Comprehensive Prevention and Control of Mastitis in Dairy Cows: From Etiology to Prevention
Simple Summary
Abstract
1. Introduction
2. Methods
3. The Causes of Mastitis
3.1. Pathogenic Microbial Infections
3.1.1. Staph. aureus
3.1.2. Strep. agalactiae
3.1.3. Strep. dysgalactiae
3.1.4. E. coli
3.1.5. CNS
3.1.6. K. pneumoniae
3.2. Environment and Management
3.3. Species and Physiological Status of Cows
3.4. Molecular Mechanisms of Mastitis
4. Diagnostic Methods for Bovine Mastitis
4.1. Monitoring of Apparent Indicators in Milk
4.1.1. CMT
4.1.2. EC
4.1.3. Other Test Indicators in Milk
| Particular Year | Biomarker | Expression in Mastitis | Description | References |
|---|---|---|---|---|
| 2023 | Procalcitonin | Upregulation | Its concentration can be used to differentiate between CM, SCM and healthy cows. | [176] |
| 2024 | miR-146a, miR-383 | Upregulation | miRNA expression profiles can serve as potential biomarkers. | [175] |
| 2021 | Lactose | Downregulation | Changes in lactose content as a diagnostic method for the prevention of SCM in dairy cattle. | [174] |
| 2021 | CATH, HP, milk amyloid A(MAA) | Upregulation | They can distinguish between healthy, SCM and CM cows. | [177] |
| 2024 | CATHs, LF, HP | Upregulation | They are good biomarker candidates for early SCM. | [178] |
| 2025 | miR-148a, miR-186 | Upregulation | They have the potential to be reliable biomarkers for SCM in buffaloes. | [179] |
| 2025 | Asymmetrical dimethylarginine | Upregulation | It can be a biomarker for detecting SCM and CM. | [180] |
| 2025 | miR-223-3p | Upregulation | Their diagnostic potential may be related to the different stages of mastitis. | [181] |
| miR-26a-5p | Downregulation | |||
| 2024 | miR-27a-3p, miR-223 | Upregulation | They are potential biomarkers for SCM, especially that caused by Staphylococcus spp. and Streptococcus spp. | [182] |
| 2022 | M-SAA, HP, CATH, LF | Upregulation | They can be used as biomarkers for mastitis. | [183] |
| 2024 | CRP, Hp, MAA, LF, CATH | Upregulation | The use of multiple markers may have the potential to differentiate mastitis pathogens. | [184] |
| α-LA | Downregulation | |||
| 2024 | miR-361 | Upregulation | They may be biomarkers of SCM. | [185] |
| miR-455, miR-1301, miR-503 | Downregulation | |||
| 2023 | DEFB-4 | Upregulation | It identifies acute mastitis and SCM in cows. | [168] |
| 2021 | IL-6, CRP | Upregulation | Their levels can be used as biomarkers to assess the severity of idiopathic granulomatous mastitis. | [170] |
4.2. Detection of Pathogenic Microorganism
4.2.1. Bacterial Culture (BC)
4.2.2. PCR
4.2.3. ELISA
4.3. Physiological Signs Monitoring
4.3.1. Ultrasound
4.3.2. IRT
4.4. Omics Technology
4.4.1. Genomics Approaches
4.4.2. Proteomics Approaches
4.4.3. Metabolomics Approaches
4.5. Emerging Technology
4.5.1. Biochips
4.5.2. Nanotechnology
5. Treatment Methods for Bovine Mastitis
5.1. Antibiotic Therapy
5.2. Herbal Therapy
| Particular Year | Herbs/Extracts | Research Target | Model Type | Finding | References |
|---|---|---|---|---|---|
| 2024 | Baicalin | BMECs | In vitro | It attenuates H2O2-induced oxidative damage in BMECs by activating the Keap1/Nrf2 signaling pathway and increasing the expression of the downstream antioxidant genes NQO1 and HO-1 as well as the antioxidant systems SOD and T-AOC. | [235] |
| 2020 | Matrine, Baicalin | BMECs | In vitro | Matrine decreased the protein expression levels of endogenous and exogenous cleaved caspase-3, cleaved caspase-8, and cleaved caspase-9, and baicalein down-regulated the expression of cleaved caspase-9 and inhibited apoptosis in BMECs. | [244] |
| 2025 | Baicalin | Kunming mice | In vitro and in vivo | LAB-CFS together with baicalein controls the activation of the NF-κB signaling pathway and thus reduces the levels of inflammatory cytokines associated with mastitis. | [236] |
| 2023 | Baicalin | BMECs | In vitro | It inhibits cofilin phosphorylation or Tau hyperphosphorylation by regulating the activation of RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β signaling pathways. | [245] |
| 2024 | Baicalein | Swiss albino mice | In vivo | Chitosan-encapsulated baicalein in a triple complex with beta-lactam antibiotics significantly reduced mean blood leukocyte and neutrophil counts and inhibited matrix MMP-9 concentrations and CRP responses. | [234] |
| 2025 | Astragalus polysaccharide | ICR mice | In vivo | It reduces Staph. aureus colonization in the mammary gland by regulating intestinal flora and short-chain fatty acid metabolism, moderates inflammatory responses, protects the BMB and prevents oxidative stress retardation. | [50] |
| 2025 | Astragalus polysaccharide | Kunming mice, MAC-T | In vitro and in vivo | It reduces the EMT process through the ROS/NLRP3 signaling pathway and inhibits breast fibrosis | [246] |
| 2022 | Astragalus polysaccharide, astragaloside IV | BMECs | In vitro | They attenuate inflammation in BMECs by modulating the Wnt/β-cyclin signaling pathway. | [247] |
| 2024 | Angelica sinensis polysaccharide (ASP) | BALB/c mice | In vivo | Oral ASP may modulate the levels of intestinal metabolites by affecting the diversity and composition of the intestinal flora, thereby reducing breast inflammation and maintaining the integrity of the BMB. | [49] |
| 2022 | Lycium barbarum polysaccharides | BMECs | In vitro | It attenuates LPS-induced inflammatory response in BMECs through PPARγ/MAPK/NF-κB pathway. | [248] |
| 2024 | Ginsenoside Rg1 | Holstein Friesian cows, MAC-T | In vitro and in vivo | It can attenuate BMB destruction by activating PPARγ to inhibit oxidative stress and subsequent excessive autophagy in subclinical bovine mastitis. | [249] |
| 2024 | Triterpenoid saponin | MRSA strain IID1677 | In vitro | It increases the susceptibility of MRSA to β-lactam and aminoglycoside antibiotics. | [250] |
| 2020 | Curcumin | mice, murine mammary epithelial cells (MMECs) | In vitro and in vivo | It ameliorates Staph. aureus-induced mastitis injury by attenuating TLR2-mediated NF-κB activation. | [251] |
| 2021 | Curcumin | MAC-T | In vitro | It attenuates LPS-induced oxidative stress, inflammation and apoptosis in BMECs through the NFE2L2 signaling pathway. | [237] |
| 2022 | Curcumin | Wistar Albino rats | In vivo | Nanocurcumin alleviates inflammation and oxidative stress in LPS-induced mastitis by activating Nrf2 and inhibiting TLR4-mediated NF-κB and HMGB1 signaling pathways. | [238] |
| 2022 | Curcumin | mice | In vivo | Administration of nanocurcumin in Staph. aureus mastitis reduces oxidative stress markers, reverses antioxidant depletion and restores the histological structure of the mammary gland. | [252] |
| 2025 | Curcumin | BALB/c mice, BMECs | In vitro and in vivo | Curcumin binds to the ZIF-8 to form ZIF-8@CCM, which attenuates Staph. aureus-induced inflammation by inhibiting activation of the TLR2-NF-κB pathway. | [239] |
| 2021 | Curcumin quercetin | Dairy herds, milk sample | In vitro and in vivo | They positively affect cell migration, ROS generation, phagocytosis and bacterial killing, as well as the release of neutrophil extracellular traps (NETs). | [240] |
| 2022 | Quercetin | BMECs | In vitro | It alleviates the LPS-induced inflammatory response in BMECs by inhibiting the TLR4-mediated NF-κB signaling pathway. | [253] |
| 2023 | Quercetin | Milk sample | In vitro | The combination of it and gentamicin effectively inhibited P. aeruginosa and its biofilm formation and showed synergistic and additive effects. | [254] |
| 2025 | Quercetin | BALB/c mice, MAC-T | In vitro and in vivo | It attenuates Staph. aureus-induced inflammation in BMEC by disrupting cell adhesion and regulating CCL5 expression through the m6A-YTHDF2-dependent pathway. | [255] |
| 2021 | Forsythoside A | BALB/c mice | In vivo | It effectively inhibited LPS-induced mammary gland inflammation in mice by attenuating the activation of NF-κB and p38 MAPK signaling pathways. | [256] |
| 2023 | Forsythoside A | BMECs | In vitro | Its protective effect on BMECs after injury. | [257] |
| 2023 | Forsythoside A | MAC-T | In vitro | It regulates autophagy and apoptosis through the AMPK/mTOR/ULK1 pathway and attenuates inflammatory damage in MAC-T cells. | [258] |
| 2023 | Forsythoside A | MAC-T | In vitro | It altered the expression of spliceosome, lysosome and oxidative stress-related genes and reduced the effects of LPS on inflammation and oxidative stress in BMECs. | [259] |
| 2024 | Forsythoside A | Kunming mice, MAC-T | In vitro and in vivo | It attenuates LPS-induced inflammatory injury through PINK1/Parkin-mediated mitochondrial autophagy. | [260] |
| 2023 | Puerarin | Dairy herds, BMECs | In vitro and in vivo | It effectively reduces oxidative stress in BMECs, enhances intercellular tight junctions, and acts as an anti-inflammatory agent. | [261] |
| 2023 | Hordenine | ICR mice, EpH4-Ev | In vitro and in vivo | It alters the composition of the gut microbiota in mice, reduces the extent of inflammatory damage and upregulates the expression of tight junction proteins. | [262] |
| 2024 | Salvia officinalis | MAC-T | In vitro | It was able to effectively inhibit biofilm formation of Staph. aureus even at sub-inhibitory concentrations. | [263] |
| 2023 | Geraniol-a | Kunming mice, Holstein cows, | In vivo | It shows comparable therapeutic rates to antibiotics and significantly inhibits pathogenic bacteria and restores microbial communities while increasing the abundance of probiotics in milk. | [264] |
| 2023 | Resveratrol | Mice | In vivo | It does this mainly by combating NF-κB and MAPK mechanisms, by Nrf2 signaling activation, which reduces pro-inflammatory cytokine production and increases antioxidant levels. | [241] |
| 2023 | Resveratrol | BMECs | In vitro | It alleviated LPS-induced apoptosis in BMECs through the PGC1α-SIRT3 axis. | [242] |
| 2025 | Resveratrol | BMECs | In vitro | It activates the PGC-1α pathway via PRKAA1 and enhances mitochondrial antioxidant capacity, thereby attenuating the inflammatory response in BMECs. | [243] |
| 2022 | Tea tree oil, thymol, carvacrol | Staph. aureus ATCC BAA976 | In vitro | The combination of the three inhibits Gram-negative bacteria and Candida albicans activity. | [265] |
| 2022 | Quercus robur, Calluna vulgaris L. | Six bovine mastitis clinical isolates | In vitro | They may be used as antimicrobial agents in bovine mastitis. | [266] |
| 2021 | Origanum vulgare L., Satureja montana L. | Holstein-Friesian cows | In vitro and in vivo | They may be the solution for mastitis treatment. | [267] |
| 2022 | Vitexin | Kunming mice, MAC-T | In vitro and in vivo | It promotes PPARγ to inhibit ROS production, increase antioxidant enzyme activity, and reduce inflammatory cytokines and apoptosis by alleviating endoplasmic reticulum stress and inactivating MAPKs and NF-κB signaling pathways. | [268] |
5.3. Bacteriophage Therapy
5.4. AMPs Therapy
5.5. Nanoparticle Therapy
5.6. Probiotics Therapy
5.7. Stem Cell Therapy
5.8. Physical Therapy
5.8.1. APT
5.8.2. Laser Radiation Therapy
5.8.3. PDT
6. Preventive Measures for Bovine Mastitis
6.1. Environmental and Hygiene Management
6.2. Cow Health Management
6.2.1. Host Factor
6.2.2. Intervention Measures
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy. Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.D.; Gillespie, B.E.; Ivey, S.; Pighetti, G.M.; Almeida, R.A.; Kerro Dego, O. Antimicrobial Resistance of Major Bacterial Pathogens from Dairy Cows with High Somatic Cell Count and Clinical Mastitis. Animals 2021, 11, 131. [Google Scholar] [CrossRef]
- Fox, L.K. Prevalence, incidence and risk factors of heifer mastitis. Vet. Microbiol. 2009, 134, 82–88. [Google Scholar] [CrossRef]
- Gruet, P.; Maincent, P.; Berthelot, X.; Kaltsatos, V. Bovine mastitis and intramammary drug delivery: Review and perspectives. Adv. Drug Deliv. Rev. 2001, 50, 245–259. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy. Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Tančin, V.; Uhrinčat, M. The effect of somatic cell on milk yield and milk flow at quarter level. Vet. Ir Zootech. 2014, 66, 69–72. [Google Scholar]
- Dalanezi, F.M.; Joaquim, S.F.; Guimarães, F.F.; Guerra, S.T.; Lopes, B.C.; Schmidt, E.M.S.; Cerri, R.L.A.; Langoni, H. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy. Sci. 2020, 103, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Razooqi, M.A.; Mounam, M.; Saleem, H.D. Article Review: Changes In Cows’ Milk Quantity And Quality Due To Bacterial Contamination. Nveo-Nat. Volatiles Essent. Oils J. 2021, 8, 2550–2561. [Google Scholar]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Pascu, C.; Herman, V.; Iancu, I.; Costinar, L. Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania. Antibiotics 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Samardžija, M.; Horvat, O.; Tomanić, D.; Radinović, M.; Bijelić, K.; Vukomanović, A.G.; Kladar, N. Is There a Relationship between Antimicrobial Use and Antibiotic Resistance of the Most Common Mastitis Pathogens in Dairy Cows? Antibiotics 2022, 12, 3. [Google Scholar] [CrossRef]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef]
- Huijps, K.; Lam, T.J.; Hogeveen, H. Costs of mastitis: Facts and perception. J. Dairy. Res. 2008, 75, 113–120. [Google Scholar] [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.; Bhadwal, M. Relationship of somatic cell count and mastitis: An overview. Asian-Australas. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Hussein, H.A.; El-Razik, K.; Gomaa, A.M.; Elbayoumy, M.K.; Abdelrahman, K.A.; Hosein, H.I. Milk amyloid A as a biomarker for diagnosis of subclinical mastitis in cattle. Vet. World 2018, 11, 34–41. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population-a review. Vet. Q. 2019, 39, 76–94. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Sękowska, A.; Kaczmarek, A.; Grudlewska, K.; Budzyńska, A.; Białucha, A.; Gospodarek-Komkowska, E. Comparison of the effectiveness of dipping agents on bacteria causing mastitis in cattle. Ann. Agric. Environ. Med. 2019, 26, 39–45. [Google Scholar] [CrossRef]
- Park, Y.K.; Fox, L.K.; Hancock, D.D.; McMahan, W.; Park, Y.H. Prevalence and antibiotic resistance of mastitis pathogens isolated from dairy herds transitioning to organic management. J. Vet. Sci. 2012, 13, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Babra, C.; Tiwari, J.G.; Pier, G.; Thein, T.H.; Sunagar, R.; Sundareshan, S.; Isloor, S.; Hegde, N.R.; de Wet, S.; Deighton, M.; et al. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiol. 2013, 58, 469–474. [Google Scholar] [CrossRef]
- Collado, R.; Prenafeta, A.; González-González, L.; Pérez-Pons, J.A.; Sitjà, M. Probing vaccine antigens against bovine mastitis caused by Streptococcus uberis. Vaccine 2016, 34, 3848–3854. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef]
- Bradley, A.J.; Breen, J.E.; Payne, B.; White, V.; Green, M.J. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. J. Dairy. Sci. 2015, 98, 1706–1720. [Google Scholar] [CrossRef] [PubMed]
- Côté-Gravel, J.; Malouin, F. Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies. J. Dairy. Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef]
- Castelani, L.; Arcaro, J.R.P.; Braga, J.E.P.; Bosso, A.S.; Moura, Q.; Esposito, F.; Sauter, I.P.; Cortez, M.; Lincopan, N. Short communication: Activity of nisin, lipid bilayer fragments and cationic nisin-lipid nanoparticles against multidrug-resistant Staphylococcus spp. isolated from bovine mastitis. J. Dairy. Sci. 2019, 102, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro Machado, G.T.; Veleirinho, M.B.; Mazzarino, L.; Machado Filho, L.C.P.; Maraschin, M.; Cerri, R.L.A.; Kuhnen, S. Development of propolis nanoparticles for the treatment of bovine mastitis: In vitro studies on antimicrobial and cytotoxic activities. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar] [CrossRef]
- Abera, M.; Demie, B.; Aragaw, K.; Regassa, F.; Regassa, A. Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J. Vet. Med. Anim. Health 2010, 2, 29–34. [Google Scholar]
- Abdalhamed, A.M.; Zeedan, G.S.G.; Zeina, H. Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Vet. World 2018, 11, 355–362. [Google Scholar] [CrossRef]
- Abdalhamed, A.M.; Ghazy, A.A.; Ibrahim, E.S.; Arafa, A.A.; Zeedan, G.S.G. Therapeutic effect of biosynthetic gold nanoparticles on multidrug-resistant Escherichia coli and Salmonella species isolated from ruminants. Vet. World 2021, 14, 3200–3210. [Google Scholar] [CrossRef]
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 166–185. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Suresh, K.P.; Jayamma, K.S.; Shome, B.R.; Patil, S.S.; Amachawadi, R.G. An Understanding of the Global Status of Major Bacterial Pathogens of Milk Concerning Bovine Mastitis: A Systematic Review and Meta-Analysis (Scientometrics). Pathogens 2021, 10, 545. [Google Scholar] [CrossRef]
- McParland, S.; Dillon, P.G.; Flynn, J.; Ryan, N.; Arkins, S.; Kennedy, A. Effect of using internal teat sealant with or without antibiotic therapy at dry-off on subsequent somatic cell count and milk production. J. Dairy. Sci. 2019, 102, 4464–4475. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A. Basic facts of mastitis in dairy animals: A review. Pak. Vet. J. 2006, 26, 204. [Google Scholar]
- Petersson-Wolfe, C.S.; Mullarky, I.K.; Jones, G.M. Staphylococcus Aureus Mastitis: Cause, Detection, and Control. 2010. Available online: http://hdl.handle.net/10919/48390 (accessed on 29 July 2025).
