Metabolic Profiling, Tissue Distribution, and Tolerance Assessment of Bopu Powder in Laying Hens Following Long-Term Dietary Administration
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagent
2.2. Solution Preparation
2.3. Birds and Grouping
2.4. Sample Collection
2.4.1. Identification of Metabolites for Allocryptopine and Protopine in Laying Hens
2.4.2. Quantification of Protopine and Allocryptopine in Laying Hens’ Tissues and Eggs
2.4.3. Serum Biochemistry
2.5. Sample Pretreatment
2.6. LC-MS Conditions
2.6.1. LC-QTOF-MS Conditions
2.6.2. LC-QQQ-MS Conditions
2.7. Comprehensive Metabolic Panel
2.8. Histopathological Examination
2.9. Data Processing
3. Results
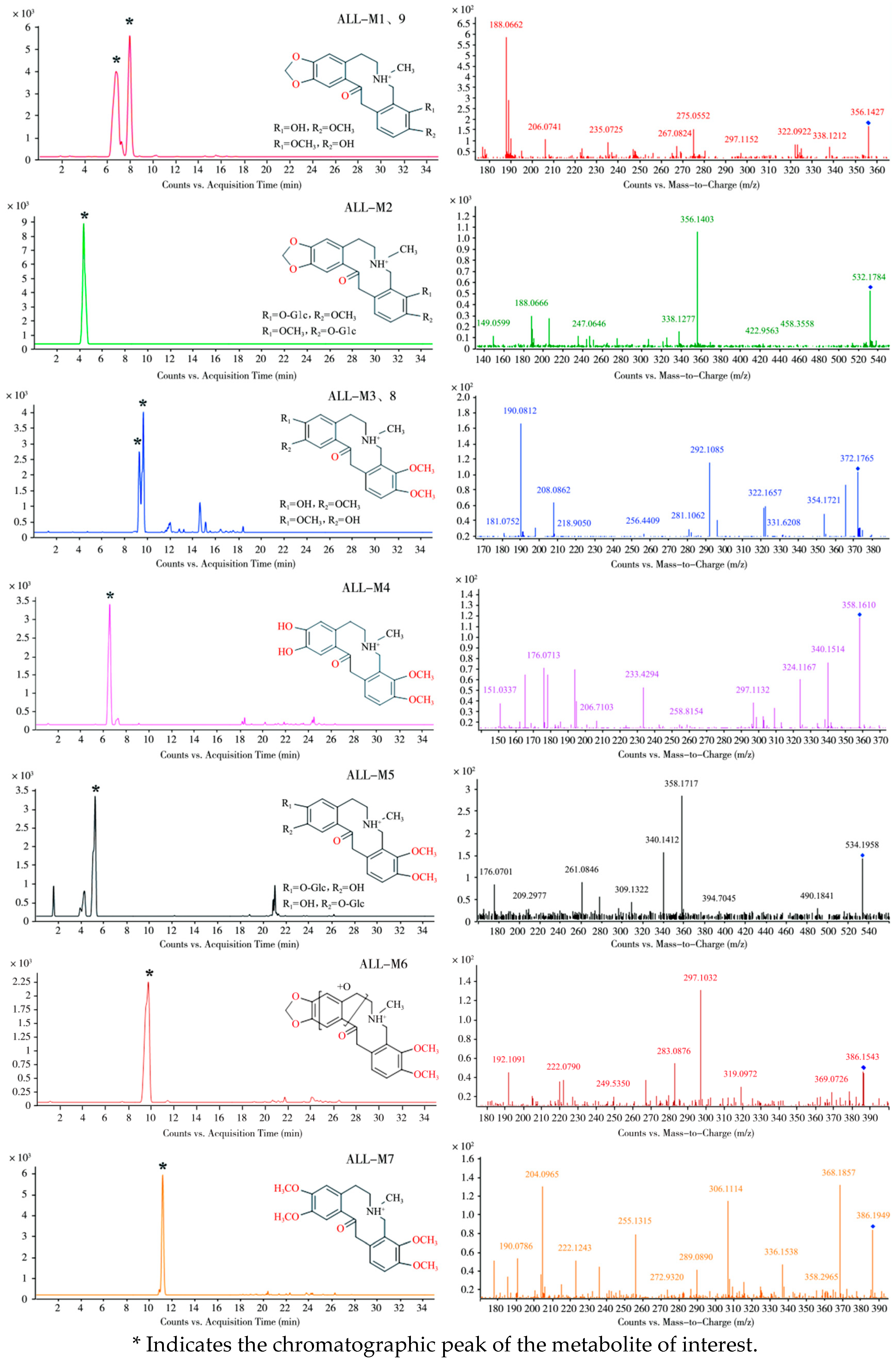
3.1. Metabolite Identification
3.1.1. Metabolic Profile of ALL in Laying Hens
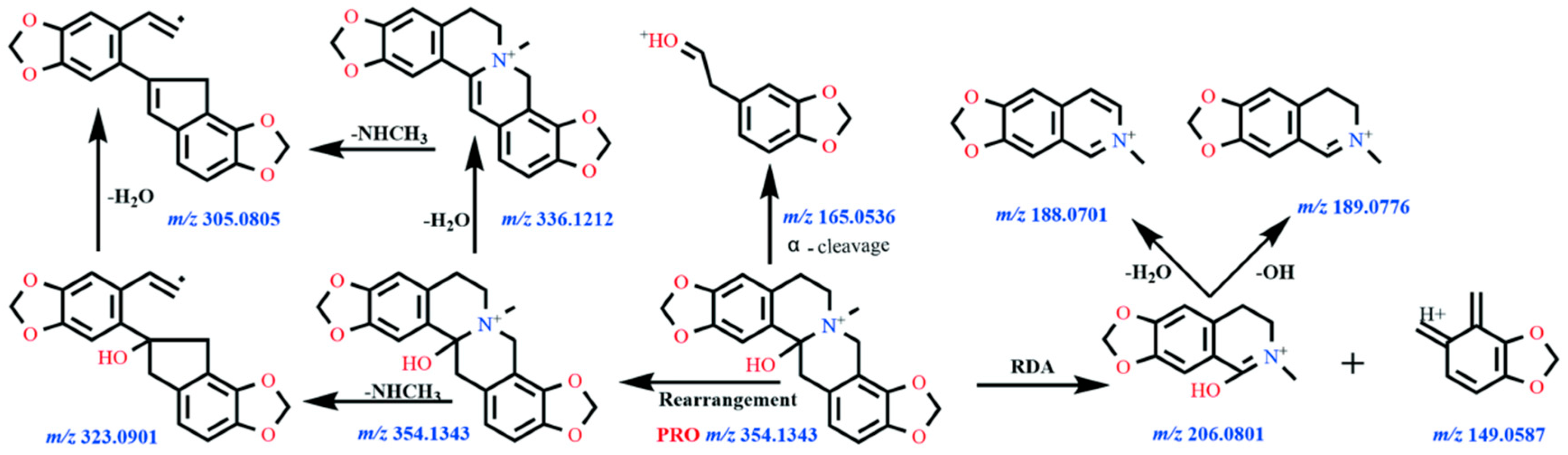
Fragmentation Pathways of ALL
Tentative Identification of ALL Metabolites in Laying Hens
- (1)
- Plasma
- (2)
- Fecal Samples
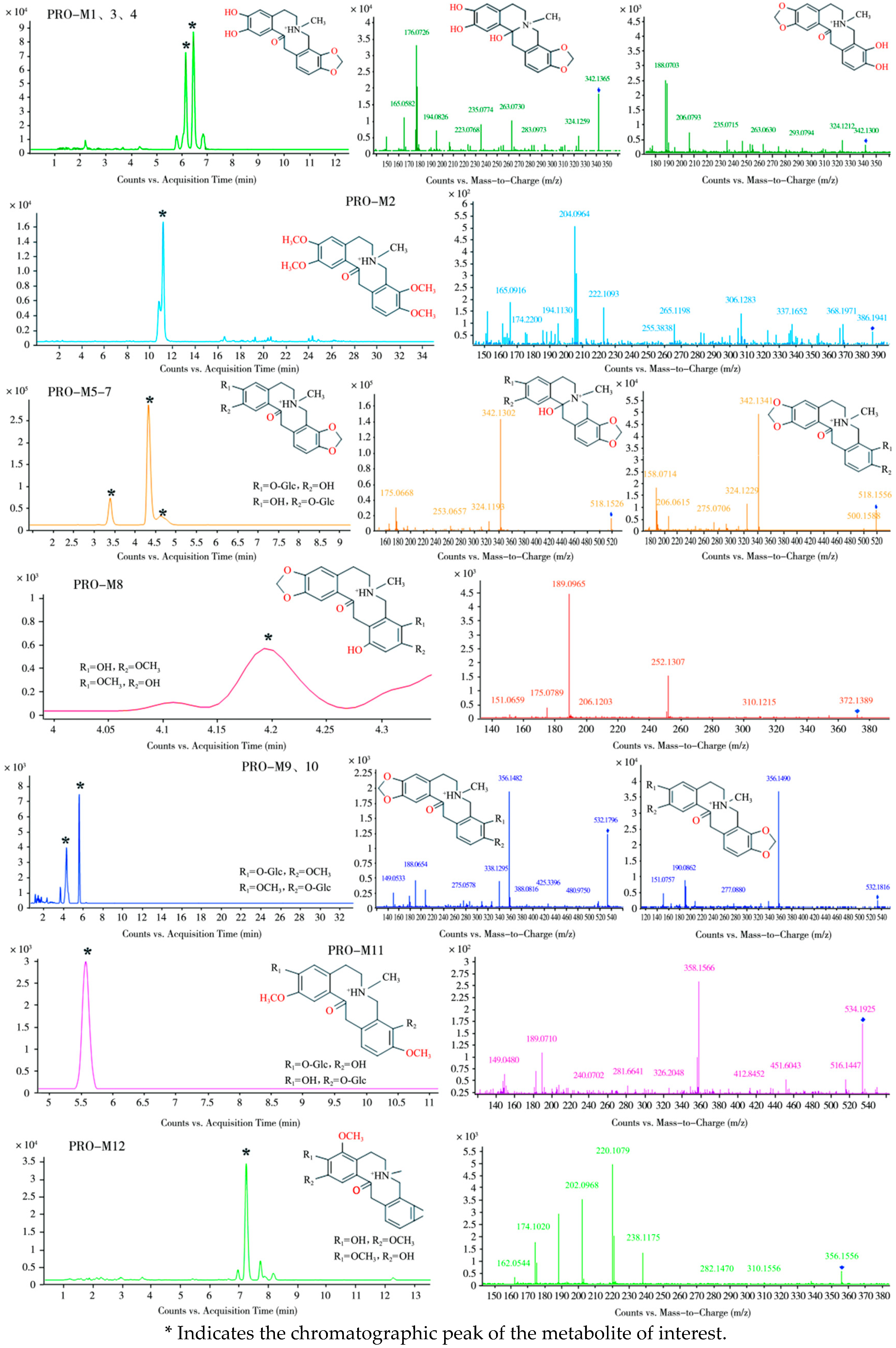
3.1.2. Metabolism of PRO in Laying Hens
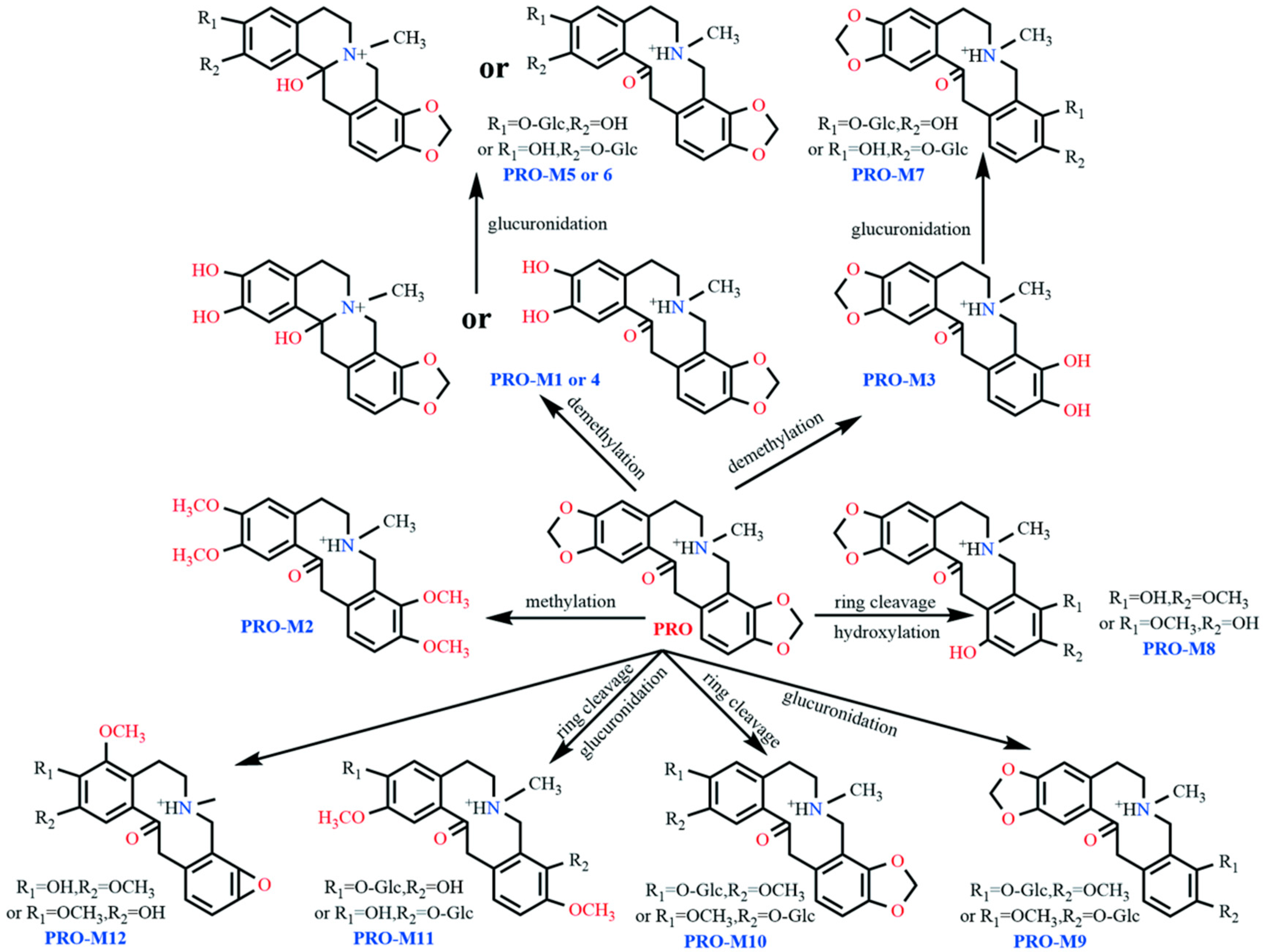
