Retrospective Analysis of Central Nervous System Diseases in Dogs, with Special Focus on Non-Suppurative Encephalomyelitis (1962–2022)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
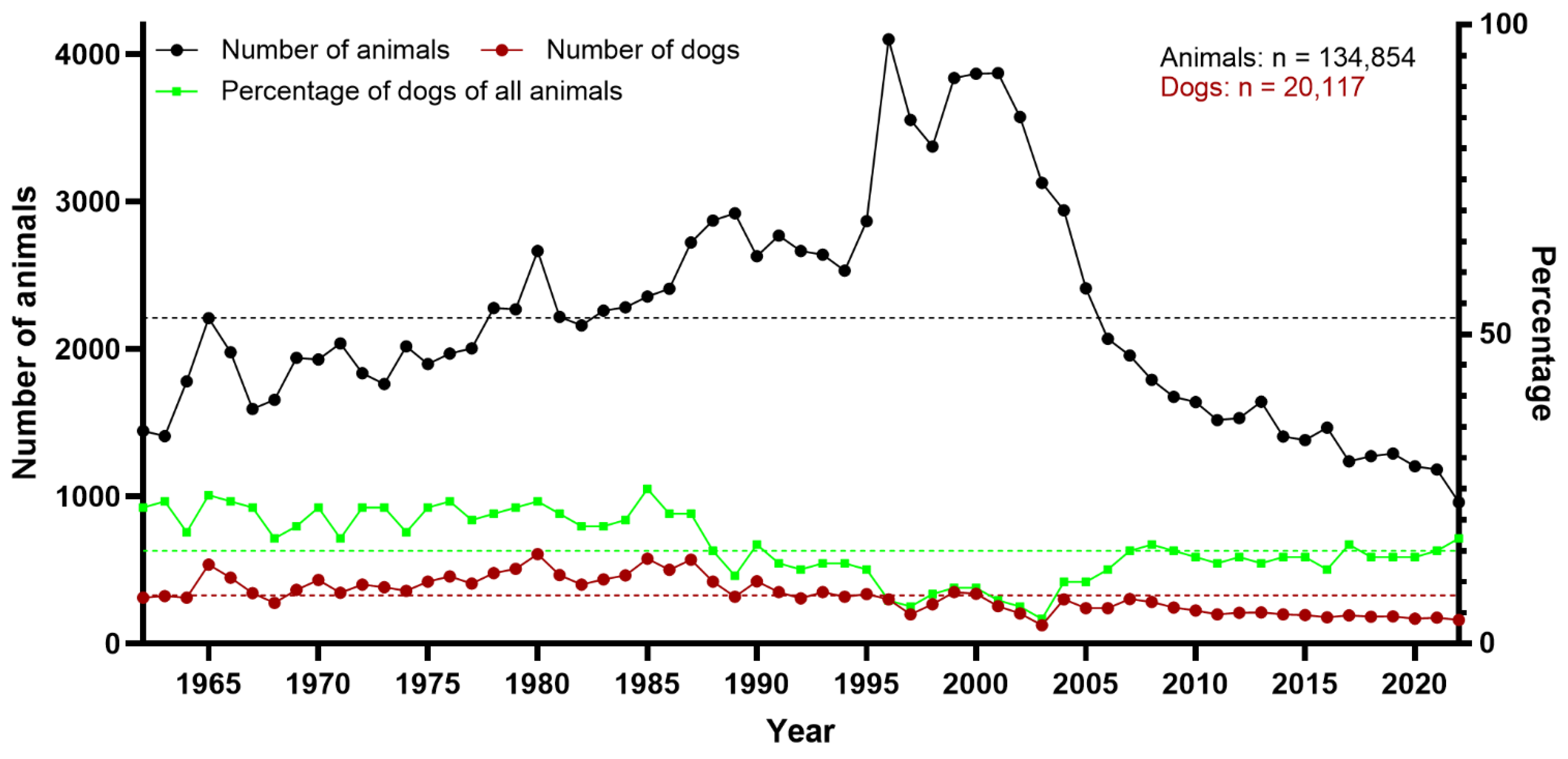
2.1. Archived Documentation of Animals
2.2. Histopathology and Formalin-Fixed, Paraffin-Embedded Material
2.3. Immunohistochemistry
3. Results
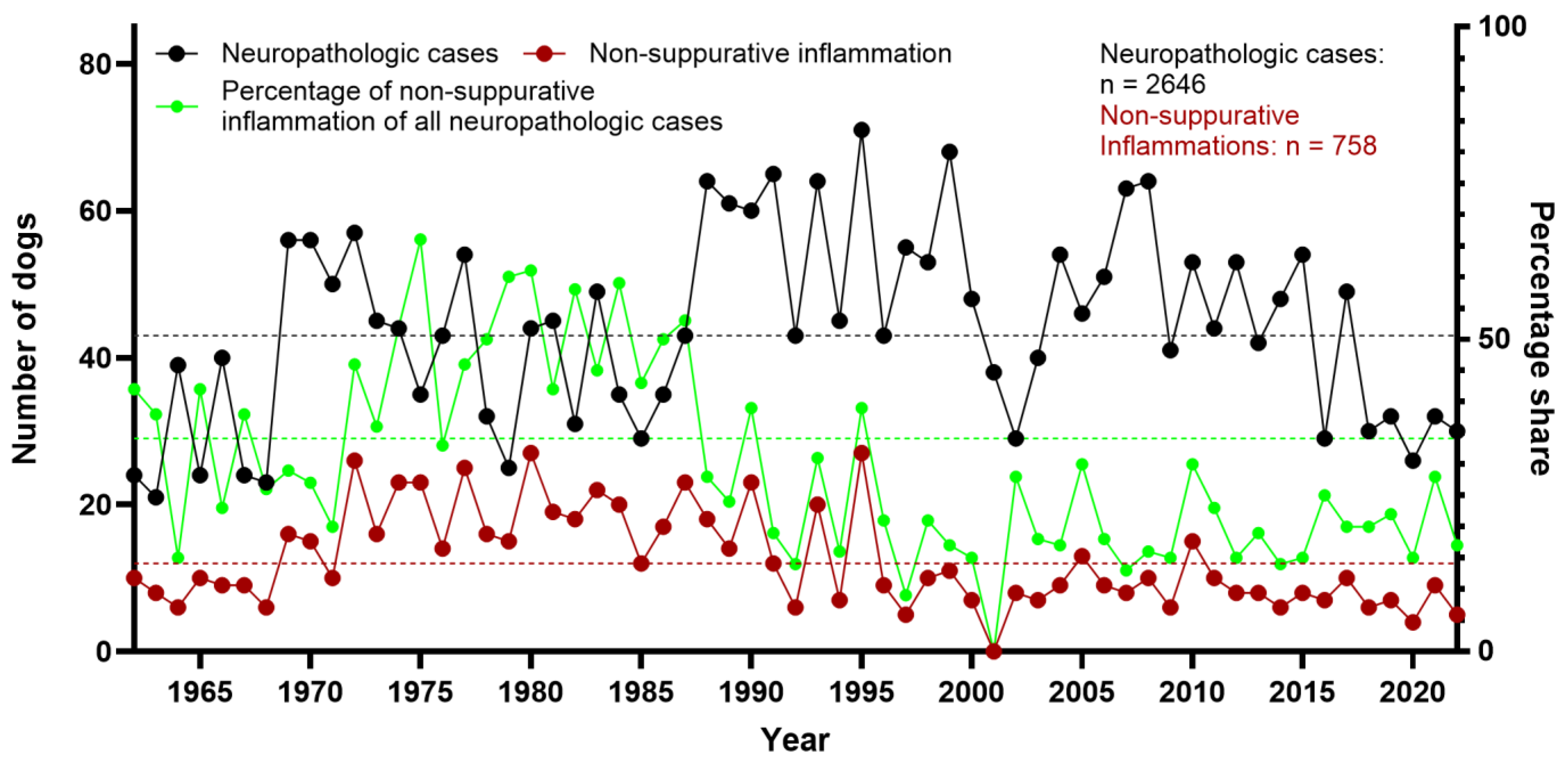
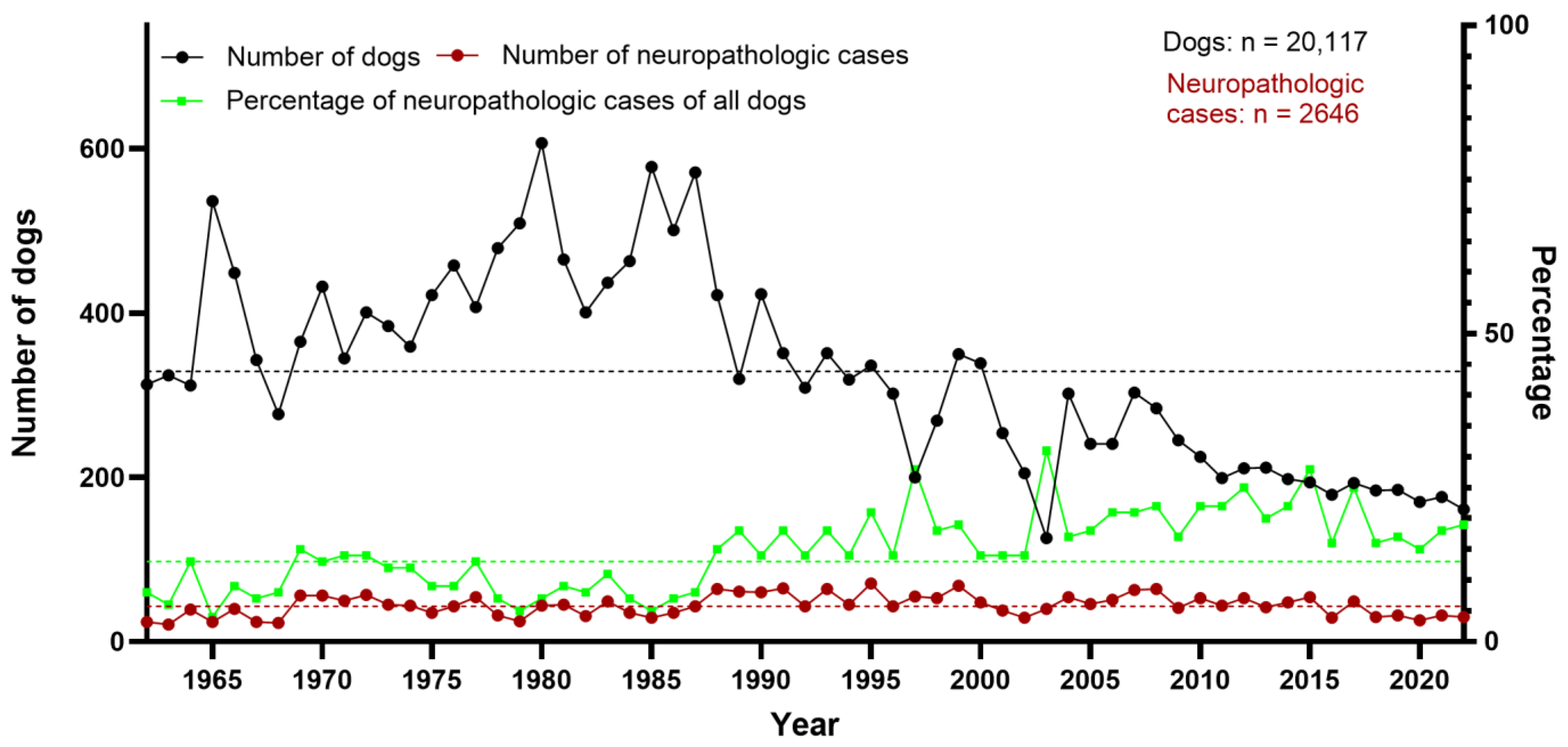
3.1. Neuropathologic Findings in Dogs
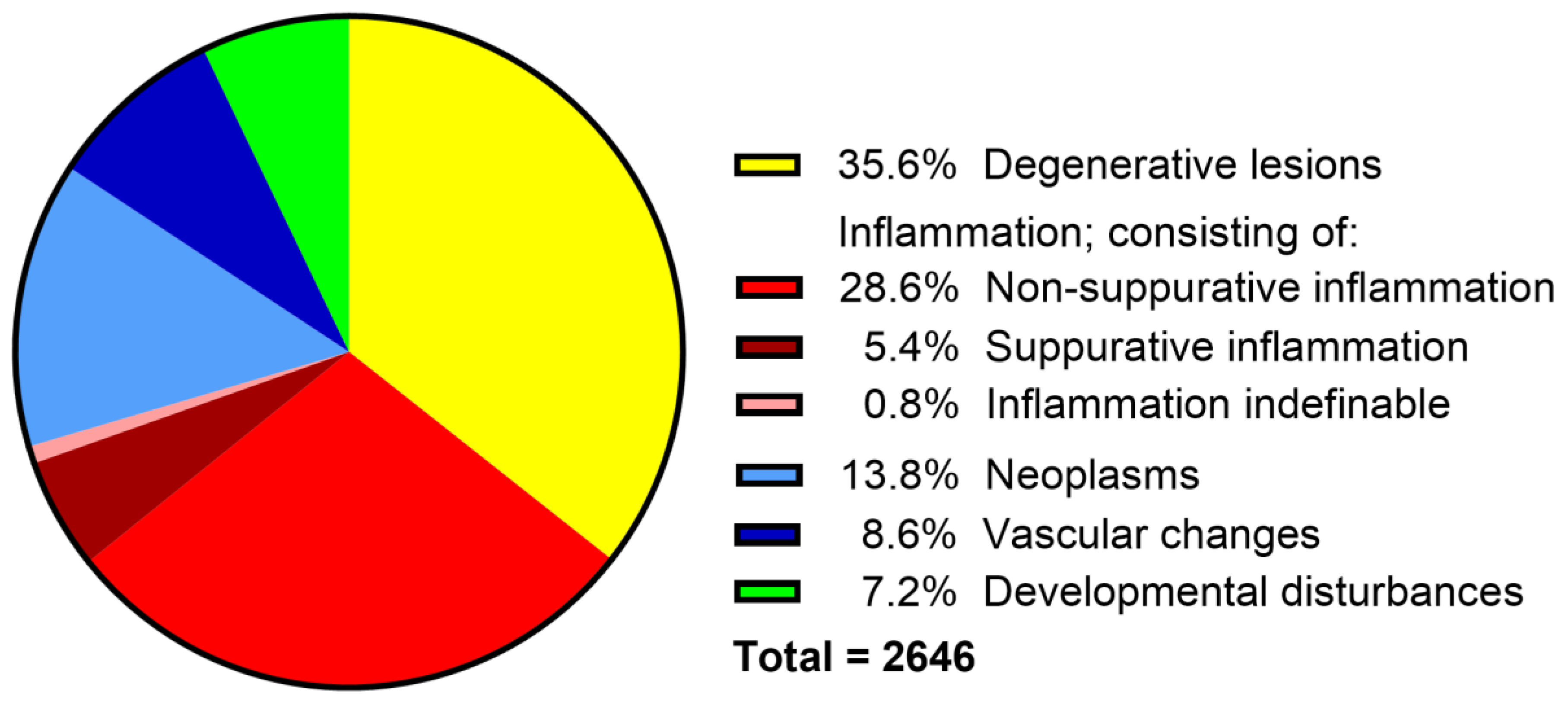
3.2. Categorization of Canine Neuropathologic Cases
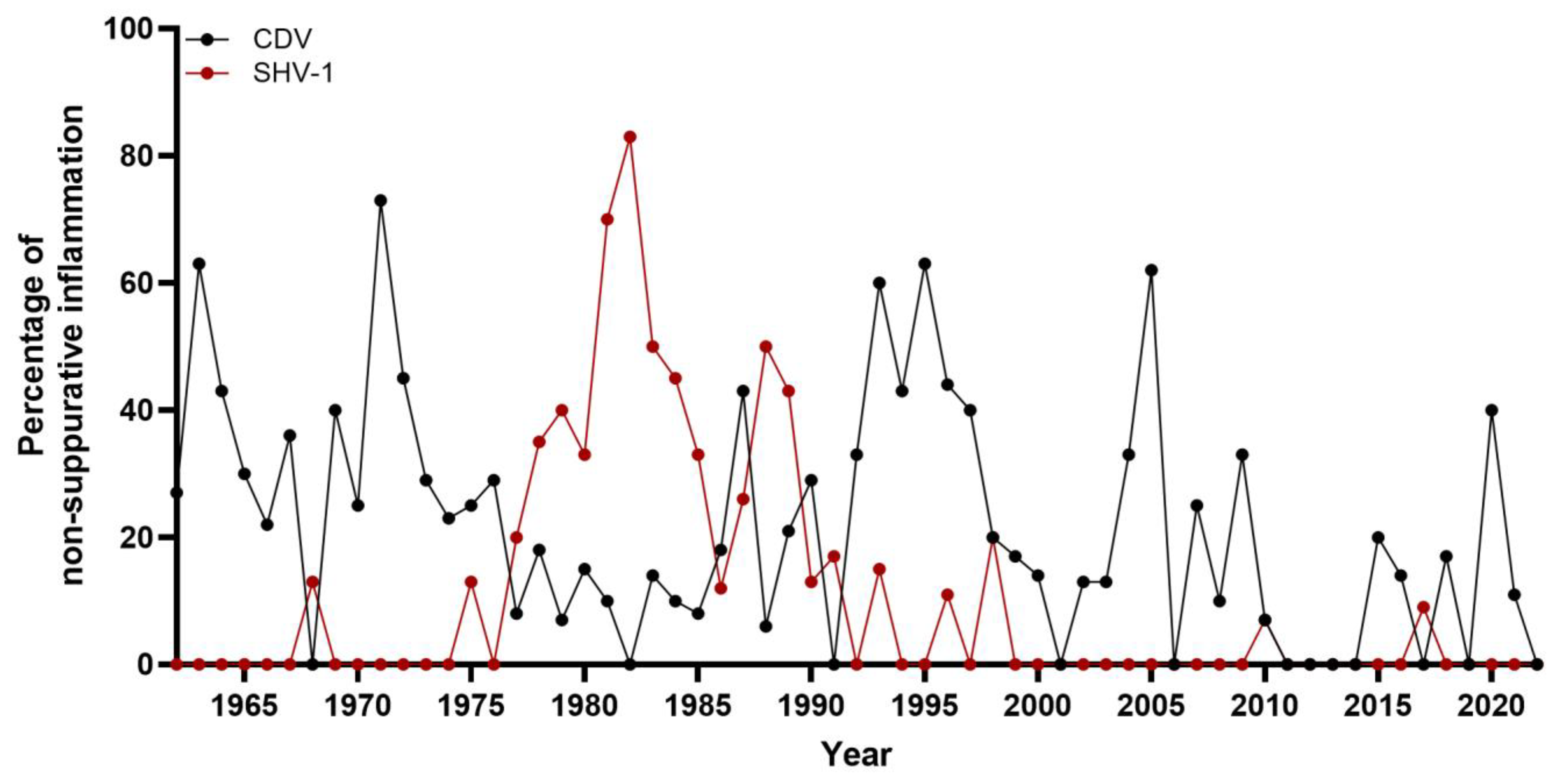
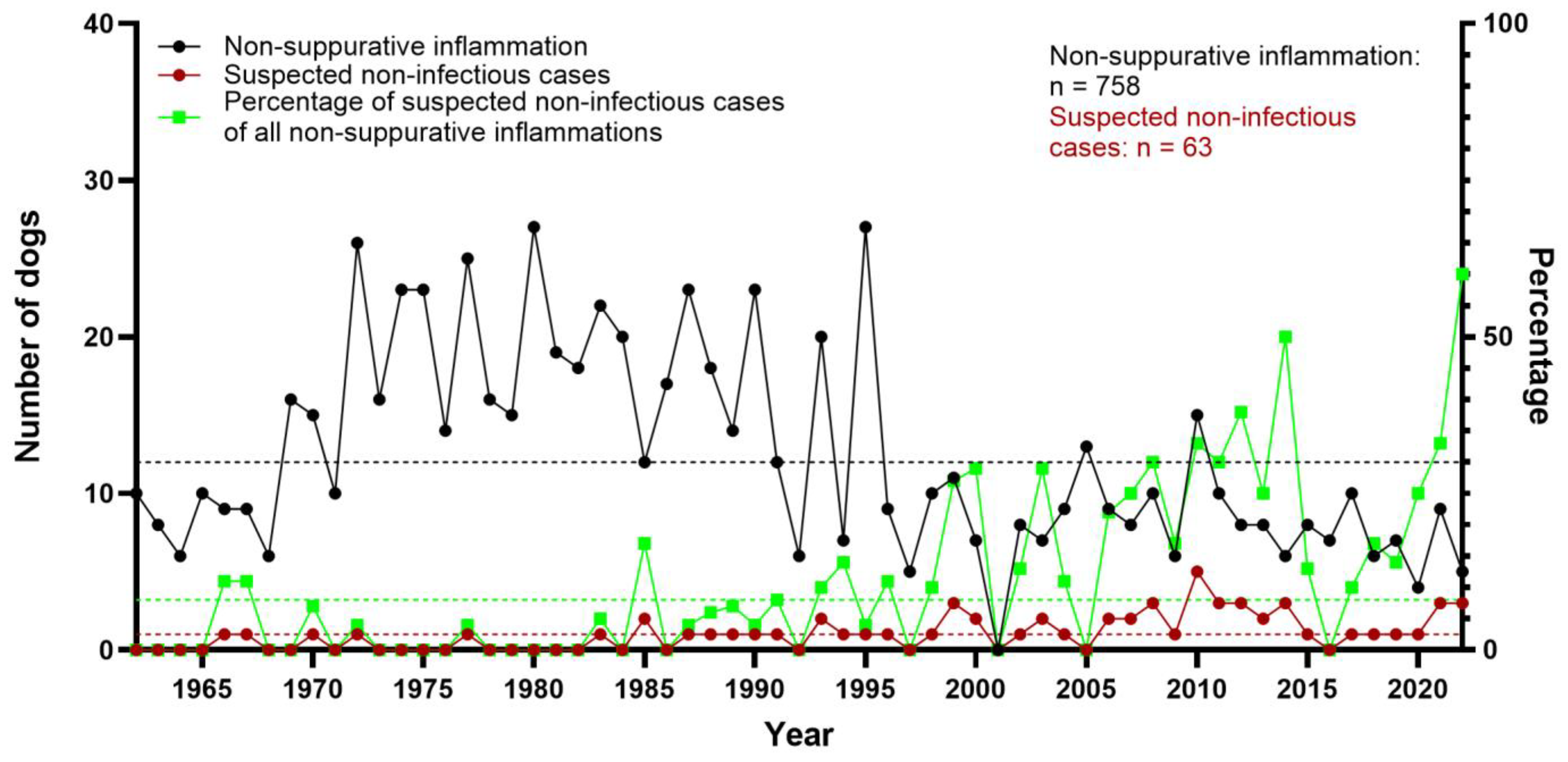
3.3. Non-Suppurative Inflammation
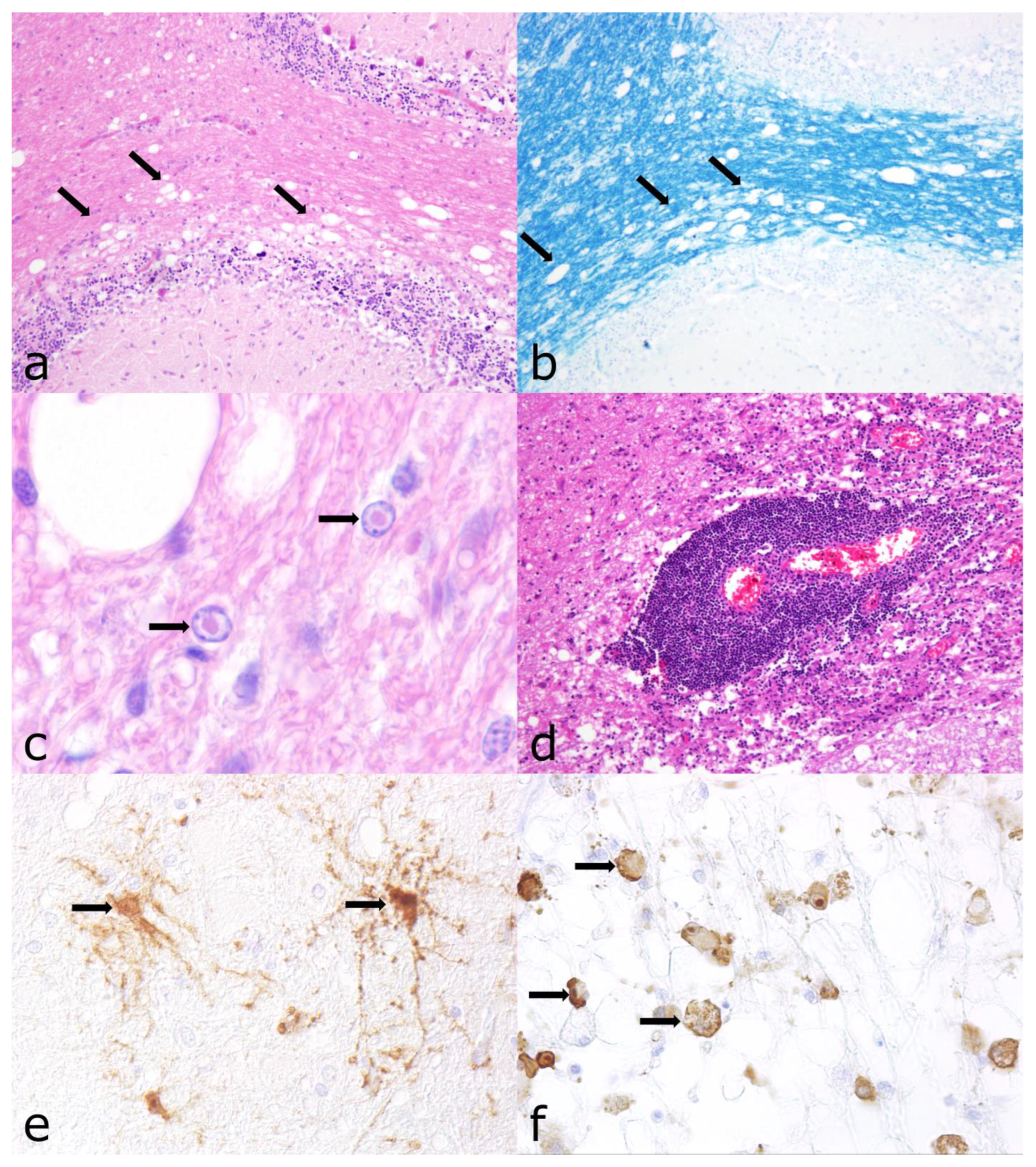
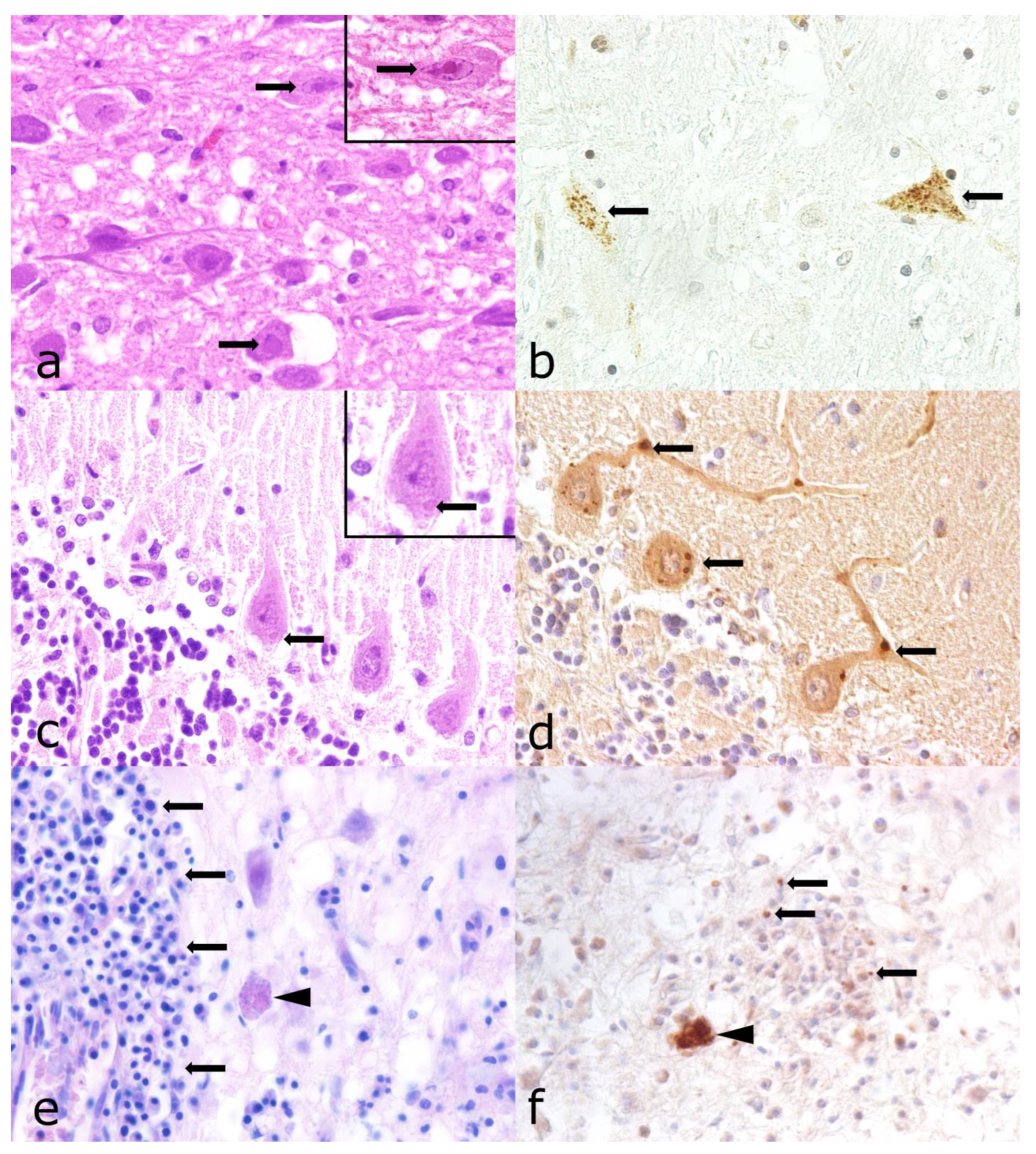
3.4. Morbillivirus Canis Infection
3.5. Varicellovirus Suidalpha1 Infection
3.6. Lyssavirus Rabies Infection
3.7. Other Detected Pathogens
3.8. Exclusion of Parvovirus Infection
3.9. Non-Infectious, Non-Suppurative Encephalomyelitis of Unknown Origin
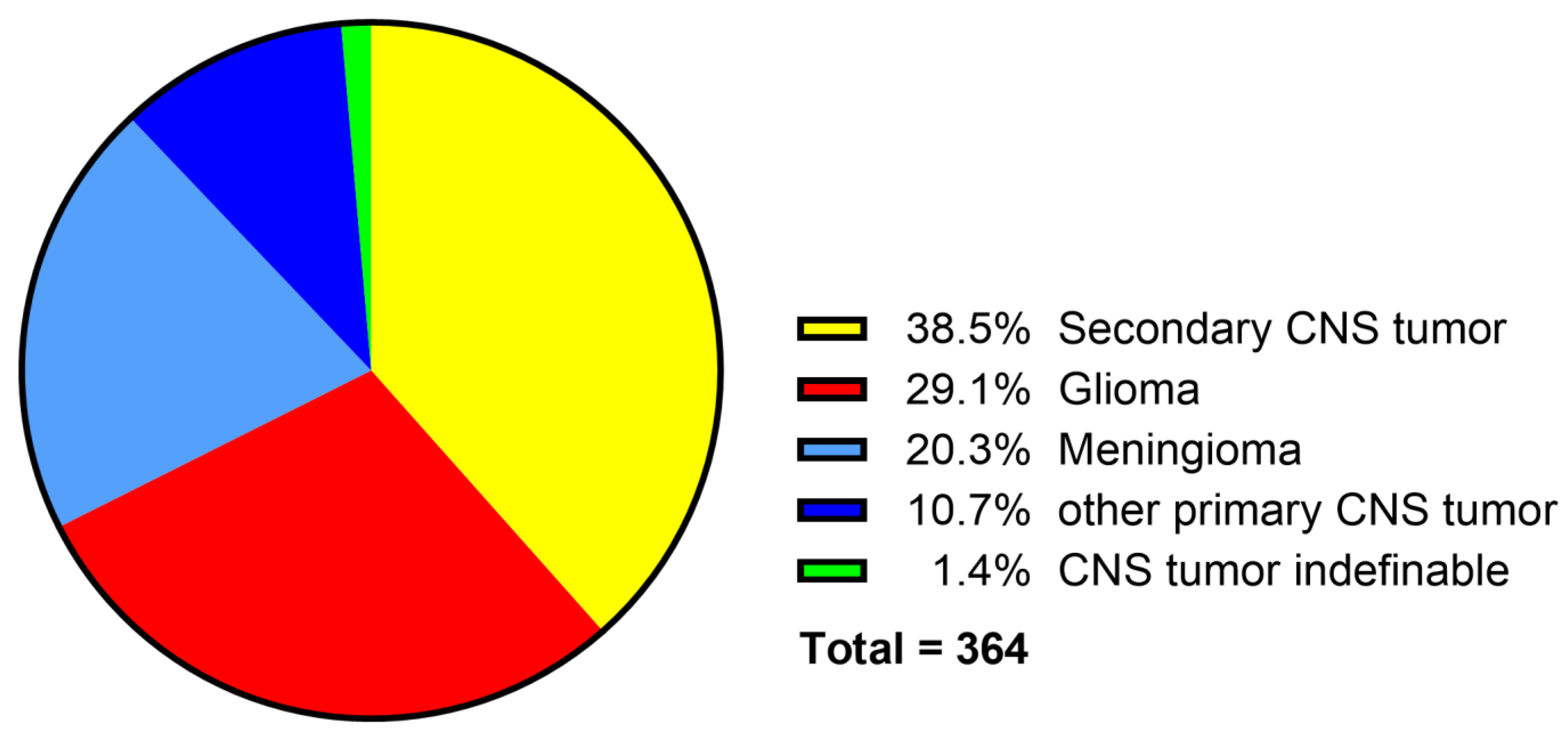
3.10. Neoplasms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- Wrzosek, M.; Giza, E.; Plonek, M.; Podgorski, P.; Vandevelde, M. Alexander disease in a dog: Case presentation of electrodiagnostic, magnetic resonance imaging and histopathologic findings with review of literature. BMC Vet. Res. 2015, 11, 115. [Google Scholar] [CrossRef]
- Hansen, H.J. A pathologic-anatomical study on disc degeneration in dog, with special reference to the so-called enchondrosis intervertebralis. Acta Orthop. Scand. Suppl. 1952, 11, 1–117. [Google Scholar] [CrossRef]
- McKee, W.M.; Downes, C.J.; Pink, J.J.; Gemmill, T.J. Presumptive exercise-associated peracute thoracolumbar disc extrusion in 48 dogs. Vet. Rec. 2010, 166, 523–528. [Google Scholar] [CrossRef]
- Maxie, M.G. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1. [Google Scholar]
- Vandevelde, M.; Higgins, R.J.; Oevermann, A. Veternary Neuropathology Essentials of Theory and Practice; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bach, F.S.; Cray, C.; Burgos, A.P.; Junior, J.A.V.; Montiani-Ferreira, F. A comparison between neurological clinical signs, cerebrospinal fluid analysis, cross-sectional CNS imaging, and infectious disease testing in 168 dogs with infectious or immune-mediated meningoencephalomyelitis from Brazil. Front. Vet. Sci. 2023, 10, 1239106. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, M.J.; Kent, M.; Glass, E.N. Noninfectious inflammatory central nervous system diseases in dogs. Compend. Contin. Educ. Vet. 2007, 29, 488–497. [Google Scholar] [PubMed]
- Schwab, S.; Herden, C.; Seeliger, F.; Papaioannou, N.; Psalla, D.; Polizopulou, Z.; Baumgärtner, W. Non-suppurative meningoencephalitis of unknown origin in cats and dogs: An immunohistochemical study. J. Comp. Pathol. 2007, 136, 96–110. [Google Scholar] [CrossRef]