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Gröhn, Y.T. Pathogen-specific effects on milk yield in repeated clinical mastitis episodes in Holstein dairy cows. J. Dairy. Sci. 2014, 97, 1465–1480. [Google Scholar] [CrossRef]
- Fagundes, H.; Barchesi, L.; Filho, A.N.; Ferreira, L.M.; Oliveira, C.A. Occurrence of Staphylococcus aureus in raw milk produced in dairy farms in São Paulo state, Brazil. Braz. J. Microbiol. 2010, 41, 376–380. [Google Scholar]
- Keefe, G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet. Clin. North. Am. Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef]
- Tomazi, T.; de Souza Filho, A.F.; Heinemann, M.B.; Dos Santos, M.V. Molecular characterization and antimicrobial susceptibility pattern of Streptococcus agalactiae isolated from clinical mastitis in dairy cattle. PLoS ONE 2018, 13, e0199561. [Google Scholar] [CrossRef]
- Wente, N.; Krömker, V. Streptococcus dysgalactiae—Contagious or environmental? Animals 2020, 10, 2185. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; Liski, E.; Heikkilä, A.M.; Pyörälä, S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J. Dairy. Sci. 2017, 100, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef]
- Zaatout, N. An overview on mastitis-associated Escherichia coli: Pathogenicity, host immunity and the use of alternative therapies. Microbiol. Res. 2022, 256, 126960. [Google Scholar] [CrossRef] [PubMed]
- El-Jakee, J.K.; Aref, N.E.; Gomaa, A.; El-Hariri, M.D.; Galal, H.M.; Omar, S.A.; Samir, A. Emerging of coagulase negative staphylococci as a cause of mastitis in dairy animals: An environmental hazard. Int. J. Vet. Sci. Med. 2013, 1, 74–78. [Google Scholar] [CrossRef]
- Deng, J.; Liu, K.; Wang, K.; Yang, B.; Xu, H.; Wang, J.; Dai, F.; Xiao, X.; Gu, X.; Zhang, L.; et al. The prevalence of coagulase-negative staphylococcus associated with bovine mastitis in China and its antimicrobial resistance rate: A meta-analysis. J. Dairy. Res. 2023, 90, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Elhaig, M.M.; El-Tarabili, R.M.; Wahdan, A. A report of coagulase-negative Staphylococci from clinically incurable cases of bovine mastitis: Prevalence, biofilm formation, and resistance profile. Iran. J. Vet. Res. 2024, 25, 279–284. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, J.; Han, B.; Barkema, H.W.; Cobo, E.R.; Kastelic, J.P.; Zhou, M.; Shi, Y.; Wang, J.; Yang, R.; et al. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cells. J. Dairy. Sci. 2020, 103, 3493–3504. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Hare, W.R.; Hope, J.C. Characterization of the bovine innate immune response to intramammary infection with Klebsiella pneumoniae. J. Dairy. Sci. 2004, 87, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Li, Y.; Guo, W.; Li, K.; Guo, W.; Wang, X.; Liu, J.; Bi, J.; Fu, S. Angelica sinensis Polysaccharide Alleviates Staphylococcus aureus-Induced Mastitis by Regulating The Intestinal Flora and Gut Metabolites. J. Agric. Food Chem. 2024, 72, 24504–24517. [Google Scholar] [CrossRef]
- Li, K.; Ran, X.; Han, J.; Ding, H.; Wang, X.; Li, Y.; Guo, W.; Li, X.; Guo, W.; Fu, S.; et al. Astragalus polysaccharide alleviates mastitis disrupted by Staphylococcus aureus infection by regulating gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2025, 286, 138422. [Google Scholar] [CrossRef]
- Liu, X.; Mi, S.; Dari, G.; Chen, S.; Song, J.; MacHugh, D.E.; Yu, Y. Functional validation to explore the protective role of miR-223 in Staphylococcus aureus-induced bovine mastitis. J. Anim. Sci. Biotechnol. 2025, 16, 34. [Google Scholar] [CrossRef]
- Ucella-Filho, J.G.M.; Ferreira, N.S.; Lacerda, E.M.F.; Neto, C.S.; de Souza Duarte, C.E.; Ferreira, I.M.; Balu, A.M.; Ignacchiti, M.D.C.; Junior, A.F.D.; Resende, J.A. Citrus sinensis waste wood pyroligneous extract as an effective agent against biofilms formed by mastitis-causing Staphylococcus aureus. Ind. Crops Prod. 2024, 218, 118854. [Google Scholar] [CrossRef]
- Wiarda, J.E.; Davila, K.M.S.; Trachsel, J.M.; Loving, C.L.; Boggiatto, P.; Lippolis, J.D.; Putz, E.J. Single-cell RNA sequencing characterization of Holstein cattle blood and milk immune cells during a chronic Staphylococcus aureus mastitis infection. Sci. Rep. 2025, 15, 12689. [Google Scholar] [CrossRef] [PubMed]
- Kerro Dego, O.; Vidlund, J. Staphylococcal mastitis in dairy cows. Front. Vet. Sci. 2024, 11, 1356259. [Google Scholar] [CrossRef]
- JOSE, K.R.; VIJAYAKUMAR, K. Antimicrobial resistance profiling of coagulase negative staphylococci isolated from bovine mastitis. 2024, 94, 308–314.
- Seker, E.; Ozenc, E.; Turedi, O.K.; Yilmaz, M. Prevalence of mecA and pvl genes in coagulase negative staphylococci isolated from bovine mastitis in smallholder dairy farms in Turkey. Anim. Biotechnol. 2023, 34, 2427–2432. [Google Scholar] [CrossRef]
- Gerez, G.; Hernandez, L.B.; Cadona, J.; Sanso, A.M.; Bustamante, A.V. Genetic diversity of Streptococcus agalactiae from dairy cattle with mastitis in Argentina. BMC Vet. Res. 2025, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.B.R.; Garcia, B.L.N.; Fidelis, C.E.; Barbosa, K.D.S.; Dantas, S.T.A.; Rall, V.L.M.; Dos Santos, M.V. Comparative analysis of MALDI-TOF MS and RAPD for grouping Streptococcus agalactiae isolated from subclinical mastitis isolates. Microb. Pathog. 2025, 204, 107538. [Google Scholar] [CrossRef]
- Guo, S.; Wang, K.; Zhang, H.; Luo, C.; Zhao, Z.; Tong, J. Matrine Attenuates Streptococcus agalactiae Virulence by Suppressing Capsular Polysaccharide Synthesis and Host Adhesion Pathways. Microorganisms 2025, 13, 1192. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Yang, J.; Gu, X.; Zhang, L.; Qu, W. Streptococcus agalactiae isolated from clinical mastitis cases on large dairy farms in north China: Phenotype, genotype of antimicrobial resistance and virulence genes. Front. Cell Infect. Microbiol. 2024, 14, 1417299. [Google Scholar] [CrossRef]
- Wataradee, S.; Boonserm, T.; Samngamnim, S.; Ajariyakhajorn, K. Characterization of Virulence Factors and Antimicrobial Susceptibility of Streptococcus agalactiae Associated with Bovine Mastitis Cases in Thailand. Animals 2024, 14, 447. [Google Scholar] [CrossRef]
- Miotti, C.; Cicotello, J.; Suarez Archilla, G.; Neder, V.; Alvarado Lucero, W.; Calvinho, L.; Signorini, M.; Camussone, C.; Zbrun, M.V.; Molineri, A.I. Antimicrobial resistance of Streptococcus uberis isolated from bovine mastitis: Systematic review and meta-analysis. Res. Vet. Sci. 2023, 164, 105032. [Google Scholar] [CrossRef]
- Thomas, C.; Linde, J.; El-Adawy, H.; Wedlich, N.; Hruschka, K.; Einax, E.; Donat, K.; Berens, C.; Tomaso, H. Whole-genome sequencing of Streptococcus uberis isolated from cows with mastitis in Thuringia. J. Med. Microbiol. 2024, 73, 001887. [Google Scholar] [CrossRef]
- Zouharova, M.; Nedbalcova, K.; Matiaskova, K.; Slama, P.; Matiasovic, J. Antimicrobial Susceptibility and Resistance Genes in Streptococcus uberis Isolated from Bovine Mastitis in the Czech Republic. Antibiotics 2023, 12, 1527. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Fu, S.; Sun, N.; Li, W.; Xu, Y.; Han, X.; Zhang, J.; Miao, J. Taurine reduction of injury from neutrophil infiltration ameliorates Streptococcus uberis-induced mastitis. Int. Immunopharmacol. 2023, 124, 111028. [Google Scholar] [CrossRef]
- Zouharová, M.; Matiašovic, J.; Gebauer, J.; Matiašková, K.; Nedbalcová, K. Survey of Genotype Diversity, Virulence, and Antimicrobial Resistance Genes in Mastitis-Causing Streptococcus uberis in Dairy Herds Using Whole-Genome Sequencing. Pathogens 2023, 12, 1378. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yang, J.; Cobo, E.R.; Wang, Y.; Xu, M.; Wang, T.; Shi, Y.; Liu, G.; Han, B. Streptococcus uberis induced expressions of pro-inflammatory IL-6, TNF-α, and IFN-γ in bovine mammary epithelial cells associated with inhibited autophagy and autophagy flux formation. Microb. Pathog. 2023, 183, 106270. [Google Scholar] [CrossRef]
- Srithanasuwan, A.; Zou, Y.; Suriyasathaporn, W.; Schukken, Y.H. Genetic diversity and molecular epidemiology of Streptococcus uberis in high-prevalence mastitis herds. J. Dairy. Sci. 2025, 108, 10173–10185. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.M.; Leigh, J.A. In vitro determination of essential genes required by Streptococcus uberis to grow in a complex biological media relating to intramammary infection. Microb. Genom. 2025, 11, 001425. [Google Scholar] [CrossRef] [PubMed]
- Rios Agudelo, P.A.; Reyes Vélez, J.; Olivera Angel, M.; Blanchard, A.M.; Cuesta Astroz, Y.; Caraballo Guzmán, A.; Torres Lindarte, G. Whole-Genome Sequencing Analysis Revealed High Genomic Variability, Recombination Events and Mobile Genetic Elements in Streptococcus uberis Strains Isolated from Bovine Mastitis in Colombian Dairy Herds. Antibiotics 2025, 14, 297. [Google Scholar] [CrossRef]
- Zouharova, M.; Nedbalcova, K.; Slama, P.; Bzdil, J.; Masarikova, M.; Matiasovic, J. Occurrence of virulence-associated genes in Streptococcus uberis and Streptococcus parauberis isolated from bovine mastitis. Vet. Med. 2022, 67, 123–130. [Google Scholar] [CrossRef]
- Crippa, B.L.; Rodrigues, M.X.; Tomazi, T.; Yang, Y.; de Oliveira Rocha, L.; Bicalho, R.C.; Silva, N.C.C. Virulence factors, antimicrobial resistance and phylogeny of bovine mastitis-associated Streptococcus dysgalactiae. J. Dairy. Res. 2023, 90, 152–157. [Google Scholar] [CrossRef]
- Farzana, Z.; Saha, A.; Siddiki, A.Z. Molecular characterization of Streptococcus agalactiae and Streptococcus dysgalactiae causing bovine mastitis in the southern region of Bangladesh. J. Adv. Vet. Anim. Res. 2023, 10, 178–184. [Google Scholar] [CrossRef]
- Klassen, A.; Dittmar, K.; Schulz, J.; Einax, E.; Donat, K. Estimation of the performance of two real-time polymerase chain reaction assays for detection of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in pooled milk samples in a field study. J. Dairy. Sci. 2023, 106, 9228–9243. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Yang, X.; Zhao, Y.; Niu, J.; Jiang, S.; Ma, J.; Zhang, X. Identification and Characterization of a Novel Prophage Lysin against Streptococcus dysgalactiae. Molecules 2024, 29, 3411. [Google Scholar] [CrossRef]
- Kang, H.J.; You, J.Y.; Kim, S.H.; Moon, J.S.; Kim, H.Y.; Kim, J.M.; Lee, Y.J.; Kang, H.M. Association with Elevated Somatic Cell Counts and Characterization of Aerococcus viridans Isolates from Bovine Mastitis Milk in South Korea. Curr. Microbiol. 2025, 82, 325. [Google Scholar] [CrossRef]
- Cybulski, P.; Kondratiuk, R.; Spiekermeier, I.; Woźniakowski, G. First isolation of Aerococcus viridans from clinical specimens collected on a pig farm in Poland. J. Vet. Res. 2024, 68, 509–514. [Google Scholar] [CrossRef]
