Antioxidant Activity of Paederia foetida Linn. Leaf Extract and Its Effect on Bovine Sperm Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. Experimental Design
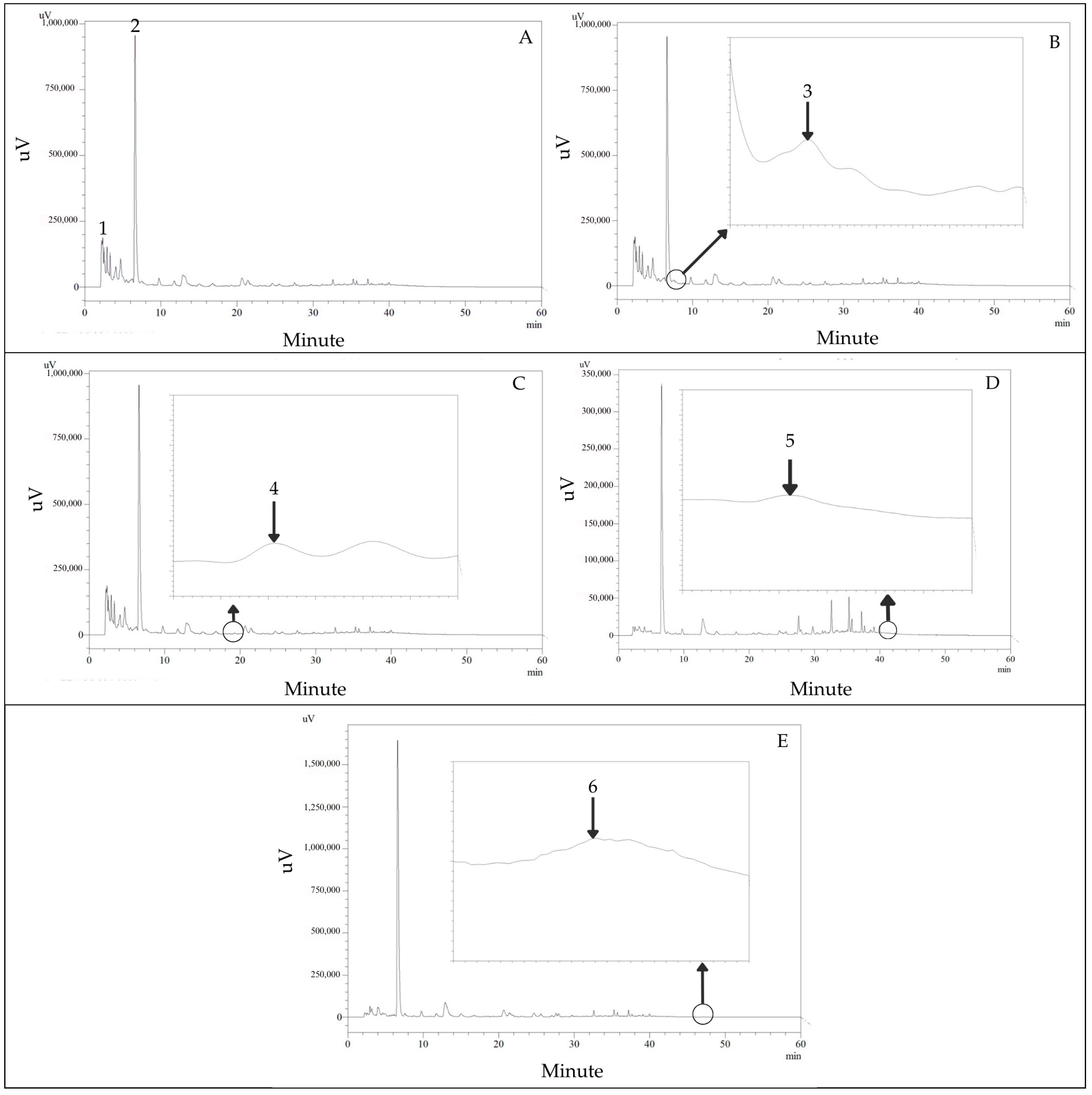
2.3. Analysis of Phytochemical Content by High-Performance Liquid Chromatography (HPLC)
2.4. Antioxidant Characteristics
2.4.1. The Inhibition of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Determination