- Tipold, A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: A retrospective study. J. Vet. Intern. Med. 1995, 9, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Beineke, A.; Baumgartner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus-an update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Njaa, B.L. Emerging viral encephalitides in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 863–878. [Google Scholar] [CrossRef]
- Rupprecht, C.E.; Hanlon, C.A.; Hemachudha, T. Rabies re-examined. Lancet Infect. Dis. 2002, 2, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, I.; Van Ham, L.; Gielen, I.; De Decker, S.; Bhatti, S.F.M. Clinical presentation, diagnostic findings, prognostic factors, treatment and outcome in dogs with meningoencephalomyelitis of unknown origin: A review. Vet. J. 2019, 244, 37–44. [Google Scholar] [CrossRef]
- Goncalves, R.; De Decker, S.; Walmsley, G.; Butterfield, S.; Maddox, T.W. Inflammatory Disease Affecting the Central Nervous System in Dogs: A Retrospective Study in England (2010–2019). Front. Vet. Sci. 2021, 8, 819945. [Google Scholar] [CrossRef]
- Nessler, J.N.; Jo, W.K.; Osterhaus, A.; Ludlow, M.; Tipold, A. Canine meningoencephalitis of unknown origin—The search for infectious agents in the cerebrospinal fluid via deep sequencing. Front. Vet. Sci. 2021, 8, 645517. [Google Scholar] [CrossRef]
- Zuraw, A.; Plog, S.; Lierz, M.; Gruber, A.D. No evidence of Sarcocystis calchasi involvement in mammalian meningoencephalitis of unknown origin. Vet. Parasitol. Reg. Stud. Reports 2016, 3–4, 49–52. [Google Scholar] [PubMed]
- Ghasemzadeh, I.; Namazi, S.H. Review of bacterial and viral zoonotic infections transmitted by dogs. J. Med. Life 2015, 8, 1–5. [Google Scholar] [PubMed]
- Tobias, K.M.; Rohrbach, B.W. Association of breed with the diagnosis of congenital portosystemic shunts in dogs: 2400 cases (1980–2002). J. Am. Vet. Med. Assoc. 2003, 223, 1636–1639. [Google Scholar] [CrossRef]
- Harkin, K.R.; Andrews, G.A.; Nietfeld, J.C. Dysautonomia in dogs: 65 cases (1993–2000). J. Am. Vet. Med. Assoc. 2002, 220, 633–639. [Google Scholar] [CrossRef]
- Soderman, L.; Harkin, K.R. Gastric Physaloptera infection in 27 dogs (1997–2019). J. Am. Anim. Hosp. Assoc. 2021, 57, 8–14. [Google Scholar] [CrossRef]
- Ling, G.V.; Ruby, A.L.; Johnson, D.L.; Thurmond, M.; Franti, C.E. Renal calculi in dogs and cats: Prevalence, mineral type, breed, age, and gender interrelationships (1981–1993). J. Vet. Intern. Med. 1998, 12, 11–21. [Google Scholar] [CrossRef]
- Barone, G.; Ziemer, L.S.; Shofer, F.S.; Steinberg, S.A. Risk factors associated with development of seizures after use of iohexol for myelography in dogs: 182 cases (1998). J. Am. Vet. Med. Assoc. 2002, 220, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Bonnett, B.N.; Olson, P.; Hedhammar, A. Gender, age and breed pattern of diagnoses for veterinary care in insured dogs in Sweden during 1996. Vet. Rec. 2000, 146, 551–557. [Google Scholar] [CrossRef]
- Gutsche, W. Sektionsstatistik der Hundekrankheiten aus dem Institut für Pathologie der Tierärztlichen Hochschule Hannover von 1938 bis 1963. Ph.D. Thesis, University of Veterinary Medicine Hanover, Hanover, Germany, 1964. [Google Scholar]