- Cui, Y.; Song, K.; Liu, X.; Xu, H.; Wang, X.; Cheng, G.; Zheng, P.; Liu, J. Research on Bacterial Diversity and Antibiotic Resistance in the Dairy Farm Environment in a Part of Shandong Province. Animals 2024, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.A.; Cullimore, C.; Hutchison, W.D.; Grimsrud, A.; Nobrega, D.; De Buck, J.; Barkema, H.W.; Wilson, E.; Pickett, B.E.; Erickson, D.L. Genes associated with fitness and disease severity in the pan-genome of mastitis-associated Escherichia coli. Front. Microbiol. 2024, 15, 1452007. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, H.; Peng, Y.; Zhang, R.; Hu, Y.; Guo, A.; Hu, C. ALKBH5 Improves the Epithelial Cell Tight Junctions to Inhibit Escherichia coli-Induced Mastitis. Cells 2025, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Park, S.; Ruffini, J.; Dussault, F.; Dufour, S.; Ronholm, J. Comparative genomic analysis of Escherichia coli isolated from cases of bovine clinical mastitis and the dairy farm environment. Microb. Genom. 2025, 11, 001436. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Guo, L.; Gong, P.; Qian, Y.; Zhang, S.; Liu, B.; Guo, W.; Bao, H.; Mao, W. Investigating the role of the mPGES-PGE2-EP4 pathway in Escherichia coli-induced mastitis in dairy cows: Insights for non-antibiotic therapeutic strategies. Front. Vet. Sci. 2025, 12, 1628028. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Liang, J.; Zhang, K.; Su, H.; Wang, D.; Zhang, M.; Zhao, F.; Sun, Z.; Wu, Z.; et al. Impact of Escherichia coli and Lipopolysaccharide on the MAPK Signaling Pathway, MMPs, TIMPs, and the uPA System in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2025, 26, 3893. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Ji, X.; Zhang, L.; Wang, R.; Wang, H.; He, T. Antimicrobial resistance and molecular epidemiology of Klebsiella pneumoniae isolated from bovine mastitis in seven provinces in China. BMC Microbiol. 2025, 25, 407. [Google Scholar] [CrossRef]
- Ren, M.; Jin, T.; Tong, J.; Song, D.; Xie, Q.; Li, X.; Li, Y.; Liu, K.; Gao, J.; Liu, M.; et al. Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation. Animals 2025, 15, 1139. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.; Peng, W.; Li, X.; Guo, G.; Chen, L.; Pang, X.; Chen, M.; Li, J.; Wei, Y.; et al. Antimicrobial potential of a novel K5-specific phage and its recombinant strains against Klebsiella pneumoniae in milk. J. Dairy. Sci. 2025, 108, 6788–6802. [Google Scholar] [CrossRef]
- Cheng, J.; Tong, J.; Li, C.; Wang, Z.; Li, H.; Ren, M.; Song, J.; Song, D.; Xie, Q.; Liu, M. Probiotic Characterization of Lactiplantibacillus paraplantarum SDN1.2 and Its Anti-Inflammatory Effect on Klebsiella pneumoniae-Infected Mammary Glands. Vet. Sci. 2025, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Pirner, L.H.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. In vitro antimicrobial resistance of Escherichia coli, Serratia marcescens, Klebsiella oxytoca, and Klebsiella pneumoniae on Bavarian dairy farms between 2014 and 2022. J. Dairy. Sci. 2024, 107, 8402–8412. [Google Scholar] [CrossRef] [PubMed]
- Abegewi, U.A.; Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Prevalence and risk factors of coliform-associated mastitis and antibiotic resistance of coliforms from lactating dairy cows in North West Cameroon. PLoS ONE 2022, 17, e0268247. [Google Scholar] [CrossRef]
- Ozdikmenli Tepeli, S.; Numanoglu Cevik, Y.; Tosun, M.N.; Taylan Yalcin, G.; Kaya, B.; Ipek, D.; Bakkaloglu, Z.; Simsek, H.; Zorba, N.N. Carbapenem resistance and biofilm formation status of Enterobacterales isolated from raw milk via molecular versus phenotypic methods. Antonie Van. Leeuwenhoek 2023, 116, 67–80. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, J.; Liu, J.; Sun, X.; Yang, Y.; Lv, Y.; Zheng, J.; Mou, X.; Li, H.; Ding, X.; et al. Prevalence and Characterization of Serratia marcescens Isolated from Clinical Bovine Mastitis Cases in Ningxia Hui Autonomous Region of China. Infect. Drug Resist. 2023, 16, 2727–2735. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Cao, H.; Zhou, Z.; Xu, T. Characterization of Pseudomonas aeruginosa Isolated from Bovine Mastitis in Northern Jiangsu Province and Correlation to Drug Resistance and Biofilm Formability. Animals 2024, 14, 3290. [Google Scholar] [CrossRef]
- Ma, H.; Menghwar, H.; Lippolis, J.D.; Sarlo Davila, K.M.; Casas, E.; Dassanayake, R.P. Draft genome sequence of a multidrug-resistant Pseudomonas aeruginosa strain isolated from a dairy cow with chronic mastitis. Microbiol. Resour. Announc. 2024, 13, e0117323. [Google Scholar] [CrossRef]
- Salem, M.; Awad, A.; Younis, G. Antibiotic susceptibility and molecular detection of virulent pseudomonas aeruginosa isolated from bovine mastitis milk in Egypt. J. Adv. Vet. Res. 2023, 13, 664–671. [Google Scholar]
- El-Karim, D.R.G.; El-Amrawi, G.A.; Salama, A.R. Serum Biochemical Changes in Response to Affection with Pseudomonas aeruginosa Mastitis in Holstein Dairy Cows. J. Adv. Vet. Res. 2023, 13, 1153–1156. [Google Scholar]
- Bedawy, Y.M.; Homouda, S.N.; Ahmed, H.A.; Abd-El Tawab, A.A. Genotyping and antibiotic resistance profile of Klebsiella pneumoniae and Corynebacterium bovis isolates recovered from clinical and subclinical mastitis milk samples. J. Adv. Vet. Res. 2024, 14, 349–355. [Google Scholar]
- Eid, R.H.; Aref, N.E.; Ibrahim, E.S. Phenotypic diagnosis and genotypic identification of Bacillus cereus causing subclinical mastitis in cows. Vet. World 2023, 16, 888–894. [Google Scholar] [CrossRef]
- Wu, F.; Xie, X.; Du, T.; Jiang, X.; Miao, W.; Wang, T. Lactococcus lactis, a bacterium with probiotic functions and pathogenicity. World J. Microbiol. Biotechnol. 2023, 39, 325. [Google Scholar] [CrossRef] [PubMed]
- Kolar, Q.K.; Goncalves, J.L.; Erskine, R.J.; Ruegg, P.L. Comparison of minimum inhibitory concentrations of selected antimicrobials for non-aureus Staphylococci, Enterococci, Lactococci, and Streptococci isolated from milk samples of cows with clinical mastitis. Antibiotics 2024, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Han, J.; Barkema, H.W.; Wang, Y.; Gao, J.; Kastelic, J.P.; Han, B.; Qin, S.; Deng, Z. Comparative Genomic Analyses of Lactococcus garvieae Isolated from Bovine Mastitis in China. Microbiol. Spectr. 2023, 11, e0299522. [Google Scholar] [CrossRef]
- Xie, X.; Pan, Z.; Yu, Y.; Yu, L.; Wu, F.; Dong, J.; Wang, T.; Li, L. Prevalence, Virulence, and Antibiotics Gene Profiles in Lactococcus garvieae Isolated from Cows with Clinical Mastitis in China. Microorganisms 2023, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Du, T.; Jiang, X.; Liu, S.; Cheng, Y.; Zhang, Z.; Miao, W.; Wang, T. Lactococcus garvieae exerts a critical role in inducing inflammation in dairy mastitis by triggering NLRP3 inflammasome-mediated pyroptosis in MAC-T cells. World J. Microbiol. Biotechnol. 2024, 40, 132. [Google Scholar] [CrossRef]
- Krukowski, H.; Lisowski, A.; Puacz, E.; Chabuz, W.; Targońska-Karasek, M. First report of the isolation and molecular characterisation of Lactococcus garvieae in dairy cattle in Poland. J. Vet. Res. 2025, 69, 213–218. [Google Scholar] [CrossRef]
- Gelgie, A.E.; Desai, S.E.; Gelalcha, B.D.; Kerro Dego, O. Mycoplasma bovis mastitis in dairy cattle. Front. Vet. Sci. 2024, 11, 1322267. [Google Scholar] [CrossRef]
- Wagner, H.; Heller, M.; Fawzy, A.; Schnee, C.; Nesseler, A.; Kaim, U.; Ewers, C.; Semmler, T.; Spergser, J.; Schultze, T.; et al. Mycoplasma mycoides subspecies capri, an uncommon mastitis and respiratory pathogen isolated in a German flock of goats. Vet. Microbiol. 2024, 290, 109996. [Google Scholar] [CrossRef]
- Gelgie, A.E.; Schneider, P.; Citti, C.; Dordet-Frisoni, E.; Gillespie, B.E.; Almeida, R.A.; Agga, G.E.; Amoah, Y.S.; Shpigel, N.Y.; Kerro Dego, O.; et al. Mycoplasma bovis 5′-nucleotidase is a virulence factor conferring mammary fitness in bovine mastitis. PLoS Pathog. 2024, 20, e1012628. [Google Scholar] [CrossRef]
- Suzuki, K.; Kaneko, F.; Matsushita, A.; Hata, E. Outbreaks of bovine mastitis caused by specific Mycoplasma bovis strains recurring at multi-year intervals. J. Vet. Diagn. Invest. 2024, 36, 457–462. [Google Scholar] [CrossRef]
- Ghazvineh, N.; Mokhtari, A.; Abadi, M.G.N.; Kadivar, A.; Shahraki, S.S. Molecular detection of selective virulence factors of mycoplasma bovis local isolates involved in bovine mastitis. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 631–639. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Q.; Han, L.; Chen, X. Molecular Characterization of Resistance and Virulence Factors of Trueperella pyogenes Isolated from Clinical Bovine Mastitis Cases in China. Infect. Drug Resist. 2024, 17, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Wente, N.; Leimbach, S.; Woudstra, S.; Krömker, V. Trueperella Pyogenes-Strain Diversity and Occurrence in Dairy Herds. Pathogens 2024, 13, 534. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Cheng, J.; Liu, M. Virulence factors and therapeutic methods of Trueperella pyogenes: A review. Virulence 2025, 16, 2467161. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.A.; Mohammadzadeh, A.; Mahmoodi, P.; Pakbin, B.; Salehi, T.Z. Antimicrobial susceptibility, virulence genes and genomic characterization of Trueperella pyogenes isolated from abscesses in dairy cattle. Res. Vet. Sci. 2023, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Božić, D.D.; Kladar, N.; Samardžija, M.; Apić, J.; Baljak, J.; Kovačević, Z. Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics 2024, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Turedi, O.K.; Seker, E. Identification of yeast in healthy and subclinical mastitis-diagnosed Anatolian buffaloes in smallholder dairy farms in Turkey. Acta Vet. Hung. 2025, 73, 104–110. [Google Scholar] [CrossRef]
- Kot, M.; Lange, A.; Jabłońska, W.; Kalińska, A.; Nasiłowska, B.; Skrzeczanowski, W.; Gołębiewski, M. Nanoparticles as New Antifungals in the Prevention of Bovine Mycotic Mastitis Caused by Candida spp. and Diutina spp.-In Vitro Studies. Molecules 2025, 30, 2086. [Google Scholar] [CrossRef]
- Fayed, H.; Elgendy, A.A.; Shoulah, S.A.; Moustafa, S.M.; Maher, A.; Hikal, A.F.; Abdeen, A.; Elmorsy, E.; Mohamed, M.E.; Hetta, H.F.; et al. Mycological and molecular identification of mycoses involved in mastitis from Holstein dairy cattle with special reference to Candida albicans. Vet. Res. Commun. 2025, 49, 187. [Google Scholar] [CrossRef]
- Jiang, C.; Fang, W.; Chen, S.; Guo, X.; Gao, X.; Liu, P.; Hu, G.; Li, G.; Mai, W.; Liu, P. Genetic framework sequencing analysis of Candida tropicalis in dairy cow mastitis and study of pathogenicity and drug resistance. BMC Microbiol. 2024, 24, 428. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hussein, A.E.-D.; El-Diasty, M. Study on mycological and molecular detection of yeast and mold isolated from bovine mastitis. SVU-Int. J. Vet. Sci. 2024, 7, 64–76. [Google Scholar] [CrossRef]
- Jabłońska, W.; Gołębiewski, M.; Kot, M.; Mardan, H.; Pawliński, B.; Kalińska, A. Perspectives and Possibilities for New Antimicrobial Agents in the Treatment and Control of Mastitis Induced by Algae of the Genus Prototheca spp.: A Review. Int. J. Mol. Sci. 2024, 25, 8219. [Google Scholar] [CrossRef]
- Kuczyńska, M.; Kot, M.; Stocki, M.; Zapora, E.; Jagielski, T.; Perlińska-Teresiak, M.; Kalińska, A. In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. Int. J. Mol. Sci. 2025, 26, 5451. [Google Scholar] [CrossRef] [PubMed]