2.4.2. The Inhibition of 2,2′-Aziobis-[3-Ethylbenzthiazoline-6-Sulfonic Acid] (ABTS) Determination
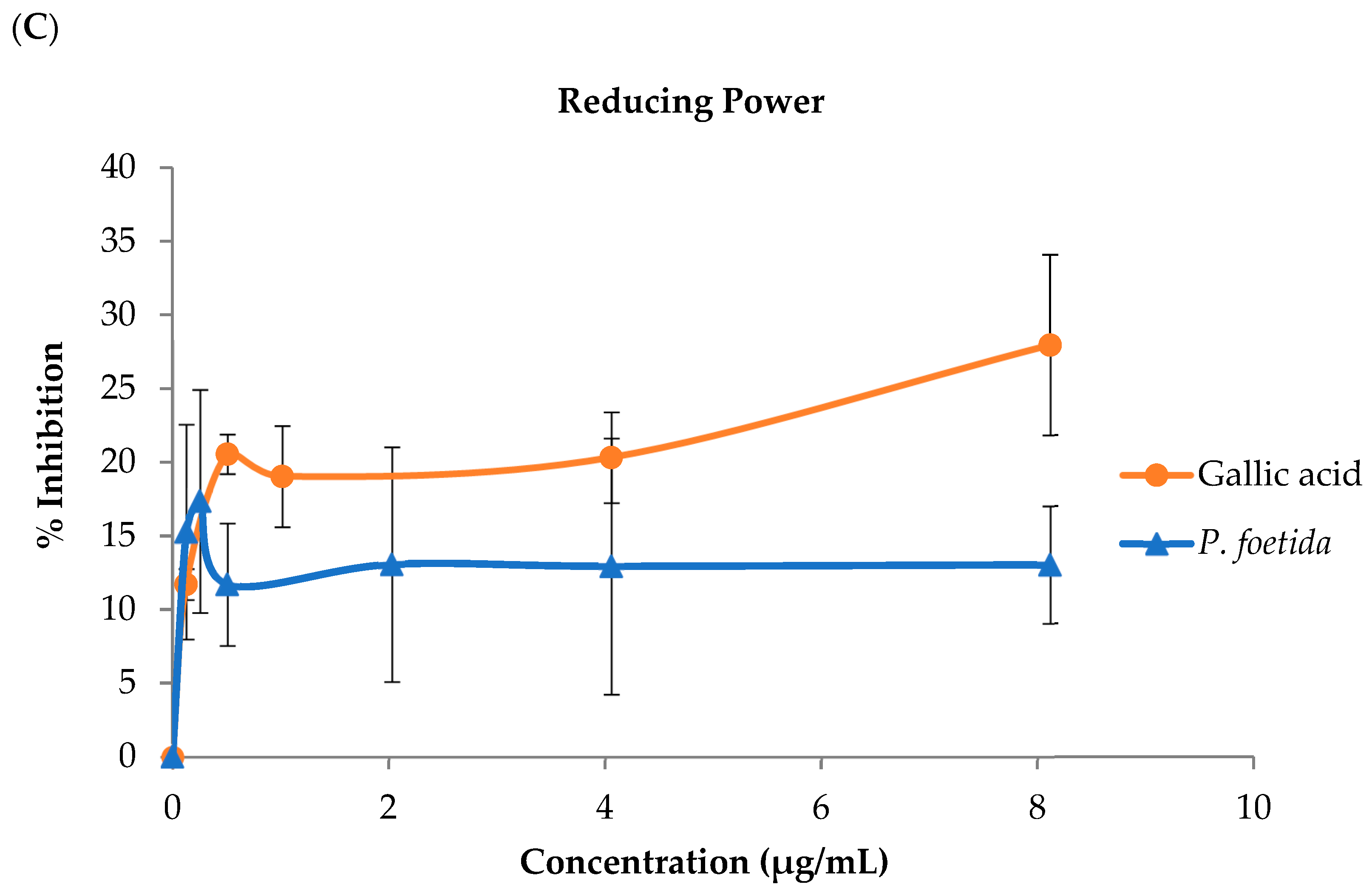
2.4.3. Reducing Power Determination
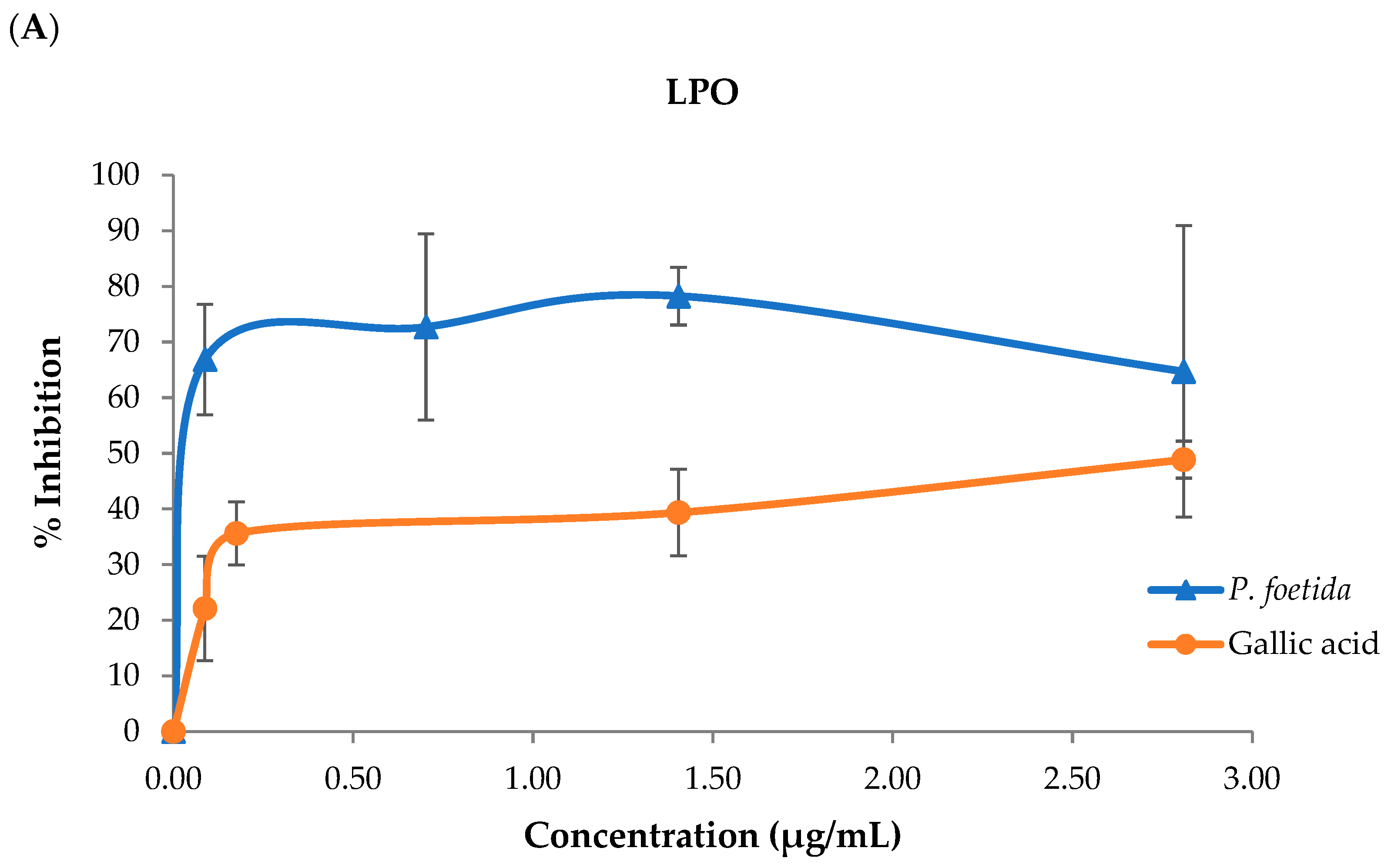
2.5. The Inhibition of Lipid Peroxidation Determination
2.6. Inhibition of Advanced Oxidation Protein Products (AOPP) Formation
2.7. Inhibition of Advanced Glycation End Product (AGE) Formation