Fragmentation Pathways of PRO
Identification of PRO Metabolites in Laying Hens
- (1)
- Plasma
- (2)
- Feces
3.2. Residues of Bopu Powder in Laying Hens’ Tissues
3.3. Serum Biochemistry and Histopathological Examination
4. Discussion
4.1. Numbers and Types of Metabolites
4.2. Metabolic Pathways
4.3. Residues of Bopu Powder in Laying Hens’ Tissues
4.4. Tolerance Evaluation of Bopu Powder in Laying Hens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Zhang, R.; Lei, S.; Zuo, H.; Chen, S.; Chang, Y. Advances in Chemical Constituents and Bioactivities of Macleaya Cordata. Chin. J. Exp. Tradit. Med. Formulae 2019, 26, 243–250. [Google Scholar] [CrossRef]
- Wang, X.; Min, C.; Han, P. Antibacterial Effect of the Extracts from Different Parts of Macleya cordata Against Coliforms. Nat. Prod. Res. Dev. 2016, 28, 247–250+288. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Liu, Y.; Ji, X.; Liang, F. An overview of natural plant-derived anti-inflammatory agents. J. Exp. Tradit. Vet. Med. 2016, 35, 24–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Ma, W.; Liu, P.; Wu, Y.; Liu, Y. Advances in the Anticancer Activities of Sanguinarine from Macleaya cordata (Bo Luo Hui) in Shennongjia. J. Hubei Univ. Med. 2018, 37, 476–483. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Yang, Q.; Xiang, J.; Tang, Y.; Xv, G. Antitumor effect of Macleaya cordata and its molecular mechanism on inducement of human telomeric DNA to form G-quadruplex. Chin. Tradit. Herb. Drugs 2011, 42, 738–742. [Google Scholar]
- Dong, Z.; Xu, Y.; Liu, Z.; Zeng, J. Evaluation of the Efficacy of the Protopine Total Alkaloids of Macleaya Cordata (Willd), R. Br. in Controlling E. Coli Infection in Broiler Chickens. bioRxiv 2024. 2024.07.03.601902. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, H.; Niu, J.-I.; Jiao, N.; Huang, L.; Jiang, S.; Guan, Q.; Yang, W.; Li, Y. Effects of dietary Bopu Powder supplementation on intestinal development and microbiota in broiler chickens. Front. Microbiol. 2022, 13, 1019130. [Google Scholar] [CrossRef]
- Liu, H.; Lin, Q.; Liu, X.; Huang, P.; Yang, Z.; Cao, M.; Liu, M.; Li, X.; Zeng, J.; He, J. Effects of Dietary Bopu Powder Supplementation on Serum Antioxidant Capacity, Egg Quality, and Intestinal Microbiota of Laying Hens. Front. Physiol. 2022, 13, 902784. [Google Scholar] [CrossRef]
- Dong, Z.; Tang, S.-S.; Ma, X.-L.; Tan, B.; Tang, Z.-S.; Li, C.-H.; Yang, Z.-H.; Zeng, J.-G. Acute, Chronic, and Genotoxic Studies on the Protopine Total Alkaloids of the Macleaya Cordata (Willd.) R. Br. in Rodents. Front. Pharmacol. 2022, 13, 987800. [Google Scholar] [CrossRef]
- Hu, W.; Yang, F.; Liu, W.; Guo, L.; Ai, L.; Zhang, X.; Sheng, Z.; Gao, C. Potential Toxicity Evaluation of Protopine in Macleaya Cordata (Willd.) R. Br.—A Bioactivity Guided Approach. Front. Vet. Sci. 2021, 8, 752767. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, J.; Li, W.; Zhang, S.; Yang, L.; Teng, Y.; Lv, K.; Liu, Y.; Su, Y.; Zhang, J.; et al. Analgesic Effect of the Main Components of Corydalis Yanhusuo (Yanhusuo in Chinese) Is Caused by Inhibition of Voltage Gated Sodium Channels. J. Ethnopharmacol. 2021, 280, 114457. [Google Scholar] [CrossRef]