- Răpuntean, S. Cow’s Mastitis produced by Unicellular Algae of the Genus Prototheca, an Emerging Disease: Treatment Evaluations (Literature Review). Int. J. For. Anim. Fish. Res. (IJFAF) 2017, 1, 23–33. [Google Scholar] [CrossRef]
- Nojo, H.; Ishijima, S.A.; Morikawa, M.; Ito, T.; Kano, R. In vitro susceptibility testing of phytochemicals from essential oils against Prototheca species. J. Vet. Med. Sci. 2024, 86, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Sekizuka, T.; Yasutomi, I.; Fujii, Y.; Uchida, I. Draft genome sequence of Prototheca bovis strain P18 isolated from cattle in Japan. Microbiol. Resour. Announc. 2025, 31, e0054425. [Google Scholar] [CrossRef]
- Bokharaeian, M.; Toghdory, A.; Ghoorchi, T.; Ghassemi Nejad, J.; Esfahani, I.J. Quantitative Associations between Season, Month, and Temperature-Humidity Index with Milk Yield, Composition, Somatic Cell Counts, and Microbial Load: A Comprehensive Study across Ten Dairy Farms over an Annual Cycle. Animals 2023, 13, 3205. [Google Scholar] [CrossRef]
- Freu, G.; Garcia, B.L.N.; Tomazi, T.; Di Leo, G.S.; Gheller, L.S.; Bronzo, V.; Moroni, P.; Dos Santos, M.V. Association between mastitis occurrence in dairy cows and bedding characteristics of compost-bedded pack barns. Pathogens 2023, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Mendonça, L.; Souza, G.; Cesar, D.; Carneiro, J.d.C.; Brito, E.; Mendonça, J.; Brito, M.; Guimarães, A. Epidemiology of mastitis and interactions of environmental factors on udder health in the compost barn system. Arq. Bras. De Med. Veterinária E Zootec. 2023, 75, 14–26. [Google Scholar] [CrossRef]
- Shahedani, M.; Ahmadi, F.; Tosun, H. The impact of bedding materials and disinfectants on udder health and mastitis control in dairy cows. J. Hell. Vet. Med. Soc. 2024, 75, 8083–8098. [Google Scholar] [CrossRef]
- Mikhalev, V.I.; Zimnikov, V.I. Automatic milking and incidence of mastisis in cows. RUDN J. Agron. Anim. Ind. 2024, 19, 507–516. [Google Scholar]
- Watters, R.; Virkler, P. Assessment of Milking Equipment in Relation to Milk Quality Issues—Including Automatic Milking Systems. Vet. Clin. North. Am. Food Anim. Pract. 2025, 41, 259–269. [Google Scholar] [CrossRef]
- Hässig, M.; Wyss, P.; Bilgery, E.; Fatzer, H.; Hausammann, M.; Schick, M. [Vibration as a risk of mastitis during milking]. Schweiz. Arch. Tierheilkd. 2024, 166, 41–48. [Google Scholar]
- Hristov, S.; Stanković, B.; Samolovac, L.; Andrić, D.; Nakov, D. Hygiene procedures before, during and after cow milking. Arch. Vet. Med. 2023, 16, 5–15. [Google Scholar] [CrossRef]
- Hansson, I.; Woudstra, S. Associations of parity and lactation stage with the order cows enter the milking parlor. JDS Commun. 2024, 5, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lungu, S.E.; Danso, F.; Dzou, C.F.; Chen, Y.; Zheng, X.; Nie, F.; Lin, H.; Chen, J.; Zhou, G. Animal health and nutrition: Metabolic disorders in cattle and improvement strategies. Front. Vet. Sci. 2025, 12, 1470391. [Google Scholar] [CrossRef]
- Khan, M.Z.; Huang, B.; Kou, X.; Chen, Y.; Liang, H.; Ullah, Q.; Khan, I.M.; Khan, A.; Chai, W.; Wang, C. Enhancing bovine immune, antioxidant and anti-inflammatory responses with vitamins, rumen-protected amino acids, and trace minerals to prevent periparturient mastitis. Front. Immunol. 2023, 14, 1290044. [Google Scholar] [CrossRef]
- Mamatsios, K.; Karatzia, M.A.; Manessis, G.; Kasapidou, E.; Bossis, I.; Basdagianni, Z. Effect of Milking Vacuum and the Supplementation of Vitamin E and Se in Milk Quantity, Quality, and Hygiene of Mammary gland in Mountainous Greek Sheep. Animals 2023, 13, 3400. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Xiao, J.; Chen, T.; Ma, J.; Liu, S.; Wang, Y.; Khan, A.; Alugongo, G.M.; Cao, Z. Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period. Antioxidant 2022, 11, 657. [Google Scholar] [CrossRef]
- Chen, Y.C.; Orellana Rivas, R.M.; Marins, T.N.; Melo, V.; Wang, Z.; Garrick, M.; Gao, J.; Liu, H.; Bernard, J.K.; Melendez, P.; et al. Effects of heat stress abatement on systemic and mammary inflammation in lactating dairy cows. J. Dairy. Sci. 2023, 106, 8017–8032. [Google Scholar] [CrossRef]
- Ferronato, G.; Simonetto, A.; Gilioli, G.; Zecconi, A. Modeling Mastitis Risk Management Effects on Dairy Milk Yield and Global Warming Potential. Animals 2024, 15, 50. [Google Scholar] [CrossRef]
- Olsen, H.E.; Anderson, K.N.; Creutzinger, K.C.; Vogel, K.D. Broken tails in Holstein dairy cattle: A cross-sectional study. JDS Commun. 2023, 4, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Gantner, V.; Popović, V.; Steiner, Z.; Gantner, R.; Potočnik, K. The differences in subclinical mastitis prevalence and effect on milk production due to cows’ breed and breeding region. In Proceedings of the 4th International Scientific Conference on Sustainable Agriculture and Rural Development, New Delhi, India, 14–15 December 2023; pp. 383–391. [Google Scholar]
- Yunusa, A.; Mai, H.; Abubaar, M. DETERMINATION OF MASTITIS PREVALENCE USING CALIFORNIA MASTITIS TEST (CMT) PADDLE ON TWO BREEDS OF DAIRY CATTLE. Niger. J. Agric. Agric. Technol. 2025, 5, 200–205. [Google Scholar] [CrossRef]
- Cai, Z.; Iso-Touru, T.; Sanchez, M.P.; Kadri, N.; Bouwman, A.C.; Chitneedi, P.K.; MacLeod, I.M.; Vander Jagt, C.J.; Chamberlain, A.J.; Gredler-Grandl, B.; et al. Meta-analysis of six dairy cattle breeds reveals biologically relevant candidate genes for mastitis resistance. Genet. Sel. Evol. 2024, 56, 54. [Google Scholar] [CrossRef] [PubMed]
- Tharwat, M.; Alkhedhairi, S.; El Tigani-Asil, E.T.A. Clinical predictive significance of biomarker molecules elevation during the transition period in cattle suffering from different pathological states: A review. Open Vet. J. 2024, 14, 1345–1357. [Google Scholar] [CrossRef]
- Nurye, M.; Eshetu, M.; Yirga, M. Prevalence of bovine mastitis and its associated risk factors under different production system in Borena district of South Wollo Zone, Amhara, Ethiopia. Cogent Food Agric. 2023, 9, 2291224. [Google Scholar] [CrossRef]
- Mramba, R.P.; Mohamed, M.A. The prevalence and factors associated with mastitis in dairy cows kept by small-scale farmers in Dodoma, Tanzania. Heliyon 2024, 10, e34122. [Google Scholar] [CrossRef]
- Spellman, M.E.; Geary, C.M.; Somula, H.; Singh, A.; Wieland, M. The association between teat shape and clinical mastitis. J. Dairy. Sci. 2025, 108, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.; Lawrence, K.E.; Ridler, A.L.; Laven, R.A. Teat and udder morphology and pathology of New Zealand dairy ewes. N. Z. Vet. J. 2025, 73, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.C.; Lopez-Villalobos, N.; Loveday, S.M.; Weeks, M.; McNabb, W. Udder and teat morphology traits associated with milk production and somatic cell score in dairy sheep from a New Zealand flock. New Zealand J. Agric. Res. 2024, 67, 348–360. [Google Scholar] [CrossRef]
- Shinefield, H.R.; Black, S. Prevention of Staphylococcus aureus infections: Advances in vaccine development. Expert. Rev. Vaccines 2005, 4, 669–676. [Google Scholar] [CrossRef]
- Rainard, P.; Foucras, G.; Fitzgerald, J.R.; Watts, J.L.; Koop, G.; Middleton, J.R. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 2018, 65 (Suppl. 1), 149–165. [Google Scholar] [CrossRef] [PubMed]
- Ferwerda, G.; Meyer-Wentrup, F.; Kullberg, B.J.; Netea, M.G.; Adema, G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008, 10, 2058–2066. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [CrossRef]
- De Jong, N.W.; Van Kessel, K.P.; Van Strijp, J.A. Immune evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7, 10.1128. [Google Scholar] [CrossRef]
- Richardet, M.; Solari, H.G.; Cabrera, V.E.; Vissio, C.; Agüero, D.; Bartolomé, J.A.; Bó, G.A.; Bogni, C.I.; Larriestra, A.J. The economic evaluation of mastitis control strategies in Holstein-Friesian dairy herds. Animals 2023, 13, 1701. [Google Scholar] [CrossRef]
- Peters, M.; Silveira, I.; Fischer, V. Impact of subclinical and clinical mastitis on sensitivity to pain of dairy cows. Animal 2015, 9, 2024–2028. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Karzis, J.; Donkin, E.F.; Webb, E.C.; Etter, E.M.; Petzer, I.-M. Somatic cell count thresholds in composite and quarter milk samples as indicator of bovine intramammary infection status. Onderstepoort J. Vet. Res. 2017, 84, 1–10. [Google Scholar]
- Rochmah, E.R.; Raharjo, D.; Hidanah, S.; Effendi, M.H.; Witaningrum, A.M.; Warsito, S.H. Effectiveness of the California mastitis test (CMT), reductase test, and alcohol test for dairy cows subclinical mastitis detection. J. Agro Vet. (Agrovet) 2024, 7, 91–97. [Google Scholar]
- Roberts, J. The California mastitis test: What is the value? Livestock 2025, 30, S4–S10. [Google Scholar] [CrossRef]
- Kaşikçi, G.; Çetin, Ö.; Bingöl, E.B.; Gündüz, M.C. Relations between electrical conductivity, somatic cell count, California mastitis test and some quality parameters in the diagnosis of subclinical mastitis in dairy cows. Turk. J. Vet. Anim. Sci. 2012, 36, 49–55. [Google Scholar] [CrossRef]
- Khatun, M.; Clark, C.E.; Lyons, N.A.; Thomson, P.C.; Kerrisk, K.L.; García, S.C. Early detection of clinical mastitis from electrical conductivity data in an automatic milking system. Anim. Prod. Sci. 2017, 57, 1226–1232. [Google Scholar] [CrossRef]
- Ramadani, X.; Kryeziu, A.; Kamberi, M.; Zogaj, M. Influence of the farm location and seasonal fluctuations on the composition and properties of the milk. Agron. Res. 2024, 22, 238–252. [Google Scholar]
- Rainard, P.; Foucras, G.; Boichard, D.; Rupp, R. Invited review: Low milk somatic cell count and susceptibility to mastitis. J. Dairy. Sci. 2018, 101, 6703–6714. [Google Scholar] [CrossRef]
- Addis, M.; Tedde, V.; Puggioni, G.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of milk cathelicidin for detection of bovine mastitis. J. Dairy. Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef]
- Le, M.N.; Kawada-Matsuo, M.; Komatsuzawa, H. Efficiency of Antimicrobial Peptides Against Multidrug-Resistant Staphylococcal Pathogens. Front. Microbiol. 2022, 13, 930629. [Google Scholar] [CrossRef]
- Neumann, S.; Siegert, S.; Fischer, A. β-defensin-4 as an endogenous biomarker in cows with mastitis. Front. Vet. Sci. 2023, 10, 1154386. [Google Scholar] [CrossRef] [PubMed]
- Veas, F. Acute Phase Proteins as Early Non-Specific Biomarkers of Human and Veterinary Diseases; BoD–Books on Demand: Norderstedt, Germany, 2011. [Google Scholar]
- Huang, Y.-M.; Lo, C.; Cheng, C.-F.; Lu, C.-H.; Hsieh, S.-C.; Li, K.-J. Serum C-reactive protein and interleukin-6 levels as biomarkers for disease severity and clinical outcomes in patients with idiopathic granulomatous mastitis. J. Clin. Med. 2021, 10, 2077. [Google Scholar] [CrossRef]
- Bochniarz, M.; Szczubiał, M.; Brodzki, P.; Krakowski, L.; Dąbrowski, R. Serum amyloid A as an marker of cow֨ s mastitis caused by Streptococcus sp. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101498. [Google Scholar]