- Fluehmann, G.; Doherr, M.G.; Jaggy, A. Canine neurological diseases in a referral hospital population between 1989 and 2000 in Switzerland. J. Small. Anim. Pract. 2006, 47, 582–587. [Google Scholar] [CrossRef]
- Schwab, S. Retrospective Studie zur Ätiologischen Abklärung Unklarer Nicht-Eitrige Meningoenzephalitiden bei Hund und Katze. Ph.D. Thesis, University of Veterinary Medicine Hanover, Hanover, Germany, 2004. [Google Scholar]
- Elbert, J.A.; Yau, W.; Rissi, D.R. Neuroinflammatory diseases of the central nervous system of dogs: A retrospective study of 207 cases (2008–2019). Can. Vet. J. 2022, 63, 178–186. [Google Scholar]
- Griot, C.; Vandevelde, M.; Schobesberger, M.; Zurbriggen, A. Canine distemper, a re-emerging morbillivirus with complex neuropathogenic mechanisms. Anim. Health Res. Rev. 2003, 4, 1–10. [Google Scholar] [CrossRef]
- Gaskell, R.; Willoughby, K. Herpesviruses of carnivores. Vet. Microbiol. 1999, 69, 73–88. [Google Scholar] [CrossRef]
- Percy, D.H.; Olander, H.J.; Carmichael, L.E. Encephalitis in the newborn pup due to a canine herpesvirus. Pathol. Vet. 1968, 5, 135–145. [Google Scholar] [CrossRef]
- Kaplan, M.M. Epidemiology of rabies. Nature 1969, 221, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Arechiga Ceballos, N.; Puebla Rodriguez, P.; Aguilar Setien, A. The new face of human rabies in Mexico, what’s next after eradicating rabies in dogs. Vector Borne Zoonotic Dis. 2022, 22, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.J.; Brookes, S.M.; Johnson, N.; Smith, J.; Mansfield, K.L.; Keene, V.L.; McElhinney, L.M.; Fooks, A.R. Rabies in North America and Europe. J. R. Soc. Med. 2002, 95, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zanoni, R.G.; Kappeler, A.; Muller, U.M.; Muller, C.; Wandeler, A.I.; Breitenmoser, U. Tollwutfreiheit der Schweiz nach 30 Jahren Fuchstollwut. Schweiz. Arch. Tierheilkd. 2000, 142, 423–429. [Google Scholar] [PubMed][Green Version]
- Müller, T.; Batza, H.J.; Freuling, C.; Kliemt, A.; Kliemt, J.; Heuser, R.; Schluter, H.; Selhorst, T.; Vos, A.; Mettenleiter, T.C. Elimination of terrestrial rabies in Germany using oral vaccination of foxes. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 178–190. [Google Scholar][Green Version]
- Sehl, J.; Teifke, J.P. Comparative pathology of pseudorabies in different naturally and experimentally infected species—A review. Pathogens 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Nägler, I.; Puff, C.; Fayyad, A.; Baumgärtner, W.; Wohlsein, P. Retrospektive Untersuchung zu nichteitrigen Entzündungen im zentralen Nervensystem bei Hunden aus den Jahren 1962 bis 2022. Tierarztl Prax 2025, 53, 182. [Google Scholar][Green Version]
- Klotz, D.; Gerhauser, I. Interferon-stimulated genes—Mediators of the innate immune response during canine distemper virus infection. Int. J. Mol. Sci. 2019, 20, 1620. [Google Scholar] [CrossRef]
- Schaudien, D.; Polizopoulou, Z.; Koutinas, A.; Schwab, S.; Porombka, D.; Baumgärtner, W.; Herden, C. Leukoencephalopathy associated with parvovirus infection in Cretan hound puppies. J. Clin. Microbiol. 2010, 48, 3169–3175. [Google Scholar] [CrossRef]
