Dis. Sci. 2016, 61, 137–148. [Google Scholar] [CrossRef]
- Thakkar, N.; Slizgi, J.R.; Brouwer, K.L.R. Effect of Liver Disease on Hepatic Transporter Expression and Function. J. Pharm. Sci. 2017, 106, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.L.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 2011, 437, 357–372. [Google Scholar] [CrossRef]
- Yin, T.; Tu, W.; Li, Y.; Huang, L.; Bai, Y.; Xu, G. Nutrients, Diet Quality, and Dietary Patterns in Patients with Inflammatory Bowel Disease: A Comparative Analysis. Nutrients 2024, 13, 3093. [Google Scholar] [CrossRef]
| Q1 → Q3 | DP | EP | CE | CXP | |
|---|---|---|---|---|---|
| Vitamin B2 | 377.2 → 172.2 | 130 | 9 | 48 | 3.7 |
| 377.2 → 196.1 | 130 | 9 | 52 | 4.6 | |
| 377.2 → 243.2 | 130 | 9 | 31 | 6 | |
| Vitamin B2-13C4,15N2 | 383.1 → 175.1 | 130 | 9 | 48 | 3.7 |
| 383.1 → 200.0 | 130 | 9 | 52 | 4.6 | |
| 383.1 → 249.0 | 130 | 9 | 31 | 6 | |
| Vitamin B3 | 123.2 → 52.9 | 90 | 9 | 42 | 3.9 |
| 123.2 → 80.0 | 90 | 9 | 27 | 6.3 | |
| 123.2 → 96.2 | 90 | 9 | 25 | 8 | |
| Vitamin B3-13C6 | 129.2 → 57.0 | 90 | 9 | 42 | 3.9 |
| 129.2 → 85.0 | 90 | 9 | 27 | 6.3 | |
| 129.2 → 101.0 | 90 | 9 | 25 | 8 | |
| Vitamin B5 | 220.3 → 71.9 | 75 | 9.3 | 33 | 5.4 |
| 220.3 → 90.0 | 75 | 9.3 | 20 | 8.1 | |
| 220.3 → 96.0 | 75 | 9.3 | 27 | 7.2 | |
| Vitamin B5 13C3,15N | 224.0 → 75.8 | 75 | 9.3 | 33 | 5.4 |
| 224.0 → 94.1 | 75 | 9.3 | 20 | 8.1 | |
| 224.0 → 126.3 | 75 | 9.3 | 30 | 7.2 |
| Parameter | CLD Dogs (n = 66) | Reference Range |
|---|---|---|
| ALP (U/L) | 782 (31–2100) | 45–250 |
| GGT (U/L) | 14.5 (0.1–184) | 2–11 |
| AST (U/L) | 44.5 (18–909) | 15–40 |
| ALT (U/L) | 139 (5–1170) | 20–70 |
| Tot Bil (mg/dL) | 0.2 (0.1–27.76) | 0.07–0.3 |
| TP (g/dL) | 6.5 (4.3–8.8) | 5.8–7.8 |
| Alb (g/dL) | 3.4 (2–4.6) | 2.6–4.1 |
| Chol (mg/dL) | 285 (115–541.6) | 120–280 |
| Trig (mg/dL) | 100 (49–213) | 25–90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habermaass, V.; Cogozzo, A.; Bartoli, F.; Vitelli, V.; Dini, R.; Marchetti, V. Water-Soluble Vitamins (Riboflavin, Niacin, Pantothenic Acid) in Dogs with Chronic Liver Disease vs. Healthy Controls. Vet. Sci. 2025, 12, 877. https://doi.org/10.3390/vetsci12090877
Habermaass V, Cogozzo A, Bartoli F, Vitelli V, Dini R, Marchetti V. Water-Soluble Vitamins (Riboflavin, Niacin, Pantothenic Acid) in Dogs with Chronic Liver Disease vs. Healthy Controls. Veterinary Sciences. 2025; 12(9):877. https://doi.org/10.3390/vetsci12090877
Chicago/Turabian StyleHabermaass, Verena, Aurora Cogozzo, Francesco Bartoli, Valentina Vitelli, Rebecca Dini, and Veronica Marchetti. 2025. "Water-Soluble Vitamins (Riboflavin, Niacin, Pantothenic Acid) in Dogs with Chronic Liver Disease vs. Healthy Controls" Veterinary Sciences 12, no. 9: 877. https://doi.org/10.3390/vetsci12090877
APA StyleHabermaass, V., Cogozzo, A., Bartoli, F., Vitelli, V., Dini, R., & Marchetti, V. (2025). Water-Soluble Vitamins (Riboflavin, Niacin, Pantothenic Acid) in Dogs with Chronic Liver Disease vs. Healthy Controls. Veterinary Sciences, 12(9), 877. https://doi.org/10.3390/vetsci12090877