- Shimazaki, K.I.; Kawai, K. Advances in lactoferrin research concerning bovine mastitis. Biochem. Cell Biol. 2017, 95, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, X.; Liu, L.; Yang, Z.; Li, C.; Bao, X.; Amantuer, A.; Wen, P.; Wang, D.; Zhang, S. The Relationship Between Protein Fraction Contents and Immune Cells in Milk. Animals 2025, 15, 1578. [Google Scholar] [CrossRef]
- Antanaitis, R.; Juozaitienė, V.; Jonike, V.; Baumgartner, W.; Paulauskas, A. Milk lactose as a biomarker of subclinical mastitis in dairy cows. Animals 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.B.; Ingole, S.D.; Bharucha, S.V.; Yoshitha, K.L.; Gaikwad, R.V.; Pharande, R.R.; Kharde, S.D. Milk miRNA expression in buffaloes as a potential biomarker for mastitis. BMC Vet. Res. 2024, 20, 150. [Google Scholar] [CrossRef]
- Neumann, S.; Siegert, S.; Fischer, A. Procalcitonin as an endogenous biomarker for mastitis in cows. Animals 2023, 13, 2204. [Google Scholar] [CrossRef] [PubMed]
- Wollowski, L.; Heuwieser, W.; Kossatz, A.; Addis, M.; Puggioni, G.; Meriaux, L.; Bertulat, S. The value of the biomarkers cathelicidin, milk amyloid A, and haptoglobin to diagnose and classify clinical and subclinical mastitis. J. Dairy Sci. 2021, 104, 2106–2122. [Google Scholar] [CrossRef]
- Farkaš, V.; Beletić, A.; Kuleš, J.; Thomas, F.C.; Rešetar Maslov, D.; Rubić, I.; Benić, M.; Bačić, G.; Mačešić, N.; Jović, I. Biomarkers for subclinical bovine mastitis: A high throughput TMT-based proteomic investigation. Vet. Res. Commun. 2024, 48, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Yoshitha, K.L.; Ingole, S.D.; Bharucha, S.V.; Bhuyan, M.; Pharande, R.R.; Gaikwad, R.V. Expression and characterization of exosomal miRNAs in healthy, sub-clinical mastitis and pasteurized milk of buffaloes. Sci. Rep. 2025, 15, 1915. [Google Scholar] [CrossRef]
- Bronzo, V.; Sala, G.; Ciabattini, I.; Orsetti, C.; Armenia, G.; Meucci, V.; De Marchi, L.; Bertelloni, F.; Sgorbini, M.; Bonelli, F. Endogenous Symmetric Dimethylarginine (SDMA) and Asymmetrical Dimethylarginine (ADMA) Levels in Healthy Cows and Cows with Subclinical and Clinical Mastitis—A Comparative Study. Animals 2025, 15, 527. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, E.; De Rensis, F.; Martignani, E.; Miretti, S.; Ala, U.; Cavalli, V.; Cipolat-Gotet, C.; Andrani, M.; Baratta, M.; Saleri, R. Differential Expression of miR-223-3p and miR-26-5p According to Different Stages of Mastitis in Dairy Cows. Biomolecules 2025, 15, 235. [Google Scholar] [CrossRef]
- Özkan, H.; Keçeli, H.H.; Kaya, U.; Dalkiran, S.; Yüksel, M.; Tek, E.; Yakan, A. Considering potential roles of selected MicroRNAs in evaluating subclinical mastitis and Milk quality in California mastitis test (+) and infected bovine milk. Anim. Sci. J. 2024, 95, e13959. [Google Scholar] [CrossRef]
- Giagu, A.; Penati, M.; Traini, S.; Dore, S.; Addis, M.F. Milk proteins as mastitis markers in dairy ruminants—A systematic review. Vet. Res. Commun. 2022, 46, 329–351. [Google Scholar] [CrossRef]
- O’Reilly, E.L.; Viora, L.; Malcata, F.; Pepler, P.T.; Zadoks, R.; Brady, N.; Hanh, H.Q.; McLaughlin, M.; Horvatic, A.; Gelemanovic, A.; et al. Biomarker and proteome analysis of milk from dairy cows with clinical mastitis: Determining the effect of different bacterial pathogens on the response to infection. Res. Vet. Sci. 2024, 172, 105240. [Google Scholar] [CrossRef]
- Cuccato, M.; Divari, S.; Giannuzzi, D.; Grange, C.; Moretti, R.; Rinaldi, A.; Leroux, C.; Sacchi, P.; Cannizzo, F.T. Extracellular vesicle miRNome during subclinical mastitis in dairy cows. Vet. Res. 2024, 55, 112. [Google Scholar] [CrossRef]
- Kahya Demirbilek, S.; Yıldız, M.; Akkoç, A.; Mutlu, A.M.; Ardıçlı, Ö.; Aner, H. Comparison of bacteriological culture method and multiplex real-time PCR for detection of mastitis. Res. Vet. Sci. 2024, 172, 105237. [Google Scholar] [CrossRef] [PubMed]
- Galfi, A.L.; Radinović, M.Ž.; Davidov, I.N.; Erdeljan, M.M.; Kovačević, Z.R. Detection of subclinical mastitis in dairy cows using California and Draminski mastitis test. Biotechnol. Anim. Husb. 2017, 33, 465–473. [Google Scholar] [CrossRef]
- Farkašová, Z.; Zigo, F.; Halás, Š.; Záhumenská, J.; Pecka-Kiełb, E.; Vargová, M. Cultivation tests for rapid detection of mastitis pathogens in dairy cows. Acta Vet. Brno 2025, 93, 37–45. [Google Scholar] [CrossRef]
- Koskinen, M.T.; Wellenberg, G.J.; Sampimon, O.C.; Holopainen, J.; Rothkamp, A.; Salmikivi, L.; van Haeringen, W.A.; Lam, T.J.; Pyörälä, S. Field comparison of real-time polymerase chain reaction and bacterial culture for identification of bovine mastitis bacteria. J. Dairy. Sci. 2010, 93, 5707–5715. [Google Scholar] [CrossRef]
- Jones, G.; Bork, O.; Ferguson, S.A.; Bates, A. Comparison of an on-farm point-of-care diagnostic with conventional culture in analysing bovine mastitis samples. J. Dairy. Res. 2019, 86, 222–225. [Google Scholar] [CrossRef]
- Keane, O.M.; Budd, K.E.; Flynn, J.; McCoy, F. Increased detection of mastitis pathogens by real-time PCR compared to bacterial culture. Vet. Rec. 2013, 173, 268. [Google Scholar] [CrossRef]
- Vitenberga-Verza, Z.; Pilmane, M.; Šerstņova, K.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Identification of inflammatory and regulatory cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-producing cells in the milk of dairy cows with subclinical and clinical mastitis. Pathogens 2022, 11, 372. [Google Scholar] [CrossRef]
- Bochniarz, M.; Zdzisińska, B.; Wawron, W.; Szczubiał, M.; Dąbrowski, R. Milk and serum IL-4, IL-6, IL-10, and amyloid A concentrations in cows with subclinical mastitis caused by coagulase-negative staphylococci. J. Dairy Sci. 2017, 100, 9674–9680. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.; Ahmad, S.B.; Rehman, M.U.; Muzamil, S.; Bhat, R.R.; Hussain, I.; Bashir, N.; Mir, M.U.R.; Paray, B.A.; Dawood, M.A. Investigations on cytokines and proteins in lactating cows with and without naturally occurring mastitis. J. King Saud. Univ. -Sci. 2020, 32, 2863–2867. [Google Scholar] [CrossRef]
- Šerstņova, K.; Pilmane, M.; Vitenberga-Verza, Z.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Expression of anti-inflammatory markers IL-2, IL-10, TGF-β1, βDEF-2, βDEF-3 and Cathelicidin LL37 in dairy cattle milk with different health status of the udder. Pol. J. Vet. Sci. 2022, 25, 237–248. [Google Scholar] [CrossRef]
- Eckersall, P.; Brady, N.; O’Reilly, E.; Viora, L.; Pepler, P.T. An Elisa for Cathelicidin for Diagnosis of Bovine Mastitis. In Proceedings of the XXI International Society for Animal Clinical Pathology Congress, Crete, Greece, 14–18 May 2024; Available online: https://eprints.gla.ac.uk/325758/ (accessed on 29 July 2025).
- Pranayapradhan, P.; Gopinath, S.; Krishankumar, K.; Joshi, R. Biomarker based detection of subclinical mastitis by liquid phase blocking ELISA. Int. J. Pharma Bio Sci. 2013, 4, B-776. [Google Scholar]
- Gad, W.A.; Osman, S.A.; Abd El-Razik, K.A.; Soror, A.H.; Fouad, E.A. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J. 2024, 14, 3388–3396. [Google Scholar] [CrossRef]
- Fasulkov, I.; Karadaev, M.; Vasilev, N.; Simeonov, R.; Urumova, V.; Mladenova, E. Ultrasound and histopathological investigations of experimentally induced Staphylococcus aureus mastitis in goats. Small Rumin. Res. 2015, 129, 114–120. [Google Scholar] [CrossRef]
- Abdullah, O.; Aslam, S.; Khan, M.; Mushtaq, H.; Hassan, M.; Akbar, H.; Hassan, N.; Ijaz, M. Diagnosis and prognosis of bovine mastitis using ultrasonography and the associated risk factors on dairy farms. South. Afr. J. Anim. Sci. 2023, 53, 626–636. [Google Scholar] [CrossRef]
- Themistokleous, K.S.; Papadopoulos, I.; Panousis, N.; Zdragas, A.; Arsenos, G.; Kiossis, E. Udder Ultrasonography of Dairy Cows: Investigating the Relationship between Echotexture, Blood Flow, Somatic Cell Count and Milk Yield during Dry Period and Lactation. Animals. 2023, 13, 1779. [Google Scholar] [CrossRef]
- Risvanli, A.; Dogan, H.; Safak, T.; Kilic, M.A.; Seker, I. The relationship between mastitis and the B-mode, colour Doppler ultrasonography measurements of supramammary lymph nodes in cows. J. Dairy Res. 2019, 86, 315–318. [Google Scholar] [CrossRef]
- Korelidou, V.; Simitzis, P.; Massouras, T.; Gelasakis, A.I. Infrared Thermography as a Diagnostic Tool for the Assessment of Mastitis in Dairy Ruminants. Animals 2024, 14, 2691. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using infrared thermography to detect subclinical mastitis in dairy cows in compost barn systems. J. Therm. Biol. 2021, 97, 102881. [Google Scholar] [CrossRef]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Zerbe, H. Infrared thermography of the udder after experimentally induced Escherichia coli mastitis in cows. Vet. J. 2015, 204, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Fang, Z.; Mu, T.; Wang, Z.; Ma, Y.; Ma, Y. Application of Metabolomics in Diagnosis of Cow Mastitis: A Review. Front. Vet. Sci. 2021, 8, 747519. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Fan, Y.; Chen, Z.; Li, M.; Mao, Y.; Karrow, N.A.; Loor, J.J.; Moore, S.; Yang, Z. Transcriptomics and iTRAQ-Proteomics Analyses of Bovine Mammary Tissue with Streptococcus agalactiae-Induced Mastitis. J. Agric. Food Chem. 2018, 66, 11188–11196. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Istiaq, A.; Rahman, M.S.; Islam, M.R.; Anwar, A.; Siddiki, A.; Sultana, M.; Crandall, K.A.; Hossain, M.A. Microbiome dynamics and genomic determinants of bovine mastitis. Genomics 2020, 112, 5188–5203. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Nkhebenyane, J.; Mnisi, Z.; Kwenda, S.; Thekisoe, O. Comprehensive whole genome analysis of Staphylococcus aureus isolates from dairy cows with subclinical mastitis. Front. Microbiol. 2024, 15, 1376620. [Google Scholar] [CrossRef]
- Nesaraj, J.; Grinberg, A.; Laven, R.; Biggs, P. Genomic epidemiology of bovine mastitis-causing Staphylococcus aureus in New Zealand. Vet. Microbiol. 2023, 282, 109750. [Google Scholar] [CrossRef]
- Wang, M.; Yang, N.; Laterrière, M.; Gagné, D.; Omonijo, F.; Ibeagha-Awemu, E.M. Multi-omics integration identifies regulatory factors underlying bovine subclinical mastitis. J. Anim. Sci. Biotechnol. 2024, 15, 46. [Google Scholar] [CrossRef]
- Narayana, S.G.; de Jong, E.; Schenkel, F.S.; Fonseca, P.A.S.; Chud, T.C.S.; Powell, D.; Wachoski-Dark, G.; Ronksley, P.E.; Miglior, F.; Orsel, K.; et al. Underlying genetic architecture of resistance to mastitis in dairy cattle: A systematic review and gene prioritization analysis of genome-wide association studies. J. Dairy. Sci. 2023, 106, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.P.; Lemma, F.; Koylass, M.; Rogers, J.; Ayling, R.D.; Worth, D.; Klita, M.; Steventon, A.; Line, K.; Wragg, P.; et al. Evaluation of MALDI-ToF as a method for the identification of bacteria in the veterinary diagnostic laboratory. Res. Vet. Sci. 2015, 101, 42–49. [Google Scholar] [CrossRef]