- Kaneko, C.; Kaneko, Y.; Sudaryatma, P.E.; Mekata, H.; Kirino, Y.; Yamaguchi, R.; Okabayashi, T. Pseudorabies virus infection in hunting dogs in Oita, Japan: Report from a prefecture free from Aujeszky’s disease in domestic pigs. J. Vet. Med. Sci. 2021, 83, 680–684. [Google Scholar] [CrossRef]
- Umeda, K.; Goto, Y.; Watanabe, K.; Ushio, N.; Fereig, R.M.; Ihara, F.; Tanaka, S.; Suzuki, Y.; Nishikawa, Y. Transcriptomic analysis of the effects of chemokine receptor CXCR3 deficiency on immune responses in the mouse brain during Toxoplasma gondii infection. Microorganisms 2021, 9, 2340. [Google Scholar] [CrossRef]
- Poch, O.; Tordo, N.; Keith, G. Sequence of the 3386 3′ nucleotides of the genome of the AVO1 strain rabies virus: Structural similarities in the protein regions involved in transcription. Biochimie 1988, 70, 1019–1029. [Google Scholar] [CrossRef]
- Raha, A.A.; Henderson, J.W.; Stott, S.R.; Vuono, R.; Foscarin, S.; Friedland, R.P.; Zaman, S.H.; Raha-Chowdhury, R. Neuroprotective effect of TREM-2 in aging and Alzheimer’s disease model. J. Alzheimers Dis. 2017, 55, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Forni, M.F.; Ramos Maia Lobba, A.; Pereira Ferreira, A.H.; Sogayar, M.C. Simultaneous isolation of three different stem cell populations from murine skin. PLoS ONE 2015, 10, e0140143. [Google Scholar] [CrossRef] [PubMed]
- Lenz, J.A.; Assenmacher, C.A.; Costa, V.; Louka, K.; Rau, S.; Keuler, N.S.; Zhang, P.J.; Maki, R.G.; Durham, A.C.; Radaelli, E.; et al. Increased tumor-infiltrating lymphocyte density is associated with favorable outcomes in a comparative study of canine histiocytic sarcoma. Cancer Immunol. Immunother. 2022, 71, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Shimojima, M.; Nagata, N.; Ami, Y.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Fukushi, S.; Watanabe, S.; Kurosu, T.; Kataoka, M.; et al. Severe fever with thrombocytopenia syndrome phlebovirus causes lethal viral hemorrhagic fever in cats. Sci. Rep. 2019, 9, 11990. [Google Scholar] [CrossRef]
- Kühl, B.; Beyerbach, M.; Baumgärtner, W.; Gerhauser, I. Characterization of microglia/macrophage phenotypes in the spinal cord following intervertebral disc herniation. Front. Vet. Sci. 2022, 9, 942967. [Google Scholar] [CrossRef]
- Gregor, K.M.; Knebel, A.; Haverkamp, A.K.; Baumgärtner, W.; Volk, H. Metastatic Canine phaeochromocytoma with unusual manifestation. J. Comp. Pathol. 2022, 192, 33–40. [Google Scholar] [CrossRef]
- Skedsmo, F.S.; Tranulis, M.A.; Espenes, A.; Prydz, K.; Matiasek, K.; Gunnes, G.; Hermansen, L.C.; Jaderlund, K.H. Cell and context-dependent sorting of neuropathy-associated protein NDRG1—Insights from canine tissues and primary Schwann cell cultures. BMC Vet. Res. 2019, 15, 121. [Google Scholar] [CrossRef]
- Eikelberg, D.; Lehmbecker, A.; Brogden, G.; Tongtako, W.; Hahn, K.; Habierski, A.; Hennermann, J.B.; Naim, H.Y.; Felmy, F.; Baumgärtner, W.; et al. Axonopathy and reduction of membrane resistance: Key features in a new murine model of human G(M1)-gangliosidosis. J. Clin. Med. 2020, 9, 1004. [Google Scholar] [CrossRef]