- Guo, C.; Jiang, Y.; Li, L.; Hong, L.; Wang, Y.; Shen, Q.; Lou, Y.; Hu, H.; Zhou, H.; Yu, L.; et al. Application of a Liquid Chromatography–Tandem Mass Spectrometry Method to the Pharmacokinetics, Tissue Distribution and Excretion Studies of Dactylicapnos Scandens in Rats. J. Pharm. Biomed. Anal. 2013, 74, 92–100. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Cheng, P.; Zhang, Z.-Y.; Tian, S.-J.; Sun, Z.-L.; Zeng, J.-G.; Liu, Z.-Y. Biotransformation and Tissue Distribution of Protopine and Allocryptopine and Effects of Plume Poppy Total Alkaloid on Liver Drug-Metabolizing Enzymes. Sci. Rep. 2018, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, J.; Jing, Y.; Yao, K.; Tang, Z.; Zeng, J.; Yang, Z. Residue of Allocryptopine and Protopine in Tissues and Eggs of Laying Hens Using Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry. Poult. Sci. 2025, 104, 105124. [Google Scholar] [CrossRef]
- GB/T 5916-2020; Formula Feed for Chicks, Laying Hens and Broilers. Standardization Administration of China (SAC): Beijing, China, 2020. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=7071118571C7E3C4412977B5392522E3 (accessed on 2 August 2025).