2.8. Sperm Quality Tests
2.8.1. Sperm Motility
2.8.2. Viability and Acrosome Integrity of Sperm Measurement
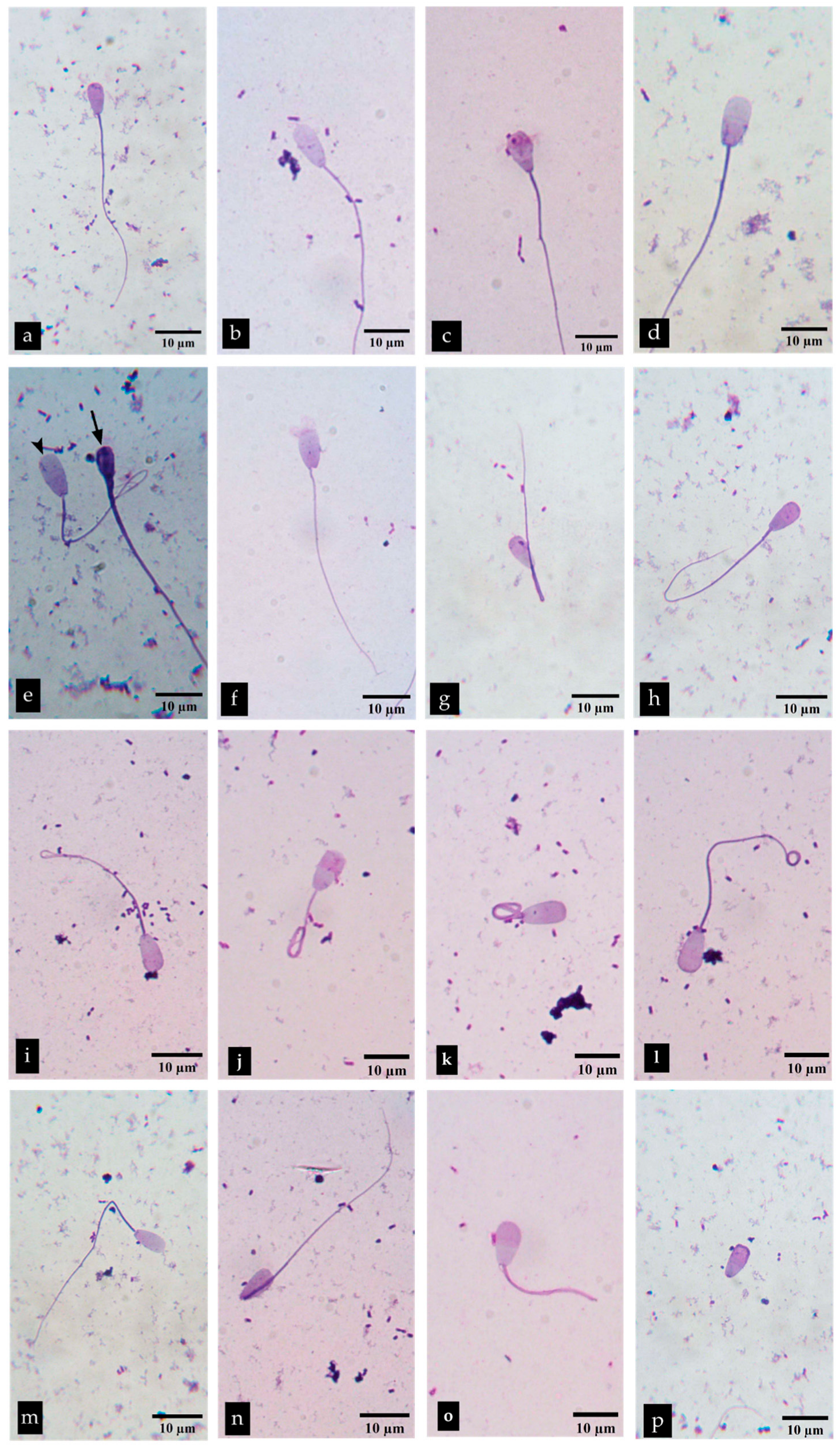
2.8.3. Sperm Morphology
2.9. Antioxidant Properties of Sperm Assay
2.9.1. Lipid Peroxidation (LPO) Assay
2.9.2. Inhibition of Formation of Advanced Oxidation Protein Products (AOPPs)
2.9.3. Suppression of Advanced Glycation End Products (AGEs) Formation
2.10. Statistical Analysis