- Jahan, N.A.; Godden, S.M.; Royster, E.; Schoenfuss, T.C.; Gebhart, C.; Timmerman, J.; Fink, R.C. Evaluation of the matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) system in the detection of mastitis pathogens from bovine milk samples. J. Microbiol. Methods 2021, 182, 106168. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.; Fidelis, C.E.; Silva, A.T.F.; Mota, R.A.; Rall, V.L.M.; Dos Santos, M.V.; Gonçalves, J.L. MALDI-TOF bacterial subtyping for rapid detection of biomarkers in Staphylococcus aureus from subclinical bovine mastitis. J. Appl. Microbiol. 2023, 134, lxad249. [Google Scholar] [CrossRef]
- Guimarães, F.F.; Moraes, G.N.; Joaquim, S.F.; Guerra, S.T.; Dalanezi, F.M.; Mioni, M.S.R.; Medeiros, F.M.H.; Lucheis, S.B.; Possebon, F.S.; Pantoja, J.C.F.; et al. Identification of Enterococcus spp. by MALDI-TOF mass spectrometry isolated from clinical mastitis and bulk tank milk samples. BMC Vet. Res. 2024, 20, 378. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef]
- Yu, J.; Liu, C.; Wang, D.; Wan, P.; Cheng, L.; Yan, X. Integrated microbiome and metabolome analysis reveals altered gut microbial communities and metabolite profiles in dairy cows with subclinical mastitis. BMC Microbiol. 2025, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tobolski, D.; Zwierzchowski, G.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. Identification of Serum-Predictive Biomarkers for Subclinical Mastitis in Dairy Cows and New Insights into the Pathobiology of the Disease. J. Agric. Food Chem. 2022, 70, 1724–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, Y.; Yang, F.; Zhang, Q.; Zhao, X.; Yang, Z.; Dao, X.; Laghi, L. Microbiome and metabolome analyses of milk and feces from dairy cows with healthy, subclinical, and clinical mastitis. Front. Microbiol. 2024, 15, 1374911. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Viveiros, S.; Rodrigues, M.; Albuquerque, D.; Martins, S.A.M.; Cardoso, S.; Martins, V.C. Multiple Bacteria Identification in the Point-of-Care: An Old Method Serving a New Approach. Sensors 2020, 20. [Google Scholar] [CrossRef]
- Yazdanpanah, H.; Mahboubi, A.; Eslamizad, S.; Karimi, Z.; Rashidi, E.; Salamzadeh, J. Validation of a multiclass method for the screening of 15 antibiotic residues in milk using biochip multi-array technology and its application to monitor real samples. Iran. J. Pharm. Res. 2021, 20, 243. [Google Scholar]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Maa, M.K.; Ahmed, S.; Huang, L.; Xie, S. Designing, structural determination and biological effects of rifaximin loaded chitosan- carboxymethyl chitosan nanogel. Carbohydr. Polym. 2020, 248, 116782. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Zhang, A.; Luo, W.; Ahmed, S.; Huang, L. Preparation of chitosan nanoparticles by ionotropic gelation technique: Effects of formulation parameters and in vitro characterization. J. Mol. Struct. 2022, 1252, 132129. [Google Scholar] [CrossRef]
- Chinnappan, R.; Al Attas, S.; Kaman, W.E.; Bikker, F.J.; Zourob, M. Development of magnetic nanoparticle based calorimetric assay for the detection of bovine mastitis in cow milk. Anal. Biochem. 2017, 523, 58–64. [Google Scholar] [CrossRef]
- Gad, W.A. Diagnosis of subclinical Staphylococcal aureus bovine mastitis using nanotechnology-based techniques. Egypt. J. Vet. Sci. 2025, 56, 721–730. [Google Scholar] [CrossRef]
- Hossain, M.; Paul, S.; Hossain, M.; Islam, M.; Alam, M. Bovine mastitis and its therapeutic strategy doing antibiotic sensitivity test. Austin J. Vet. Sci. Anim. Husb. 2017, 4, 1030. [Google Scholar]
- Svennesen, L.; Skarbye, A.P.; Farre, M.; Astrup, L.B.; Halasa, T.; Krömker, V.; Denwood, M.; Kirkeby, C. Treatment of mild to moderate clinical bovine mastitis caused by gram-positive bacteria: A noninferiority randomized trial of local penicillin treatment alone or combined with systemic treatment. J. Dairy. Sci. 2023, 106, 5696–5714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Anika, T.T.; Noman, Z.A.; Islam, M.S.; Sultana, N.; Ashraf, M.N.; Pervin, M.; Islam, M.A.; Hossain, M.M.; Rahman, M.T.; Khan, M. Draft genome sequence of multidrug-resistant Escherichia coli MAHK_SCM_BAU_30A strain isolated from a subclinical mastitis cow in Bangladesh. Microbiol. Resour. Announc. 2023, 12, e0071323. [Google Scholar] [CrossRef] [PubMed]
- Kløve, D.C.; Jensen, V.F.; Astrup, L.B. First Finding of a Methicillin-Resistant Staphylococcus aureus (MRSA) t304/ST6 from Bovine Clinical Mastitis. Antibiotics 2022, 11, 1393. [Google Scholar] [CrossRef]
- Pașca, C.; Mărghitaș, L.; Dezmirean, D.; Bobiș, O.; Bonta, V.; Chirilă, F.; Matei, I.; Fiț, N. Medicinal Plants Based Products Tested on Pathogens Isolated from Mastitis Milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef]
- Soni, S.; Mukherjee, R.; De, U.K.; Bharti, D.; Singh, M.; Paul, B.R.; Sarkar, V.K.; Sharun, K.; Barkathullah, N.; Saminathan, M. Therapeutic Efficacy of Baicalein Green Biomolecule in Methicillin-Resistant Staphylococcus aureusMurine Mastitis Model. J. Pure Appl. Microbiol. 2024, 18, 1546–1557. [Google Scholar] [CrossRef]
- Kong, X.; Wang, M.; Guo, Z.; Yang, X.; Lian, H.; Gao, T.; Zhang, L.; Fu, T. Evaluation the protective role of baicalin against H2O2-driven oxidation, inflammation and apoptosis in bovine mammary epithelial cells. Front. Vet. Sci. 2024, 11, 1504887. [Google Scholar] [CrossRef]
- Mao, Y.-n.; Ma, Y.-j.; Wang, G.-q. Synergistic Antibacterial Effect of Lactic Acid Bacteria and Baicalin Against Staphylococcus aureus In Vitro and In Vivo. Foodborne Pathog. Dis. 2025, 22, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, H.; Shen, J.; Jin, Y.; Zhao, Y.; Wang, R.; Fu, Y.; Tian, Y.; Yu, H.; Zhang, J. Curcumin Alleviates LPS-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Toxins 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; Elmassry, I.H.; Taha, N.M.; Elfeky, M.S. Nanocurcumin alleviates inflammation and oxidative stress in LPS-induced mastitis via activation of Nrf2 and suppressing TLR4-mediated NF-κB and HMGB1 signaling pathways in rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 8294–8305. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Li, Z.; Wang, X.; Qiu, Y.; Shen, X.; Chang, G. Protective effect of curcumin-loaded zeolitic imidazolate framework-8-based pH-responsive drug delivery system against Staphylococcus aureus infection. Microb. Pathog. 2025, 200, 107336. [Google Scholar] [CrossRef] [PubMed]
- Disbanchong, P.; Punmanee, W.; Srithanasuwan, A.; Pangprasit, N.; Wongsawan, K.; Suriyasathaporn, W.; Chuammitri, P. Immunomodulatory Effects of Herbal Compounds Quercetin and Curcumin on Cellular and Molecular Functions of Bovine-Milk-Isolated Neutrophils toward Streptococcus agalactiae Infection. Animals 2021, 11, 3286. [Google Scholar] [CrossRef]
- Malik, M.U.U.H.; Hashmi, N.; Khan, M.; Aabdin, Z.u.; Sami, R.; Aljahani, A.H.; Al-Eisa, R.A.; Moawadh, M.S.; Algehainy, N.A. Nutraceutical effect of resveratrol on the mammary gland: Focusing on the NF-κb/nrf2 signaling pathways. Animals 2023, 13, 1266. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, H.; Liu, Z.; Tian, Y.; Gao, X.; Peng, T.; Wang, Z.; Lan, X.; Shen, W.; Xiao, D.; et al. Resveratrol inhibits LPS-induced apoptosis in bovine mammary epithelial cells: The role of PGC1α-SIRT3 axis. Vitr. Cell Dev. Biol. Anim. 2023, 59, 264–276. [Google Scholar] [CrossRef]
- Hu, C.; An, Y.; Ma, X.; Feng, X.; Ma, Y.; Ma, Y. Resveratrol activates PGC-1α pathway via PRAKK1 to regulate mitochondrial biogenesis and alleviate inflammatory responses in bovine mammary epithelial cells. Anim. Nutr. 2025, 2, e2. [Google Scholar] [CrossRef]
- Jia, F.; Ma, W.; Zhang, X.; Wang, D.; Zhou, X. Matrine and baicalin inhibit apoptosis induced by Panton-Valentine leukocidin of Staphylococcus aureus in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 2731–2742. [Google Scholar] [CrossRef]
- Yang, J.; Hai, Z.; Hou, L.; Liu, Y.; Zhang, D.; Zhou, X. Baicalin Attenuates Panton-Valentine Leukocidin (PVL)-Induced Cytoskeleton Rearrangement via Regulating the RhoA/ROCK/LIMK and PI3K/AKT/GSK-3β Pathways in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 4520. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Yang, T.; Duan, H.; Xiao, L.; Zhang, Q.; Zhang, Y.; Dong, W.; Zhao, X. Mechanism of Astragalus Polysaccharide in Alleviating Bovine Mammary Fibrosis Through ROS/NLRP3 Inhibition and EMT Regulation. Antioxidants 2025, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jia, F.; Liu, Y.; Zhou, X. Astragalus polysaccharides and astragaloside IV alleviate inflammation in bovine mammary epithelial cells by regulating Wnt/β-catenin signaling pathway. PLoS ONE 2022, 17, e0271598. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, R.; Lu, X.; Wu, X.; Heneberg, P.; Mao, Y.; Jiang, Q.; Loor, J.; Yang, Z. Lycium barbarum polysaccharides alleviate LPS-induced inflammatory responses through PPARγ/MAPK/NF-κB pathway in bovine mammary epithelial cells. J. Anim. Sci. 2022, 100, skab345. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fang, Z.; Duan, H.; Dong, W.; Xiao, L. Ginsenoside Rg1 Alleviates Blood–Milk Barrier Disruption in Subclinical Bovine Mastitis by Regulating Oxidative Stress-Induced Excessive Autophagy. Antioxidants 2024, 13, 1446. [Google Scholar] [CrossRef]
- Tsutamoto, S.; Iwasaki, Y.; Shinohara, A.; Imamiya, R.; Samukawa, K.; Kawada-Matsuo, M.; Komatsuzawa, H.; Yamada, Y.; Mandokoro, K.; Iwao, H.; et al. Triterpenoid saponin from Panax ginseng increases the sensitivity of methicillin-resistant Staphylococcus aureus to β-lactam and aminoglycoside antibiotics. Microbiol. Spectr. 2024, 12, e0322723. [Google Scholar] [CrossRef]
- Xu, J.; Jia, Z.; Chen, A.; Wang, C. Curcumin ameliorates Staphylococcus aureus-induced mastitis injury through attenuating TLR2-mediated NF− κB activation. Microb. Pathog. 2020, 142, 104054. [Google Scholar] [CrossRef]
- Suresh, S.; Sankar, P.; Kalaivanan, R.; Telang, A.G. Ameliorative effect of nanocurcumin on Staphylococcus aureus-induced mouse mastitis by oxidative stress suppression. Inorg. Nano-Metal. Chem. 2022, 52, 1003–1011. [Google Scholar] [CrossRef]
- Jiang, M.; Lv, Z.; Huang, Y.; Cheng, Z.; Meng, Z.; Yang, T.; Yan, Q.; Lin, M.; Zhan, K.; Zhao, G. Quercetin alleviates lipopolysaccharide-induced inflammatory response in bovine mammary epithelial cells by suppressing TLR4/NF-κB signaling pathway. Front. Vet. Sci. 2022, 9, 915726. [Google Scholar] [CrossRef]
- Luo, S.; Kang, X.; Luo, X.; Li, C.; Wang, G. Study on the inhibitory effect of quercetin combined with gentamicin on the formation of Pseudomonas aeruginosa and its bioenvelope. Microb. Pathog. 2023, 182, 106274. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, W.; Shokrollahi, B.; Wang, W.; Abdel-Shafy, H.; Deng, T. Role of Quercetin in Modulating Inflammation and Epigenetic Regulation of Staphylococcus aureus-Induced Bovine Mastitis. J. Agric. Food Chem. 2025, 73, 8784–8797. [Google Scholar] [CrossRef]