- Meuthen, D.J. Tumors in Domestic Animals, 5th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Blixenkrone-Moller, M.; Svansson, V.; Have, P.; Orvell, C.; Appel, M.; Pedersen, I.R.; Dietz, H.H.; Henriksen, P. Studies on manifestations of canine distemper virus infection in an urban dog population. Vet. Microbiol. 1993, 37, 163–173. [Google Scholar] [CrossRef]
- Ek-Kommonen, C.; Sihvonen, L.; Pekkanen, K.; Rikula, U.; Nuotio, L. Outbreak off canine distemper in vaccinated dogs in Finland. Vet. Rec. 1997, 141, 380–383. [Google Scholar] [CrossRef]
- Cook, S.D.; Dowling, P.C.; Norman, J.; Jablon, S. Multiple sclerosis and canine distemper in Iceland. Lancet 1979, 1, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I. Grease, anthraxgate, and kennel cough: A revisionist history of early veterinary vaccines. Adv. Vet. Med. 1999, 41, 7–24. [Google Scholar]
- Iwatsuki, K.; Miyashita, N.; Yoshida, E.; Gemma, T.; Shin, Y.S.; Mori, T.; Hirayama, N.; Kai, C.; Mikami, T. Molecular and phylogenetic analyses of the haemagglutinin (H) proteins of field isolates of canine distemper virus from naturally infected dogs. J. Gen. Virol. 1997, 78 Pt 2, 373–380. [Google Scholar] [CrossRef]
- Gorham, J.R. Canine distemper (la maladie de Carré). Adv. Vet. Sci. 1960, 6, 287–351. [Google Scholar]
- Evans, J.M. Protection Against Canine Distemper. Vet. Rec. 1967, 81, 163–166. [Google Scholar] [CrossRef]
- Howell, D.G. Vaccination of the dog. Vet. Rec. 1961, 73, 46–54. [Google Scholar]
- Müller, T.; Batza, H.J.; Schluter, H.; Conraths, F.J.; Mettenleiter, T.C. Eradication of Aujeszky’s disease in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 207–213. [Google Scholar] [CrossRef]
- Hara, M.; Shimizu, T.; Fukuyama, M.; Nomura, Y.; Shirota, K.; Une, Y.; Hirota, A.; Yago, K.; Yamada, H.; Ishihara, M. Natural case of Aujeszky’s disease in the dog in Japan. Nihon Juigaku Zasshi 1987, 49, 645–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freuling, C.M.; Muller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef] [PubMed]
- OIE Handistatus II, Germany/Aujeszky’s Disease. Available online: https://web.oie.int/hs2/sit_pays_mald_pl.asp?c_pays=49&c_mald=21 (accessed on 26 June 2025).
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef]
- Pieracci, E.G.; Wallace, R.; Maskery, B.; Brouillette, C.; Brown, C.; Joo, H. Dogs on the move: Estimating the risk of rabies in imported dogs in the United States, 2015–2022. Zoonoses Public Health 2024, 71, 620–628. [Google Scholar] [CrossRef]
- Riccardi, N.; Giacomelli, A.; Antonello, R.M.; Gobbi, F.; Angheben, A. Rabies in Europe: An epidemiological and clinical update. Eur. J. Intern. Med. 2021, 88, 15–20. [Google Scholar] [CrossRef]
- Vandevelde, M.; Kristensen, B.; Greene, C.E. Primary reticulosis of the central nervous system in the dog. Vet. Pathol. 1978, 15, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Vuia, O.; Mehraein, P. Primary reticulosis of the central nervous system. J. Neurol. Sci. 1971, 14, 469–482. [Google Scholar] [CrossRef]