3. Results
3.1. High-Performance Liquid Chromatography (HPLC) Analysis
3.2. Antioxidant Properties
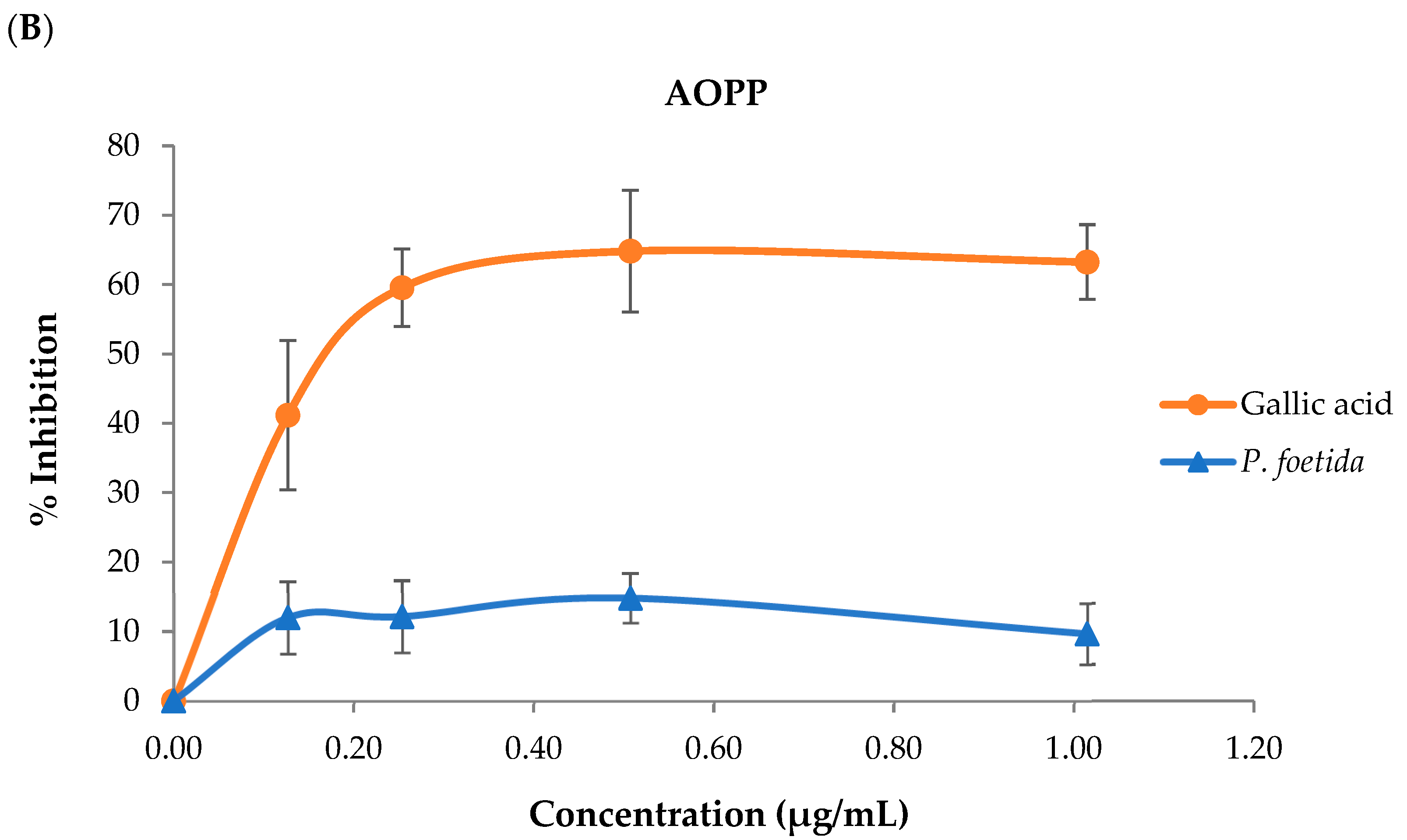
3.3. Lipid Peroxidation (LPO), Advanced Oxidation Protein Products (AOPPs), and Advanced Glycation End Products (AGEs)
3.4. Sperm Quality Test
3.4.1. The Effect of P. foetida on Sperm Motility
3.4.2. The Effect of P. foetida on Sperm Viability and Acrosome Integrity