- Guidelines for Target Animal Tolerance Testing of Feed and Feed Additives in Livestock and Poultry; Ministry of Agriculture and Rural Affairs Announcement No. 424; Ministry of Agriculture and Rural Affairs: Beijing, China, 2021. Available online: https://www.moa.gov.cn/govpublic/XMYS/201107/t20110720_2064934.htm (accessed on 2 August 2025).
- AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 2 August 2025).
- Liu, M.; Hu, N.; Zou, X.; Yang, Z.; Zeng, J. Simultaneous Determination of 4 Isoquinoline Alkaloids in Broiler Tissues by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. China Anim. Husb. Vet. Med. 2022, 49, 4843–4853. [Google Scholar] [CrossRef]
- Huang, A.; Chi, Y.; Ou, L.; Chen, W.; Zhan, R.; Xv, H. Analysis of Metabolites of α-Allocryptopine in Rats Based on UPLC-Q-TOF-MS. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 1454–1461. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Wang, L.; Sun, N.; Yang, Z.; Zeng, J. Research on the Metabolites and Key Metabolic Enzymes of Allocryptopine in Chicken Liver Microsomes via Stable Isotope Tracing Technology. J. Pharm. Biomed. Anal. 2025, 255, 116667. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, M.; Shi, X.; Li, X.; Chen, X.; He, Z.; Li, J.; Xu, G.; Zheng, J. T329S Mutation in the FMO3 Gene Alleviates Lipid Metabolic Diseases in Chickens in the Late Laying Period. Animals 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zeng, M.; Li, M.; Kan, Y.; Li, B.; Xu, R.; Wu, Y.; Wang, S.; Zheng, X.; Feng, W. Protopine Protects Mice against LPS-Induced Acute Kidney Injury by Inhibiting Apoptosis and Inflammation via the TLR4 Signaling Pathway. Molecules 2019, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Huailing Wei, G.L. Protective Effects of Corydaline, Acetylcorydaline, and Protopine on Experimental Liver Injury in Mice. Acta Pharm. Sin. 1997, 32, 12–17. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. An Assessment of the Potential of Protopine to Inhibit Microsomal Drug Metabolising Enzymes and Prevent Chemical-Induced Hepatotoxicity in Rodents. Pharmacol. Res. 1998, 38, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhao, Z.; Tang, S.; Liu, H.; Wang, Q.; Xie, X.; Zhang, Y.; Song, L.; Xv, X. The reproductive toxicity test of Bopu Total Alkali Powder on breeding chickens. J. Yangzhou Univ. (Agric. Life Sci. Ed.) 2019, 40, 65–69. [Google Scholar] [CrossRef]





| Items | Diets |
|---|---|
| Ingredients, % | |
| Corn | 58 |
| Soybean meal | 27 |
| Soybean oil | 2.2 |
| Wheat bran | 1.5 |
| Limestone | 8.28 |
| CaHPO4·2H2O | 1.5 |
| NaCl | 0.2 |
| Na2SO4 | 0.2 |
| Met | 0.12 |
| 50% | 0.1 |
| Premix 1) | 1 |
| Total | 100.00 |
| Nutrient Contents 2) | |
| Metabolic energy, MJ/kg | 11.37 |
| Crude protein, % | 16.86 |
| Crude fiber, % | 3.03 |
| Ca, % | 3.63 |
| Total phosphorus, % | 0.61 |
| Available phosphorus, % | 0.35 |
| Lys, % | 0.87 |
| Met, % | 0.38 |
| Met + Cys, % | 0.66 |
| Analyte | Retention Time (min) | Monitored Ion Pair (m/z) | Fragmentor (V) | Collision Energy (eV) |
|---|---|---|---|---|
| TET | 5.5 | 356.0→192 * 356.0→165.0 | 142 | 26 |