- Boellaard, J.W. Über einen Fall von Granulomencephalitis mit ungewöhnlicher Lokalisation. Zugleich ein Beitrag zur Frage der Reticulose des ZNS. Arch. Psychiatr. Nervenkr. 1971, 214, 17–32. [Google Scholar] [CrossRef]
- Cordy, D.R. Canine granulomatous meningoencephalomyelitis. Vet. Pathol. 1979, 16, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zdora, I.; Raue, J.; Sobbeler, F.; Tipold, A.; Baumgärtner, W.; Nessler, J.N. Case report: Lympho-histiocytic meningoencephalitis with central nervous system vasculitis of unknown origin in three dogs. Front. Vet. Sci. 2022, 9, 944867. [Google Scholar] [CrossRef]
- Lotti, D.; Capucchio, M.T.; Gaidolfi, E.; Merlo, M. Necrotizing encephalitis in a Yorkshire Terrier: Clinical, imaging, and pathologic findings. Vet. Radiol. Ultrasound 1999, 40, 622–626. [Google Scholar] [CrossRef]
- Cantile, C.; Chianini, F.; Arispici, M.; Fatzer, R. Necrotizing meningoencephalitis associated with cortical hippocampal hamartia in a Pekingese dog. Vet. Pathol. 2001, 38, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Stalis, I.H.; Chadwick, B.; Dayrell-Hart, B.; Summers, B.A.; Van Winkle, T.J. Necrotizing meningoencephalitis of Maltese dogs. Vet. Pathol. 1995, 32, 230–235. [Google Scholar] [CrossRef]
- Levine, J.M.; Fosgate, G.T.; Porter, B.; Schatzberg, S.J.; Greer, K. Epidemiology of necrotizing meningoencephalitis in Pug dogs. J. Vet. Intern. Med. 2008, 22, 961–968. [Google Scholar] [CrossRef]
- Uchida, K.; Park, E.; Tsuboi, M.; Chambers, J.K.; Nakayama, H. Pathological and immunological features of canine necrotising meningoencephalitis and granulomatous meningoencephalitis. Vet. J. 2016, 213, 72–77. [Google Scholar] [CrossRef]
- Song, R.B.; Vite, C.H.; Bradley, C.W.; Cross, J.R. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J. Vet. Intern. Med. 2013, 27, 1143–1152. [Google Scholar] [CrossRef]
- Kishimoto, T.E.; Uchida, K.; Chambers, J.K.; Kok, M.K.; Son, N.V.; Shiga, T. A retrospective survey on canine intracranial tumors between 2007 and 2017. J. Vet. Med. Sci. 2020, 82, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Merickel, J.L.; Pluhar, G.E.; Rendahl, A.; O’Sullivan, M.G. Prognostic histopathologic features of canine glial tumors. Vet. Pathol. 2021, 58, 945–951. [Google Scholar] [CrossRef]
- Fuchs, C.; Meyer-Lindenberg, A.; Wohlsein, P.; Nolte, I. Computertomographic characteristics of primary brain tumors in dogs and cats. Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 436–442. [Google Scholar]
- Röthlisberger, A.; Lehmbecker, A.; Beineke, A.; Mischke, R.; Dziallas, P.; Meyer-Lindenberg, A.; Tipold, A. Suspected primary glioblastoma multiforme in the canine spinal cord. J. Small Anim. Pract. 2012, 53, 604–607. [Google Scholar] [CrossRef]