3.4.3. The Effect of P. foetida on Sperm Morphology
3.5. Antioxidant Characteristics in Sperm
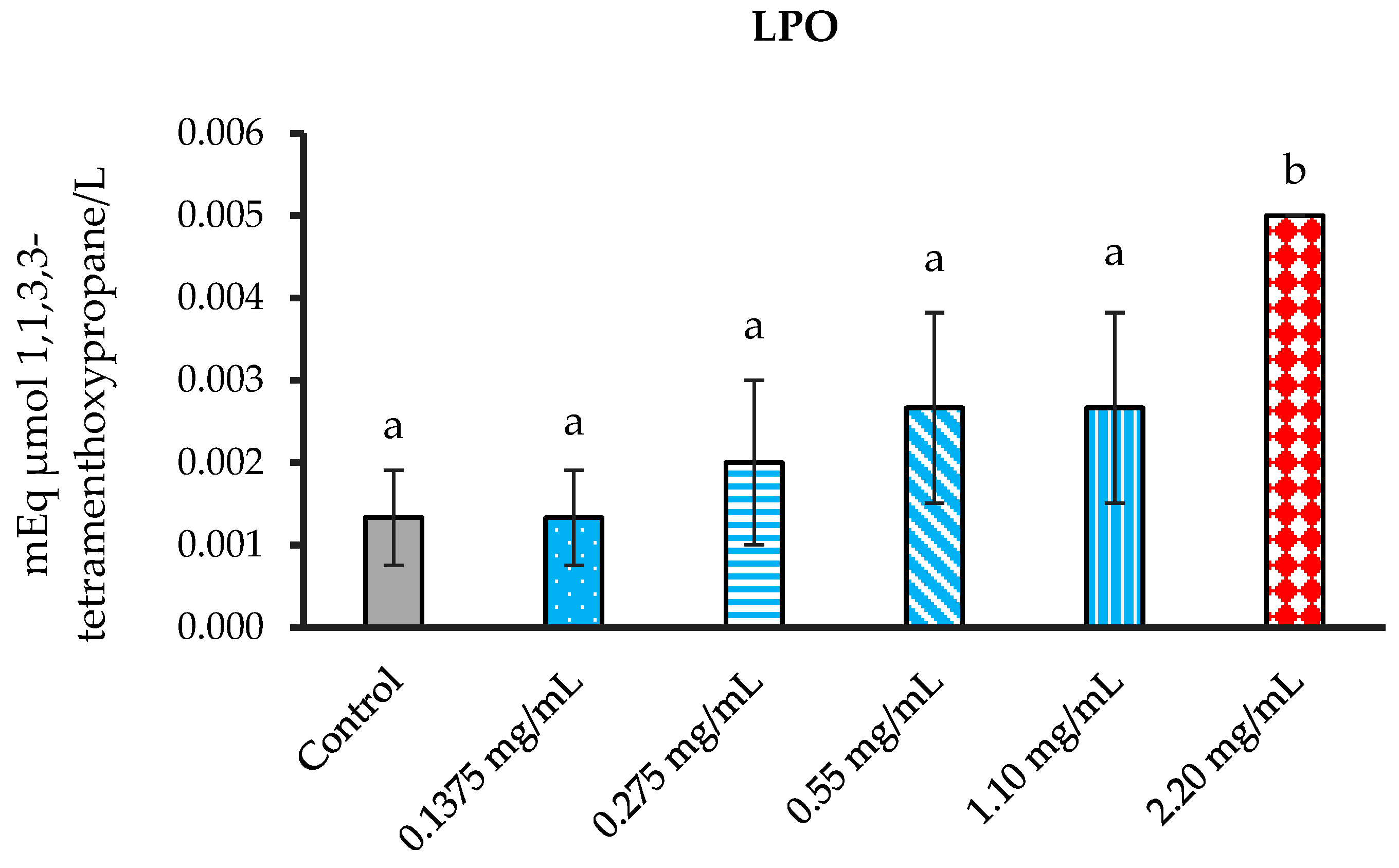
3.5.1. The Inhibition of Lipid Peroxidation (LPO)
3.5.2. Suppression of Advanced Oxidation Protein Products (AOPPs) Formation
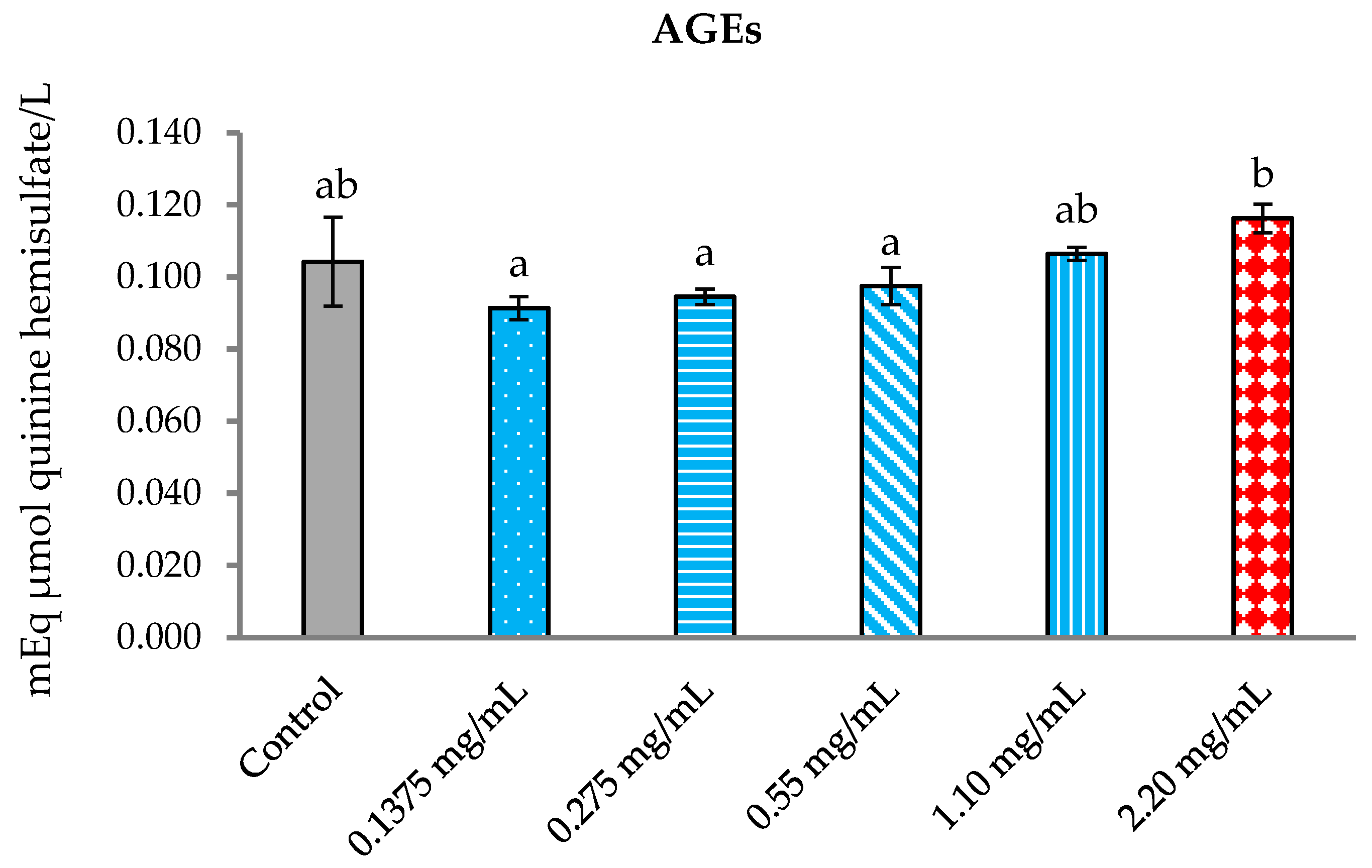
3.5.3. Inhibition of Formation of Advanced Glycation End Products (AGEs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darbandi, S.; Darbandi, M.; Khorshid, H.R.K.; Sadeghi, M.R.; Heidari, M.; Cheshmi, G.; Akhondi, M.M. The effect of paternal age on semen quality and fertilization outcome in men with normal sperm DNA compaction, reactive oxygen species, and total antioxidant capacity levels. Turk. J. Urol. 2019, 45, 164–170. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://iris.who.int/handle/10665/44261 (accessed on 10 November 2024).