| PRO | 3.8 | 354.1→189.0 * 354.1→149 | 128 | 34 |
| ALL | 5.0 | 370.1→188.1 * 370.1→290 | 114 | 22 |
| Metabolite | Retention Time | Proposed Formula | Measured | Calculated | Error (ppm) | Fragment Ions | Fragmentation Pathway |
|---|---|---|---|---|---|---|---|
| ALL | 11.3 | C21H24NO5+ | 370.1634 | 370.1649 | −4.05 | 352.1527, 206.079, 165.0897, 188.0692 | Absent |
| ALL-M1 | 6.7 | C20H22NO5+ | 356.1427 | 356.1492 | −18.25 | 338.1212, 275.055, 188.0662, 189.0785 | Demethylation |
| ALL-M2 | 4.1 | C26H30NO11+ | 532.1784 | 532.1813 | −5.45 | 356.1403, 338.1277, 188.0666, 189.0783 | Glucuronidation |
| ALL-M3 | 9.2 | C21H26NO5+ | 372.1765 | 372.1805 | −10.75 | 354.1721, 208.0862, 208.0862, 149.0607 | Ring Cleavage |
| ALL-M4 | 6.4 | C20H24NO5+ | 358.1627 | 358.1649 | −6.14 | 340.1428, 194.0762, 165.0854, 176.0685 | Ring Cleavage and Demethylation |
| ALL-M5 | 5.1 | C26H32NO11+ | 534.1958 | 534.1970 | −2.25 | 358.1717, 340.1412, 176.0701 | Glucuronidation |
| ALL-M6 | 9.7 | C21H24NO6+ | 386.1543 | 386.1593 | −12.95 | 368.1453, 297.1032, 222.0790, 204.0682 | Oxidation |
| ALL-M7 | 11.1 | C22H28NO5+ | 386.1949 | 386.1962 | −3.37 | 368.1857, 306.1114, 222.1243, 204.0965 | Methylation |
| ALL-M8 | 9.5 | C21H26NO5+ | 372.1727 | 372.1805 | −20.96 | 354.1720, 208.0797, 189.0758, 149.0607 | Ring Cleavage |
| ALL-M9 | 7.9 | C20H22NO5+ | 356.1445 | 356.1492 | −13.20 | 338.1220, 275.0637, 188.0701, 189.0780 | Demethylation |
| Metabolite | Retention Time | Proposed Formula | Measured | Calculated | Error (ppm) | Fragment Ions | Fragmentation Pathway |
|---|---|---|---|---|---|---|---|
| PRO | 10.3 | C20H20NO5+ | 354.1343 | 354.1336 | 1.98 | 336.124, 149.0594, 206.0797 165.053, 189.0773, 188.0696 | Absent |
| PRO-M1 | 5.6 | C19H20NO5+ | 342.1342 | 342.1336 | 1.75 | 324.126, 194.0732, 149.1772, 176.0626, 177.0768 | Ring cleavage, demethylation, or rearrangement; ring cleavage and demethylation |
| PRO-M2 | 11.1 | C22H28NO5+ | 386.1941 | 386.1962 | −5.44 | 368.197, 222.1093, 165.0916, 204.0964, 205.1066 | Methylation |
| PRO-M3 | 6.1 | C19H20NO5+ | 342.1300 | 342.1336 | −10.52 | 324.121, 206.0793, 188.0703, 189.0786 | Ring cleavage and demethylation |
| PRO-M4 | 6.4 | C19H20NO5+ | 342.1365 | 342.1336 | 8.48 | 324.123, 194.0730, 149.1692, 176.061, 177.0765 | Ring cleavage, demethylation, or rearrangement; ring cleavage and demethylation |
| PRO-M5 | 3.3 | C25H28NO11+ | 518.1633 | 518.1657 | −4.63 | 500.155, 342.1335, 324.1236, 176.0701 | Glucuronidation |
| PRO-M6 | 4.3 | C25H28NO11+ | 518.1626 | 518.1657 | −5.98 | 342.130, 324.1193, 194.0785, 176.0668 | Glucuronidation |
| PRO-M7 | 4.6 | C25H28NO11+ | 518.1656 | 518.1657 | −0.19 | 342.134, 324.1229, 206.0816, 188.0714 | Glucuronidation |
| PRO-M8 | 4.2 | C20H22NO6+ | 372.1389 | 372.1442 | −14.24 | 206.120, 189.0965, 151.0659, 167.0703 | Ring cleavage and hydroxylation |
| PRO-M9 | 4.3 | C26H30NO11+ | 532.1796 | 532.1813 | −3.19 | 356.148, 338.1295, 206.0812, 188.0654, 189.0785 | Glucuronidation |
| PRO-M10 | 5.4 | C26H30NO11+ | 532.1816 | 532.1813 | 0.56 | 356.148, 338.1295, 208.0968, 190.0862, 191.0941 | Glucuronidation |