- Talarico, L.R.; Schatzberg, S.J. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: A review and future perspectives. J. Small Anim. Pract. 2010, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- de le Roi, M.; Nägler, I.; Rubbenstroth, D.; Beer, M.; Höper, D.; Barth, S.A.; Fayyad, A.; Puff, C.; Baumgärtner, W.; Wohlsein, P. Retrospective analysis of clustered neuroinflammatory and neurodegenerative diseases in captive lions in the early 1970s. Vet. Pathol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Reid, A.H.; Krafft, A.E.; Bijwaard, K.E.; Fanning, T.G. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science 1997, 275, 1793–1796. [Google Scholar] [CrossRef]
- Gryseels, S.; Wattsm, T.D.; Kabongo Mpolesha, J.M.; Larsen, B.B.; Lemey, P.; Muyemebe-Tamfub, J.J.; Teuwen, D.E.; Worobey, M. A near full-length HIV-1 genome from 1966 recovered from formalin-fixed paraffin-embedded tissue. Proc. Natl. Acad. Sci. USA 2020, 117, 12222–12229. [Google Scholar] [CrossRef]
- von Ahlfen, S.; Missel, A.; Bendrat, K.; Schlumpberger, M. Determinants of RNA quality from FFPE samples. PLoS ONE 2007, 2, e1261. [Google Scholar] [CrossRef]
- Bodewes, R.; van Run, P.R.; Schurch, A.C.; Koopmans, M.P.; Osterhaus, A.D.; Baumgärtner, W.; Kuiken, T.; Smits, S.L. Virus characterization and discovery in formalin-fixed paraffin-embedded tissues. J. Virol. Methods 2015, 214, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002, 161, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Kocjan, B.J.; Hosnjak, L.; Poljak, M. Detection of alpha human papillomaviruses in archival formalin-fixed, paraffin-embedded (FFPE) tissue specimens. J. Clin. Virol. 2016, 76 (Suppl. 1), S88–S97. [Google Scholar] [CrossRef] [PubMed]
| Category | Type of Lesion According to Vandevelde et al. [6] | Modified for the Current Neuropathological Classification | |
|---|---|---|---|
| V | Vascular diseases | Vasculitis, hemorrhage, infarction | Same categories |
| I | Inflammatory diseases | Parenchymal invasion of blood-derived leucocytes around blood vessels, infiltrating into the parenchyma, proliferation of endogenous microglia | Same categories |
| T | Trauma | Mechanical disruption of tissue compounded by traumatic injury to blood vessels | Not used; included in the category vascular and/or degenerative diseases |
| A | Anomalies | Abnormal development of the CNS in utero | Same categories |
| M | Metabolic/toxic diseases | Loss of architecture of nervous tissue caused by deficiencies and toxins | Not used; categorized as degenerative disease, included in category D |
| I | Idiopathic changes | Abnormal functional neurological signs without morphologically detectable changes in the CNS tissue | Not used, not applicable for this neuropathological study |
| N | Neoplasia | Destructive invasion or compression by tumor cell proliferation by primary or secondary CNS tumors | Destructive invasion or cell proliferation by primary or secondary CNS tumors; compression of tumors outside the CNS were classified as degenerative disease |
| D | Degenerative diseases | Progressive degeneration of various specific cell types in the nervous system in a bilaterally symmetrical and restricted anatomical localization, mostly connected to genetic defects | Encephalo- and myelomalacia; neuronopathies and axonopathies; spongy encephalomyelopathies; degenerations caused by vertrebral disc herniations; cerebellar abiotrophy; disruption of tissue |