- Dai, C.; Zhang, Z.; Shan, G.; Chu, L.T.; Huang, Z.; Moskovtsev, S.; Librach, C.; Jarvi, K.; Sun, Y. Advances in sperm analysis: Techniques, discoveries and applications. Nat. Rev. Urol. 2021, 18, 447–467. [Google Scholar] [CrossRef]
- Sedha, S.; Kumar, S.; Shukla, S. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urol. J. 2015, 12, 2304–2316. [Google Scholar]
- Zakaria, M.; Al-ibraheemi, A.; Ennaji, M.; Senhaji, W.R.; Natiq, A.; Boutiche, R.; Mbaye, M.M.; Zarqaoui, M.; Hassan, M.F.; Mossa, H.A.; et al. Clinical guidelines for sperm DNA fragmentation (SDF) is associated with male infertility from reactive oxygen species (ROS). Gynecol. Reprod. Health 2021, 5, 1–7. [Google Scholar] [CrossRef]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative stress and assisted reproduction: A comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Durairajanayagam, D. Chapter 1.8—Physiological Role of Reactive Oxygen Species in Male Reproduction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Henkel, R., Samanta, L., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 65–78. [Google Scholar]
- Pappalardo, C.; Cosci, I.; Moro, G.; Stortini, A.M.; Sandon, A.; De Angelis, C.; Galdiero, G.; Trifuoggi, M.; Pivonello, R.; Pedrucci, F.; et al. Seminal cadmium affects human sperm motility through stable binding to the cell membrane. Front. Cell Dev. Biol. 2023, 11, 1134304. [Google Scholar] [CrossRef]
- Budihastuti, U.R.; Melinawati, E.; Prakosa, T.; Angelia Ratnasari, A.; Hadi, C.; Laqif, A.; Pangestu, M.; Oktadiani Putri, L.; Murti, B.; Nurwati, I. Influence of age, obesity, smoking, sleep duration, and sleep quality on concentration, morphology, and sperm motility: A cross-sectional study. Int. J. Fertil. Steril. 2024, 18, 240–247. [Google Scholar]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Effect of Nelumbo nucifera petals extract on antioxidant activity and sperm quality in Charolais cattle sperm induced by mancozeb. Plants 2022, 11, 637. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical screening, antioxidant and sperm viability of Nelumbo nucifera petal extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Nuchniyom, P.; Intui, K.; Laoung-On, J.; Jaikang, C.; Quiggins, R.; Photichai, K.; Sudwan, P. Effects of Nelumbo nucifera Gaertn. petal tea extract on hepatotoxicity and oxidative stress induced by mancozeb in rat model. Toxics 2023, 11, 480. [Google Scholar] [CrossRef]
- Intui, K.; Nuchniyom, P.; Laoung-On, J.; Jaikang, C.; Quiggins, R.; Sudwan, P. Neuroprotective effect of white Nelumbo nucifera Gaertn. petal tea in rats poisoned with mancozeb. Foods 2023, 12, 2175. [Google Scholar] [CrossRef]
- Laoung-On, J.; Nuchniyom, P.; Intui, K.; Jaikang, C.; Saenphet, K.; Boonyapranai, K.; Konguthaithip, G.; Outaitaveep, N.; Phankhieo, S.; Sudwan, P. The potential effect of bualuang (white Nelumbo nucifera Gaertn.) extract on sperm quality and metabolomic profiles in mancozeb-induced oxidative stress in male rats. Life 2024, 15, 6. [Google Scholar] [CrossRef]
- Sakr, S.A.; Okdah, Y.A.; Eladly, E.K. Effect of ginger (Zingiber officinale) on mancozeb fungicide induced testicular damage in albino rats. Aust. J. Basic Appl. Sci. 2009, 3, 1328–1333. [Google Scholar]
- Sudwan, P.; Saenphet, K.; Aritajat, S.; Sitasuwan, N. Effects of Boesenbergia rotunda (L.) Mansf. on sexual behaviour of male rats. Asian J. Androl. 2007, 9, 849–855. [Google Scholar] [CrossRef]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Wongsawad, C. Sperm density and ultrastructure of Sertoli cells in male rats treated with Kaempferia parviflora Wall. Ex Baker extract. Southeast Asian J. Trop. Med. Public Health 2007, 38, 249–254. [Google Scholar]
- Laoung-On, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. leaf tea on sexual behavior and reproductive function in male rats. Plants 2021, 10, 2019. [Google Scholar] [CrossRef]
- Laoung-on, J.; Ounjaijean, S.; Sudwan, P.; Boonyapranai, K. Phytochemical screening, antioxidant effect and sperm quality of the Bomba ceiba stamen extracts on Charolais cattle sperm induced by ferrous sulfate. Plants 2024, 13, 960. [Google Scholar] [CrossRef]