- Tong, C.; Chen, T.; Chen, Z.; Wang, H.; Wang, X.; Liu, F.; Dai, H.; Wang, X.; Li, X. Forsythiaside a plays an anti-inflammatory role in LPS-induced mastitis in a mouse model by modulating the MAPK and NF-κB signaling pathways. Res. Vet. Sci. 2021, 136, 390–395. [Google Scholar] [CrossRef]
- Shen, P.; Yu, J.; Long, X.; Huang, X.; Tong, C.; Wang, X. Effect of forsythoside A on the transcriptional profile of bovine mammary epithelial cells challenged with lipoteichoic acid. Reprod. Domest. Anim. 2023, 58, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gao, Y.; Hao, Z.; Liu, J.; Zhou, G.; Liu, F.; Li, X.; Tong, C.; Wang, X. Forsythoside A regulates autophagy and apoptosis through the AMPK/mTOR/ULK1 pathway and alleviates inflammatory damage in MAC-T cells. Int. Immunopharmacol. 2023, 118, 110053. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Zhang, H.; Zhang, X.; Gui, R.; Zhang, K.; Li, Y.; Zhou, M.; Tong, C.; Huang, S.C.; et al. Transcriptomic profiling of lipopolysaccharide-challenged bovine mammary epithelial cells treated with forsythoside A. Anim. Biotechnol. 2023, 34, 4523–4537. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, Y.; Zhang, H.; Hao, Z.; Zhou, G.; Wen, H.; Su, Q.; Tong, C.; Yang, X.; Wang, X. Forsythiaside A attenuates mastitis via PINK1/Parkin-mediated mitophagy. Phytomedicine 2024, 125, 155358. [Google Scholar] [CrossRef]
- Lyu, C.; Yuan, B.; Meng, Y.; Cong, S.; Che, H.; Ji, X.; Wang, H.; Chen, C.; Li, X.; Jiang, H.; et al. Puerarin Alleviates H(2)O(2)-Induced Oxidative Stress and Blood-Milk Barrier Impairment in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 7742. [Google Scholar] [CrossRef]
- Xie, Y.; Li, X.; Xu, D.; He, D.; Wang, J.; Bi, J.; Liu, J.; Fu, S. Hordenine Alleviates Lipopolysaccharide-Induced Mastitis by Suppressing Inflammation and Oxidative Stress, Modulating Intestinal Microbiota, and Preserving the Blood–Milk Barrier. J. Agric. Food Chem. 2024, 72, 21503–21519. [Google Scholar] [CrossRef]
- Purgato, G.A.; Píccolo, M.S.; Moreira, M.A.S.; Pizziolo, V.R.; Diaz-Muñoz, G.; Rossi, C.C.; Diaz, M.A.N. Isolation and identification of antimicrobial multicyclic terpenoids from the medicinal plant Salvia officinalis and development of a formulation against clinical Staphylococcus aureus strains. Lett. Appl. Microbiol. 2024, 77, ovae077. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Qiu, M.; Pu, Z.; Long, N.; Yang, M.; Ren, K.; Ning, R.; Zhang, S.; Peng, F.; Sun, F.; et al. Geraniol-a potential alternative to antibiotics for bovine mastitis treatment without disturbing the host microbial community or causing drug residues and resistance. Front. Cell Infect. Microbiol. 2023, 13, 1126409. [Google Scholar] [CrossRef] [PubMed]
- Corona-Gómez, L.; Hernández-Andrade, L.; Mendoza-Elvira, S.; Suazo, F.M.; Ricardo-González, D.I.; Quintanar-Guerrero, D. In vitro antimicrobial effect of essential tea tree oil (Melaleuca alternifolia), thymol, and carvacrol on microorganisms isolated from cases of bovine clinical mastitis. Int. J. Vet. Sci. Med. 2022, 10, 72–79. [Google Scholar] [CrossRef]
- Šukele, R.; Skadiņš, I.; Koka, R.; Bandere, D. Antibacterial effects of oak bark (Quercus robur) and heather herb (Calluna vulgaris L.) extracts against the causative bacteria of bovine mastitis. Vet. World 2022, 15, 2315–2322. [Google Scholar] [CrossRef]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum vulgare L. and Satureja montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Huang, Z.; Yin, B.; Umar, T.; Yang, C.; Zhang, X.; Jing, H.; Guo, S.; Guo, M.; et al. Vitexin Mitigates Staphylococcus aureus-Induced Mastitis via Regulation of ROS/ER Stress/NF-κB/MAPK Pathway. Oxid. Med. Cell Longev. 2022, 2022, 7977433. [Google Scholar] [CrossRef]
- Carvalho, C.; Costa, A.R.; Silva, F.; Oliveira, A. Bacteriophages and their derivatives for the treatment and control of food-producing animal infections. Crit. Rev. Microbiol. 2017, 43, 583–601. [Google Scholar] [CrossRef]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z.; et al. Bacteriophage Cocktails Protect Dairy Cows Against Mastitis Caused By Drug Resistant Escherichia coli Infection. Front. Cell Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef]
- Ribeiro, K.V.G.; Ribeiro, C.; Dias, R.S.; Cardoso, S.A.; de Paula, S.O.; Zanuncio, J.C.; de Oliveira, L.L. Bacteriophage Isolated from Sewage Eliminates and Prevents the Establishment of Escherichia Coli Biofilm. Adv. Pharm. Bull. 2018, 8, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Horiuk, Y. Therapeutic efficacy of bacteriophage drug Fagomast in clinical mastitis of cows. Sci. Messenger LNU Vet. Med. Biotechnologies. Ser. Vet. Sci. 2022, 24, 89–93. [Google Scholar] [CrossRef]
- Mohammadian, F.; Rahmani, H.K.; Bidarian, B.; Khoramian, B. Isolation and evaluation of the efficacy of bacteriophages against multidrug-resistant (MDR), methicillin-resistant (MRSA) and biofilm-producing strains of Staphylococcus aureus recovered from bovine mastitis. BMC Vet. Res. 2022, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef]
- Popitool, K.; Wataradee, S.; Wichai, T.; Noitang, S.; Ajariyakhajorn, K.; Charoenrat, T.; Boonyaratanakornkit, V.; Sooksai, S. Potential of Pm11 antimicrobial peptide against bovine mastitis pathogens. Am. J. Vet. Res. 2022, 84, ajvr.22.06.0096. [Google Scholar] [CrossRef]
- Orozco, R.M.Q.; Oshiro, K.G.N.; Pinto, I.B.; Buccini, D.F.; Almeida, C.V.; Marin, V.N.; de Souza, C.M.; Macedo, M.L.R.; Cardoso, M.H.; Franco, O.L. Employment of mastoparan-like peptides to prevent Staphylococcus aureus associated with bovine mastitis. J. Bacteriol. 2024, 206, e0007124. [Google Scholar] [CrossRef]
- Shah, P.; Shrivastava, S.; Gogoi, P.; Saxena, S.; Srivastava, S.; Singh, R.J.; Godara, B.; Kumar, N.; Gaur, G.K. Wasp venom peptide (Polybia MP-1) shows antimicrobial activity against multi drug resistant bacteria isolated from mastitic cow milk. Int. J. Pept. Res. Ther. 2022, 28, 44. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Lu, X.; Zhao, R.; Chen, Z.; Li, M.; Xu, T.; Mao, Y.; Yang, Y.; Yang, Z. Directed Expression of Tracheal Antimicrobial Peptide as a Treatment for Bovine-Associated Staphylococcus Aureus-Induced Mastitis in Mice. Front. Vet. Sci. 2021, 8, 700930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, J.; Liu, T.; Zhu, J.; Ma, Y.; Li, N.; Fan, W.; Wang, J.; Shen, H. Evaluation of the Therapeutic Effect of Fly Maggot Antimicrobial Peptide in Staphylococcus aureus-Induced Mouse Mastitis Model. Open Vet. J. 2025, 15, 2540–2550. [Google Scholar] [CrossRef]
- Cho, H.S.; Kim, D.; Jeon, H.; Somasundaram, P.; Soundrarajan, N.; Park, C. Bactericidal activities and biochemical features of 16 antimicrobial peptides against bovine-mastitis causative pathogens. Vet. Res. 2024, 55, 150. [Google Scholar] [CrossRef]
- Bennett, S.; Ben Said, L.; Lacasse, P.; Malouin, F.; Fliss, I. Susceptibility to Nisin, Bactofencin, Pediocin and Reuterin of Multidrug Resistant Staphylococcus aureus, Streptococcus dysgalactiae and Streptococcus uberis Causing Bovine Mastitis. Antibiotics 2021, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Motrenko, M.; Lange, A.; Kalińska, A.; Gołębiewski, M.; Kunowska-Slósarz, M.; Nasiłowska, B.; Czwartos, J.; Skrzeczanowski, W.; Orzeszko-Rywka, A.; Jagielski, T.; et al. Green Nanoparticle Synthesis in the Application of Non-Bacterial Mastitis in Cattle. Molecules 2025, 30, 1369. [Google Scholar] [CrossRef]
- Lange, A.; Grzenia, A.; Wierzbicki, M.; Strojny-Cieslak, B.; Kalińska, A.; Gołębiewski, M.; Radzikowski, D.; Sawosz, E.; Jaworski, S. Silver and Copper Nanoparticles Inhibit Biofilm Formation by Mastitis Pathogens. Anim. 2021, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Daraba, O.M.; Ichim, D.L.; Popescu, I.; Solcan, C.; Solcan, G. Silver Nanoparticles Biocomposite Films with Antimicrobial Activity: In Vitro and In Vivo Tests. Int. J. Mol. Sci. 2022, 23, 10671. [Google Scholar] [CrossRef]
- Mostafa Abdalhamed, A.; Zeedan, G.S.G.; Ahmed Arafa, A.; Shafeek Ibrahim, E.; Sedky, D.; Abdel Nabey Hafez, A. Detection of Methicillin-Resistant Staphylococcus aureus in Clinical and Subclinical Mastitis in Ruminants and Studying the Effect of Novel Green Synthetized Nanoparticles as One of the Alternative Treatments. Vet. Med. Int. 2022, 2022, 6309984. [Google Scholar] [CrossRef]
- Panchal, R.; Shukla, V.; Agrawal, Y. Nanoparticles in Managing Bovine Mastitis Pathogens—Isolation, Identification, and Therapeutic Strategies. BioNanoScience 2024, 14, 2466–2474. [Google Scholar] [CrossRef]
- Orellano, M.S.; Bohl, L.P.; Breser, M.L.; Isaac, P.; Falcone, R.D.; Porporatto, C. A comparative study of antimicrobial activity of differently-synthesized chitosan nanoparticles against bovine mastitis pathogens. Soft Matter 2021, 17, 694–703. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Wu, N.; Rong, N.; Kang, C.; Jian, S.; Chen, C.; Chen, C.; Zhang, X. Synthesis of Escherichia coli OmpA Oral Nanoparticles and Evaluation of Immune Functions against the Major Etiologic Agent of Cow Mastitis. Vaccines 2021, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Emam, A.N.; Hefny, E.G.; Koraney, N.F.; Mansour, A.S.; Salama, A.M.; Ali, S.F.; Aboolo, S.H.; Shahein, M.A. Silver nanoparticles enhance the effectiveness of traditional antibiotics against S. aureus causing bovine mastitis within the safety limit. J. Nanoparticle Res. 2021, 23, 243. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, A.B.; Gaur, P.; Mishra, V.; Huma, Z.I.; Sharma, N.; Son, Y.O. Bioengineered Ciprofloxacin-Loaded Chitosan Nanoparticles for the Treatment of Bovine Mastitis. Biomedicines 2022, 10, 3282. [Google Scholar] [CrossRef]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcón Godoy, C.; Cayupe Rivas, B.; González-Casanova, J.; Rojas-Gómez, D.; Caro Fuentes, N. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Godoy, C.A.; Balic, I.; Moreno, A.A.; Diaz, O.; Arenas Colarte, C.; Bruna Larenas, T.; Gamboa, A.; Caro Fuentes, N. Antimicrobial and Antibiofilm Activity of Chitosan Nanoparticles Against Staphylococcus aureus Strains Isolated from Bovine Mastitis Milk. Pharmaceutics 2025, 17, 186. [Google Scholar] [CrossRef]
- Kosaristanova, L.; Bytesnikova, Z.; Fialova, T.; Pekarkova, J.; Svec, P.; Ondreas, F.; Jemelikova, V.; Ridoskova, A.; Makovicky, P.; Sivak, L.; et al. In vivo evaluation of selenium-tellurium based nanoparticles as a novel treatment for bovine mastitis. J. Anim. Sci. Biotechnol. 2024, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, Y.; Kang, X.; Liu, P.; Wang, G. Research progress on the association between mastitis and gastrointestinal microbes in dairy cows and the effect of probiotics. Microb. Pathog. 2022, 173, 105809. [Google Scholar] [CrossRef]
- Steinberg, R.S.; Silva, L.; de Souza, M.R.; Reis, R.B.; Bicalho, A.F.; Nunes, J.P.S.; Dias, A.A.M.; Nicoli, J.R.; Neumann, E.; Nunes, Á.C. Prospecting of potentially probiotic lactic acid bacteria from bovine mammary ecosystem: Imminent partners from bacteriotherapy against bovine mastitis. Int. Microbiol. 2022, 25, 189–206. [Google Scholar] [CrossRef]