| Specificity | Target | Dilution | Pretreatment | Clonality, Host Species | Source |
|---|---|---|---|---|---|
| CDV nucleoprotein (D110) | CDV | 1:1000 | Citrate buffer (0.01 mol/L), MW | Monoclonal, mouse | Kindly provided by University of Bern, Bern, Switzerland |
| SHV-1-antigen | SHV1 | 1:100 | TRIS-EDTA buffer (0.01 mol/L; 0.001 mol/L), MW | Polyclonal, rabbit | Abcam, Cambridge, UK |
| T. gondii, cross-reaction with other apicomplexa | Apicomplexian parasites | 1:75 | None | Polyclonal, rabbit | Quartett, Potsdam, Germany |
| Rabies virus nucleoprotein | Rabies virus | 1:500 | Proteinase K | Polyclonal, rabbit | Flarebio Biotech LLC, Washington, DC, USA |
| CPV-antigen, cross-reaction with FPV; PPV and MPV | CPV | 1:500 | Proteinase K | Monoclonal, mouse | Custom Monoclonal International, Sacramento, CA, USA |
| PAX5 | B-lymphocytes | 1:500 | Citrate buffer (0.01 mol/L), MW | Monoclonal, mouse | BD, Franklin Lakes, NJ, USA |
| CD3 | T-lymphocytes | 1:200 | Citrate buffer (0.01 mol/L), MW | Monoclonal, rat | Bio Rad, Hercules, CA, USA |
| CD204 | Histiocytic cells | 1:500 | Citrate buffer (0.01 mol/L), MW | Monoclonal, mouse | Abnova, Taipei City, Taiwan |
| IBA 1 | Histiocytic cells | 1:1000 | Citrate buffer (0.01 mol/L), MW | Polyclonal, rabbit | Thermo Electron LED GmbH, Langenselbold, Germany |
| Olig 2 | Oligodendrocytes | 1:100 | Citrate buffer (0.01 mol/L), MW | Monoclonal, rabbit | Abcam, Cambridge, UK |
| GFAP | Astrocytes | 1:1000 | None | Polyclonal, rabbit | Dako, Santa Clara, CA, USA |
| Synaptophysin | Neuro-endocrine cells | 1:500 | Citrate buffer (0.01 mol/L), MW | Monoclonal, mouse | Dako, Santa Clara, CA, USA |
| CPNase | Oligodendrocytes | 1:500 | Citrate buffer (0.01 mol/L), MW | Monoclonal, mouse | Chemicon International, Temecula, CA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nägler, I.M.; Fayyad, A.; Puff, C.; Baumgärtner, W.; Wohlsein, P. Retrospective Analysis of Central Nervous System Diseases in Dogs, with Special Focus on Non-Suppurative Encephalomyelitis (1962–2022). Vet. Sci. 2025, 12, 869. https://doi.org/10.3390/vetsci12090869
Nägler IM, Fayyad A, Puff C, Baumgärtner W, Wohlsein P. Retrospective Analysis of Central Nervous System Diseases in Dogs, with Special Focus on Non-Suppurative Encephalomyelitis (1962–2022). Veterinary Sciences. 2025; 12(9):869. https://doi.org/10.3390/vetsci12090869
Chicago/Turabian StyleNägler, Inga Marie, Adnan Fayyad, Christina Puff, Wolfgang Baumgärtner, and Peter Wohlsein. 2025. "Retrospective Analysis of Central Nervous System Diseases in Dogs, with Special Focus on Non-Suppurative Encephalomyelitis (1962–2022)" Veterinary Sciences 12, no. 9: 869. https://doi.org/10.3390/vetsci12090869
APA StyleNägler, I. M., Fayyad, A., Puff, C., Baumgärtner, W., & Wohlsein, P. (2025). Retrospective Analysis of Central Nervous System Diseases in Dogs, with Special Focus on Non-Suppurative Encephalomyelitis (1962–2022). Veterinary Sciences, 12(9), 869. https://doi.org/10.3390/vetsci12090869