| PRO-M11 | 5.6 | C26H32NO11+ | 534.1925 | 534.1970 | −8.42 | 358.1566 | Glucuronidation |
| PRO-M12 | 7.2 | C20H22NO5+ | 356.1556 | 356.1492 | 17.97 | 238.1479, 220.1079, 202.0968, 188.0983 | Methylation, etc. |
| Tissues | Analytes | CN | BP | BPX |
|---|---|---|---|---|
| Eggs | PRO | ND | ND | 26.86 ± 15.12 |
| ALL | ND | ND | 12.29 ± 4.69 | |
| Breast muscle | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Thigh muscle | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Skin with fat | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Abdominal fat | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Gizzard | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Plasma | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Liver | PRO | ND | ND | ND |
| ALL | ND | ND | 56.14 ± 9.39 | |
| Kidney | PRO | ND | 11.21 ± 8.87 | 23.62 ± 6.94 |
| ALL | ND | 6.59 ± 4.61 | 7.92 ± 4.12 | |
| Jejunum | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Ileum | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Ovary | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Oviduct | PRO | ND | ND | ND |
| ALL | ND | ND | ND | |
| Uterus | PRO | ND | ND | ND |
| ALL | ND | ND | ND |
| Item | CN | BP | BPX | SEM | p-Value |
|---|---|---|---|---|---|
| ALT, U/L | 62.48 b | 38.30 a | 32.65 a | 7.738 | 0.032 |
| AST, U/L | 194.10 b | 130.62 a | 126.62 a | 5.58 | 0.035 |
| LDH, U/L | 347.38 | 236.50 | 235.80 | 17.202 | 0.161 |
| T-Bil, μmol/L | 46.40 | 47.90 | 49.26 | 0.506 | 0.077 |
| ALB, g/L | 5.97 | 9.22 | 8.63 | 0.669 | 0.192 |
| GLU, mmol/L | 9.04 | 11.69 | 9.71 | 0.624 | 0.320 |
| UREA, mmol/L | 1.06 | 2.06 | 0.97 | 0.221 | 0.205 |
| TG, mmol/L | 16.67 b | 5.71 a | 10.01 ab | 1.722 | 0.039 |
| TC, mmol/L | 6.36 b | 3.82 a | 5.44 ab | 0.305 | 0.008 |
| HDL-C, mmol/L | 0.156 b | 0.216 a | 0.164 b | 0.007 | <0.001 |
| LDL-C, mmol/L | 0.664 | 0.501 | 0.434 | 0.098 | 0.713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, X.; Xu, J.; Yang, Z.; Dong, Z.; Zeng, J.; Liu, H. Metabolic Profiling, Tissue Distribution, and Tolerance Assessment of Bopu Powder in Laying Hens Following Long-Term Dietary Administration. Vet. Sci. 2025, 12, 848. https://doi.org/10.3390/vetsci12090848
Wang H, Wang X, Xu J, Yang Z, Dong Z, Zeng J, Liu H. Metabolic Profiling, Tissue Distribution, and Tolerance Assessment of Bopu Powder in Laying Hens Following Long-Term Dietary Administration. Veterinary Sciences. 2025; 12(9):848. https://doi.org/10.3390/vetsci12090848
Chicago/Turabian StyleWang, Hongting, Xinhao Wang, Jiaxin Xu, Zihui Yang, Zhen Dong, Jianguo Zeng, and Hua Liu. 2025. "Metabolic Profiling, Tissue Distribution, and Tolerance Assessment of Bopu Powder in Laying Hens Following Long-Term Dietary Administration" Veterinary Sciences 12, no. 9: 848. https://doi.org/10.3390/vetsci12090848
APA StyleWang, H., Wang, X., Xu, J., Yang, Z., Dong, Z., Zeng, J., & Liu, H. (2025). Metabolic Profiling, Tissue Distribution, and Tolerance Assessment of Bopu Powder in Laying Hens Following Long-Term Dietary Administration. Veterinary Sciences, 12(9), 848. https://doi.org/10.3390/vetsci12090848