- Izhar, M.Z.; Nawaz, M.; Yaqub, T.; Avais, M. Effect of probiotic Lactobacillus plantarum CM49 on microbial profile and lactobacilli counts in milk of mastitic cattle. BMC Microbiol. 2025, 25, 109. [Google Scholar] [CrossRef]
- Sharma, N.; Jeong, D.K. Stem cell research: A novel boulevard towards improved bovine mastitis management. Int. J. Biol. Sci. 2013, 9, 818–829. [Google Scholar] [CrossRef]
- Yousefi Dehbidi, M.; Goodarzi, N.; Azhdari, M.H.; Doroudian, M. Mesenchymal stem cells and their derived exosomes to combat Covid-19. Rev. Med. Virol. 2022, 32, e2281. [Google Scholar] [CrossRef]
- Pokorska, J.; Sawicki, S.; Gabryś, J.; Kułaj, D.; Bauer, E.A.; Lenart-Boroń, A.; Bulanda, K.; Kuchta-Gładysz, M.; Grzesiakowska, A.; Kemilew, J.; et al. The use of stem cells in the treatment of mastitis in dairy cows. Sci. Rep. 2024, 14, 10349. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Saini, S.; Ansari, S.; Verma, V.; Chopra, S.; Sharma, V.; Devi, P.; Malakar, D. Allogenic umbilical cord blood-mesenchymal stem cells are more effective than antibiotics in alleviating subclinical mastitis in dairy cows. Theriogenology 2022, 187, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Cai, Y.; Wang, Y.; Sun, N.; An, N.; Yang, J.; Li, Y.; Qin, S.; Du, R. Mitigative Effect and Mechanism of Caffeic Acid Combined with Umbilical Cord-Mesenchymal Stem Cells on LPS-Induced Mastitis. J. Agric. Food Chem. 2024, 72, 23271–23285. [Google Scholar] [CrossRef] [PubMed]
- Peralta, O.A.; Carrasco, C.; Vieytes, C.; Tamayo, M.J.; Muñoz, I.; Sepulveda, S.; Tadich, T.; Duchens, M.; Melendez, P.; Mella, A.; et al. Safety and efficacy of a mesenchymal stem cell intramammary therapy in dairy cows with experimentally induced Staphylococcus aureus clinical mastitis. Sci. Rep. 2020, 10, 2843. [Google Scholar] [CrossRef]
- Leitner, G.; Zilberman, D.; Papirov, E.; Shefy, S. Assessment of acoustic pulse therapy (APT), a non-antibiotic treatment for dairy cows with clinical and subclinical mastitis. PLoS ONE 2018, 13, e0199195. [Google Scholar] [CrossRef]
- Leitner, G.; Papirov, E.; Gilad, D.; Haran, D.; Arkin, O.; Zuckerman, A.; Lavon, Y. New treatment option for clinical and subclinical mastitis in dairy cows using acoustic pulse technology (APT). Dairy. 2021, 2, 256–269. [Google Scholar] [CrossRef]
- Blum, S.E.; Krifuks, O.; Weisblith, L.; Fleker, M.; Lavon, Y.; Zuckerman, A.; Hefer, Y.; Goldhor, O.; Gilad, D.; Schcolnic, T.; et al. Evaluation of acoustic pulse technology as a non-antibiotic therapy for bovine intramammary infections: Assessing bacterial cure vs. recovery from inflammation. Front. Vet. Sci. 2023, 10, 1079269. [Google Scholar] [CrossRef]
- Merin, U.; Gilad, D.; Jacoby, S.; Keynan, B.; Hefer, Y.; Lavon, Y.; Leitner, G. Retrospective evaluation of udder recovery of cows with subclinical mastitis following treatment with acoustic pulse technology (APT) on commercial dairy farms and its economic impact. PLoS ONE 2024, 19, e0303947. [Google Scholar] [CrossRef]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Moreira, S.H.; Pazzini, J.M.; Álvarez, J.L.G.; Cassino, P.C.; Bustamante, C.C.; Bernardes, F.J.L.; Kajiura, C.Y.; De Nardi, A.B. Evaluation of angiogenesis, inflammation, and healing on irradiated skin graft with low-level laser therapy in rats (Rattus norvegicus albinus wistar). Lasers Med. Sci. 2020, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, M.; da Rosa Fraga, D.; Cidral-Filho, F.J.; Veiga, E.A.; Secco, T.R.; Trada, A.L.; Torquetti, F.J.; de Lima Gomes, M.; Teixeira, K.S.; Machado, T.B. Effect of Photobiomodulation With Laser on The Composition, Production and Quality of The Milk of Cows with Clinical Mastitis. Rev. De Gestão Social. E Ambient. 2024, 18, 1–13. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Silva, L.O.; da Silva Souza, K.L.; de Jesus Beloti, L.; Neto, W.M.R.; Núñez, S.C.; Frias, D.F.R. Use of photodynamic therapy and photobiomodulation as alternatives for microbial control on clinical and subclinical mastitis in sheep. Lasers Med. Sci. 2022, 37, 2305–2310. [Google Scholar] [CrossRef]
- Junior, R.; Campanholi, K.; Maciel, B.C.; Pinto, L.A.M.; de Morais, F.A.P.; Rando, F.D.S.; Pereira, P.C.S.; Pozza, M.; Nakamura, C.V.; Caetano, W. Natural photosensitizer-loaded in micellar copolymer to prevent bovine mastitis: A new post-dipping protocol on milking. Photodiagnosis Photodyn. Ther. 2023, 42, 103337. [Google Scholar] [CrossRef]
- Mendina, G.R.; Damián, J.P.; Meikle, A.; Chilibroste, P.; Bentancur, O.; Adrien, M.L. Udder Hygiene and Mastitis Indicators in Contrasting Environmental Conditions during Half-Time Confinement in Pasture-Based Dairy Systems. Animals 2023, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Duniere, L.; Frayssinet, B.; Achard, C.; Chevaux, E.; Plateau, J. Conditioner application improves bedding quality and bacterial composition with potential beneficial impacts for dairy cow’s health. Microbiol. Spectr. 2024, 12, e0426323. [Google Scholar] [CrossRef]
- Curone, G.; Filipe, J.; Cremonesi, P.; Trevisi, E.; Amadori, M.; Pollera, C.; Castiglioni, B.; Turin, L.; Tedde, V.; Vigo, D.; et al. What we have lost: Mastitis resistance in Holstein Friesians and in a local cattle breed. Res. Vet. Sci. 2018, 116, 88–98. [Google Scholar] [CrossRef]
- Schunig, R.; Busanello, M.; Nogara, K.F.; Zopollatto, M. Cow-level risk factors associated with the increase in somatic cell count and the occurrence of subclinical mastitis in Brazilian Holstein and Jersey dairy cows. Prev. Vet. Med. 2024, 227, 106208. [Google Scholar] [CrossRef] [PubMed]
- Jukna, V.; Meškinytė, E.; Antanaitis, R.; Juozaitienė, V. Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows. Animals 2024, 14, 2065. [Google Scholar] [CrossRef]
- Vidlund, J.; Gelalcha, B.D.; Gillespie, B.E.; Agga, G.E.; Schneider, L.; Swanson, S.M.; Frady, K.D.; Kerro Dego, O. Efficacy of novel staphylococcal surface associated protein vaccines against Staphylococcus aureus and non-aureus staphylococcal mastitis in dairy cows. Vaccine 2024, 42, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, E.V.; Kapustin, A.V.; Avduevskaya, N.N. Study of the vaccination effects against Staphylococcus aureus, causing mastitis and endometritis in cows. Vet. Sci. Today 2024, 13, 360–365. [Google Scholar] [CrossRef]
- Zeng, X.; Vidlund, J.; Gillespie, B.; Cao, L.; Agga, G.E.; Lin, J.; Dego, O.K. Evaluation of immunogenicity of enterobactin conjugate vaccine for the control of Escherichia coli mastitis in dairy cows. J. Dairy. Sci. 2023, 106, 7147–7163. [Google Scholar] [CrossRef]
- Urakawa, M.; Zhuang, T.; Sato, H.; Takanashi, S.; Yoshimura, K.; Endo, Y.; Katsura, T.; Umino, T.; Tanaka, K.; Watanabe, H.; et al. Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis. Anim. Sci. J. 2022, 93, e13764. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.C.; Wang, Y.; Li, H.; Wang, X.M.; Wu, Y.; Zhang, D.R.; Gao, S.; Qi, Z.L. Impact of yeast and lactic acid bacteria on mastitis and milk microbiota composition of dairy cows. AMB Express 2020, 10, 22. [Google Scholar] [CrossRef]
- Poindexter, M.B.; Kweh, M.F.; Zimpel, R.; Zuniga, J.; Lopera, C.; Zenobi, M.G.; Jiang, Y.; Engstrom, M.; Celi, P.; Santos, J.E.P.; et al. Feeding supplemental 25-hydroxyvitamin D(3) increases serum mineral concentrations and alters mammary immunity of lactating dairy cows. J. Dairy. Sci. 2020, 103, 805–822. [Google Scholar] [CrossRef]
- de Aguiar, S.C.; Cottica, S.M.; Dos Santos, S.T.; da Fonseca, J.M.; da Silva Leite, L.; da Silva, M.L. Antioxidant Activity, Phenolic Acid, and Flavonoid Composition of an Antiseptic Ointment Based on Aloe and Green Propolis and Its Potential for Preventing Mastitis in Dairy Cows. Vet. Sci. 2025, 12, 248. [Google Scholar] [CrossRef]
- Silva, C.; Vidal, C.S.; Filho, S.M.A.; Agatão, I.M.; Berbert, L.C.; Salles, J.B.; Cardoso, A.M.; Kuster, R.M.; Victório, C.P.; Assis, M.C. Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle. Molecules 2025, 30, 2387. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Mathew, D.; Theresa, M.; Sandhya, C.; Radhakrishnan, E.K. Development of Zinc Oxide Nanoparticle and Lemongrass Essential Oil Incorporated Nanoformulation-Based External Teat Sealant to Prevent Mastitis in Dairy Cows. BioNanoScience 2025, 15, 204. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Kot, M.; Radzikowski, D.; Smulski, S.; Gołębiewski, M. Silver and Copper Nanoparticles as the New Biocidal Agents Used in Pre- and Post-Milking Disinfectants with the Addition of Cosmetic Substrates in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 1658. [Google Scholar] [CrossRef]
- Feng, Y.; Niu, H.; Wang, F.; Ivey, S.J.; Wu, J.J.; Qi, H.; Almeida, R.A.; Eda, S.; Cao, Q. SocialCattle: IoT-based mastitis detection and control through social cattle behavior sensing in smart farms. IEEE Internet Things J. 2021, 9, 10130–10138. [Google Scholar] [CrossRef]
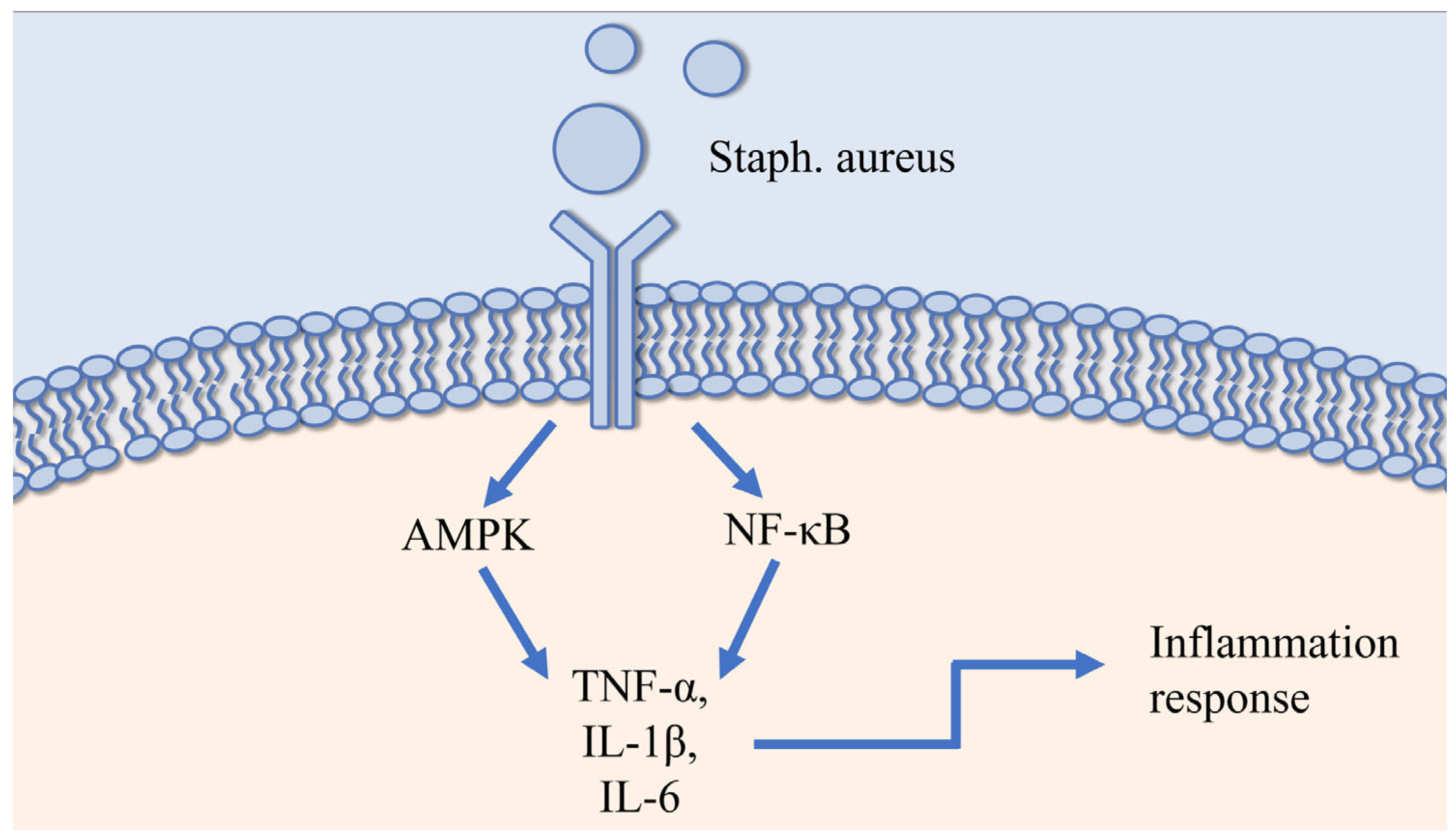
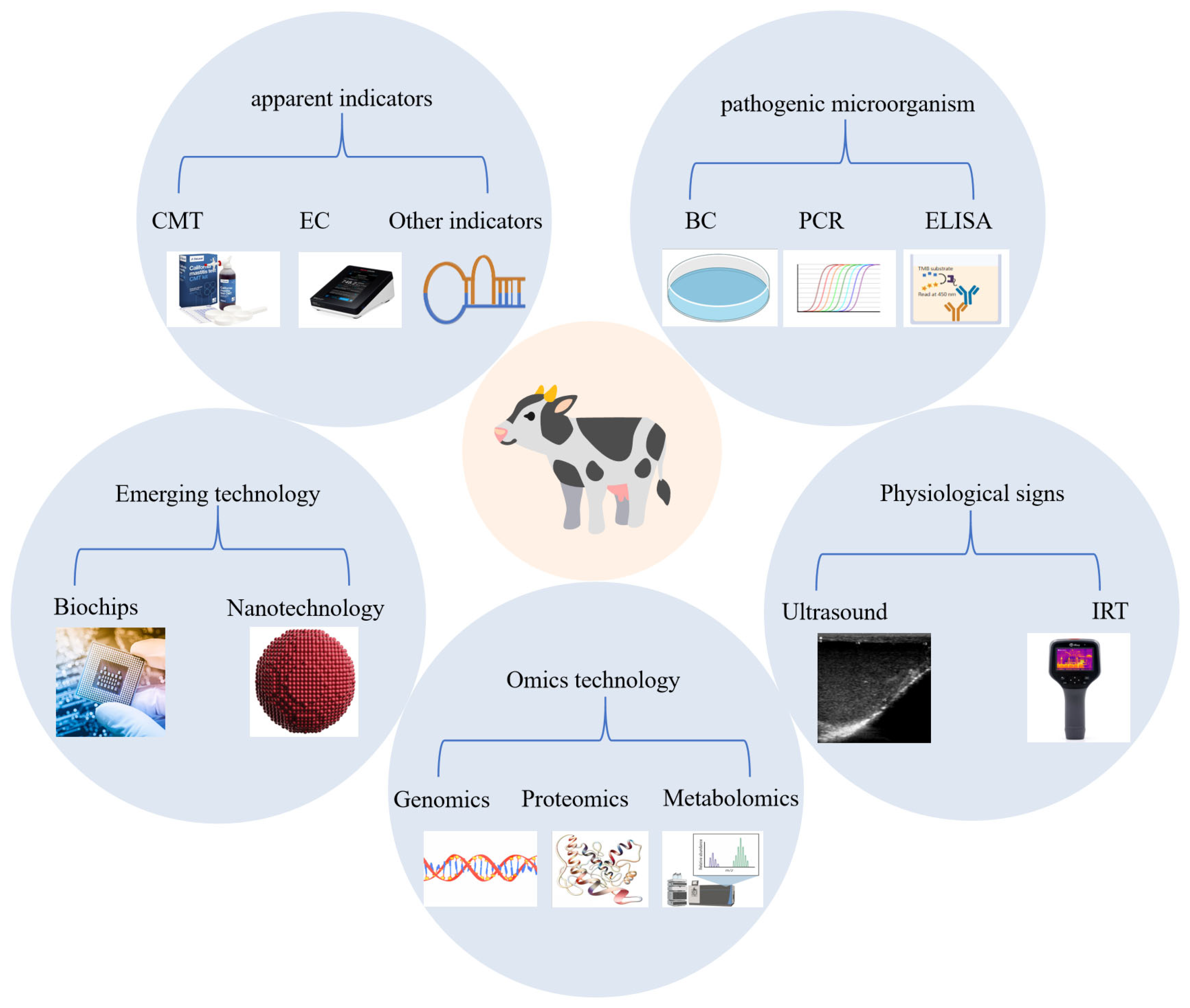
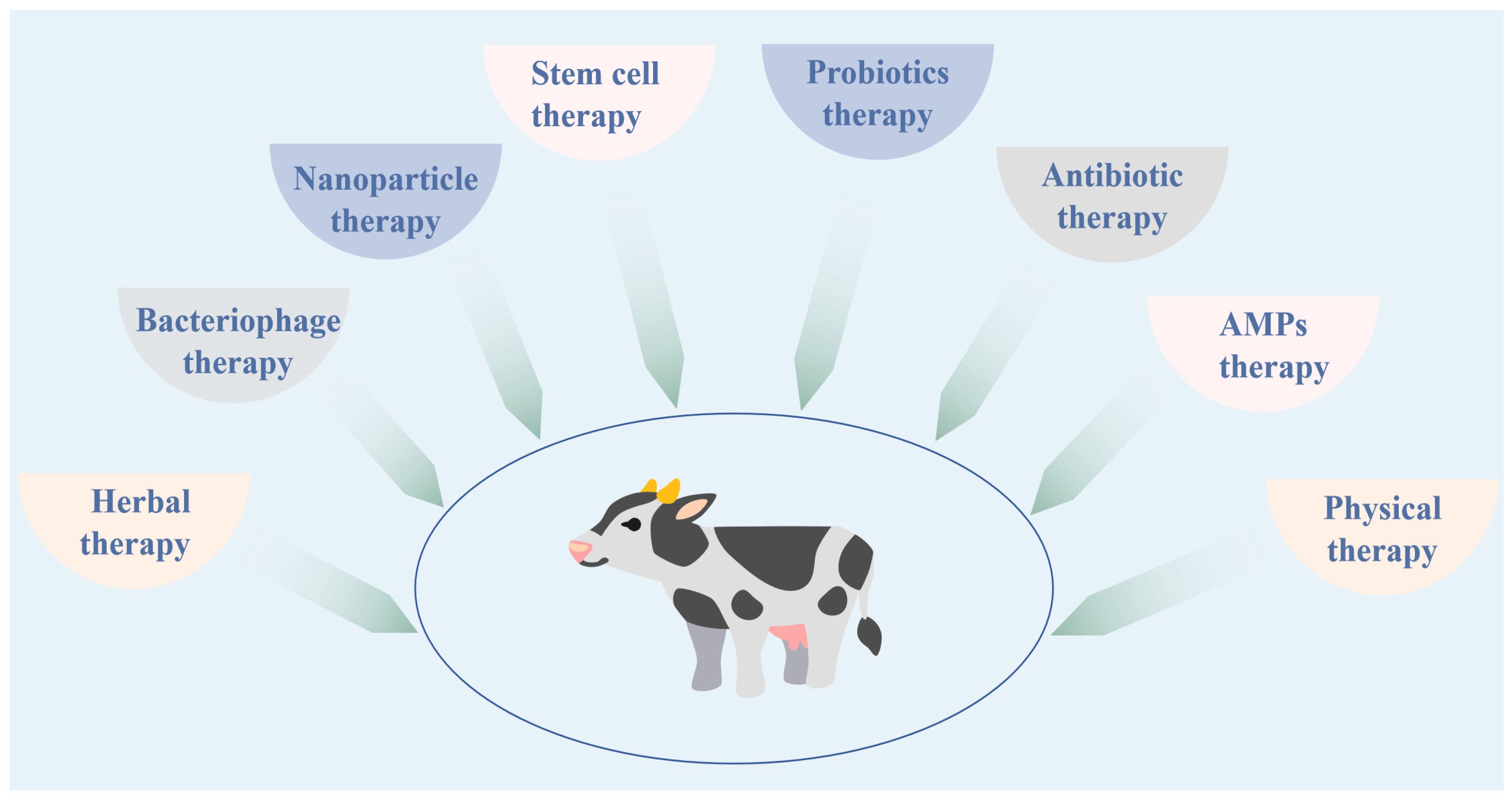
| Pathogen Genus | Name of Pathogen | References |
|---|---|---|
| Staphylococcus spp. | Staph. aureus | [49,50,51,52,53] |
| CNS | [45,46,54,55,56] | |
| Streptococcus spp. | Strep. agalactiae | [57,58,59,60,61] |
| Strep. uberis | [62,63,64,65,66,67,68,69,70,71] | |
| Strep. dysgalactiae | [72,73,74,75] | |
| Strep. parauberis | [71] | |
| Aerococcus viridans | [76,77,78] | |
| Enterobacteriaceae spp. | E. coli | [79,80,81,82,83] |
| K. pneumoniae | [84,85,86,87,88] | |
| K. oxytoca | [88,89,90] | |
| Enterobacter cloacae | [89,90] | |
| Serratia marcescens | [88,91,92] | |
| Pseudomonas spp. | Pseudomonas aeruginosa (P. aeruginosa) | [93,94,95,96] |
| Corynebacterium spp. | Corynebacteriumbovis | [97] |
| Bacillus spp. | Bacillus cereus | [98] |
| Lactococcus spp. | Lactococcus lactis | [99,100] |
| Lactococcus garvieae | [101,102,103,104] | |
| Mycoplasma spp. | Mycoplasma spp. | [105,106,107,108,109] |
| Actinomyces spp. | T. pyogenes | [110,111,112,113] |
| Fungi spp. | Yeasts spp. | [114,115,116,117,118] |
| Mold spp. | [117,119] | |
| Algae spp. | Prototheca spp. | [120,121,122,123,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Zhang, Z.; Wang, Z.; Lin, X.; Dong, X.; Hou, Q. Comprehensive Prevention and Control of Mastitis in Dairy Cows: From Etiology to Prevention. Vet. Sci. 2025, 12, 800. https://doi.org/10.3390/vetsci12090800
Yu W, Zhang Z, Wang Z, Lin X, Dong X, Hou Q. Comprehensive Prevention and Control of Mastitis in Dairy Cows: From Etiology to Prevention. Veterinary Sciences. 2025; 12(9):800. https://doi.org/10.3390/vetsci12090800
Chicago/Turabian StyleYu, Wenjing, Zixuan Zhang, Zhonghua Wang, Xueyan Lin, Xusheng Dong, and Qiuling Hou. 2025. "Comprehensive Prevention and Control of Mastitis in Dairy Cows: From Etiology to Prevention" Veterinary Sciences 12, no. 9: 800. https://doi.org/10.3390/vetsci12090800
APA StyleYu, W., Zhang, Z., Wang, Z., Lin, X., Dong, X., & Hou, Q. (2025). Comprehensive Prevention and Control of Mastitis in Dairy Cows: From Etiology to Prevention. Veterinary Sciences, 12(9), 800. https://doi.org/10.3390/vetsci12090800