- Seth, A.; Malik, J.; Gupta, S.K.; Shyam, D.; Bhaskar, S.; Tiwari, K.; Pathak, S.K. Evaluation of effect of cytotoxic and antioxidant activity of Paederia foetida Linn. Leaf Extract. Int. J. Pharm. Sci. Rev. Res. 2024, 84, 190–197. [Google Scholar] [CrossRef]
- Dutta, P.P.; Marbaniang, K.; Sen, S.; Dey, B.K.; Talukdar, N.C. A review on phytochemistry of Paederia foetida Linn. Phytomed. Plus 2023, 3, 100411. [Google Scholar] [CrossRef]
- Khan, S.; Zahan, D.; Das, D.; Nasrin, D.; Ahsan, S.; Ahmed, R.; Sadat, A.F.M.; Bashar, A.B.M.A.; Nahar, N.; Rahmatullah, M. Antihyperglycemic activity studies with methanol extract of Madhuca indica J.F. Gmel. leaves and Paederia foetida L. stems in mice. Adv. Nat. Appl. Sci. 2011, 5, 122–126. [Google Scholar]
- Soni, R.K.; Irchhaiya, R.; Dixit, V.; Alok, S. Paederia foetida Linn: Phytochemistry, pharmacological and traditional uses. Int. J. Pharm. Sci. Res. 2013, 4, 4525–4530. [Google Scholar]
- Borgohain, M.P.; Chowdhury, L.; Ahmed, S.; Bolshette, N.; Devasani, K.; Das, T.J.; Mohapatra, A.; Lahkar, M. Renoprotective and antioxidative effects of methanolic Paederia foetida leaf extract on experimental diabetic nephropathy in rats. J. Ethnopharmacol. 2017, 198, 451–459. [Google Scholar] [CrossRef]
- Upadhyaya, S. Screening of phytochemicals, nutritional status, antioxidant and antimicrobial activity of Paederia foetida Linn. from different localities of Assam, India. J. Pharm. Res. 2013, 7, 139–141. [Google Scholar] [CrossRef]
- Chanda, S.; Sarethy, I.P.; De, B.; Singh, K. Paederia foetida—A promising ethno-medicinal tribal plant of northeastern India. J. For. Res. 2013, 24, 801–808. [Google Scholar] [CrossRef]
- Srianta, I.; And, H.; Epriliati, I. Ethnobotany, nutritional composition and DPPH radical scavenging of leafy vegetables of wild Paederia foetida and Erechtites hieracifolia. Int. Food Res. J. 2012, 19, 245–250. [Google Scholar]
- Kumar, V.; Anwar, F.; Ahmed, D.; Verma, A.; Ahmed, A.; Damanhouri, Z.A.; Mishra, V.; Ramteke, P.W.; Bhatt, P.C.; Mujeeb, M. Paederia foetida Linn. leaf extract: An antihyperlipidemic, antihyperglycaemic and antioxidant activity. BMC Complement. Altern. Med. 2014, 14, 76. [Google Scholar] [CrossRef]
- Kumar, V.; Pankajkumar S, Y.; Pratap Singh, U.; Raj Bhatt, H.; Zaman, K.; Ali, M. Isolation of new racemic sugar (D/L galacturonic acid) from leaves of Paederia foetida Linn. Nat. Prec. 2011. [Google Scholar] [CrossRef]
- Hossain, M.M.; Ali, M.S.; Saha, A.; Alimuzzaman, M. Antinociceptive activity of whole plant extracts of Paederia foetida. Dhaka Univ. J. Pharm. Sci. 2007, 5, 67–69. [Google Scholar] [CrossRef]
- Afroz, S.; Alamgir, M.; Khan, M.T.; Jabbar, S.; Nahar, N.; Choudhuri, M.S. Antidiarrhoeal activity of the ethanol extract of Paederia foetida Linn. (Rubiaceae). J. Ethnopharmacol. 2006, 105, 125–130. [Google Scholar] [CrossRef]
- De, S.; Ravishankar, B.; Bhavsar, G.C. Investigation of the anti-inflammatory effects of Paederia foetida. J. Ethnopharmacol. 1994, 43, 31–38. [Google Scholar] [CrossRef]
- Uddin, B.; Nahar, T.; Khalil, M.; Hossain, S. In vitro antibacterial activity of the ethanol extract of Paederia foetida L. (Rubiaceae) leaves. Bangladesh J. Life Sci. 2007, 19, 141–143. [Google Scholar]
- Ahmed, A.M.A.; Islam, M.M.; Rahman, M.A.; Hossain, M.A. Thrombolytic, cytotoxic and antidiabetic effects of Paederia foetida L. leaf extract. J. Adv. Med. Med. Res. 2013, 4, 1244–1256. [Google Scholar] [CrossRef]
- Chanda, S.; Deb, L.; Tiwari, R.K.; Singh, K.; Ahmad, S. Gastroprotective mechanism of Paederia foetida Linn. (Rubiaceae)—A popular edible plant used by the tribal community of North-East India. BMC Complement. Altern. Med. 2015, 15, 304. [Google Scholar] [CrossRef]
- Thakur, M.; Thompson, D.; Connellan, P.; Deseo, M.A.; Morris, C.; Dixit, V.K. Improvement of penile erection, sperm count and seminal fructose levels in vivo and nitric oxide release in vitro by ayurvedic herbs. Andrologia 2011, 43, 273–277. [Google Scholar] [CrossRef]
- Osman, H.; Rahim, A.A.; Isa, N.M.; Bakhir, N.M. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecules 2009, 14, 970–978. [Google Scholar] [CrossRef]
- Ishikura, N.; Yang, Z.-q.; Yoshitama, K.; Kurosawa, K. Flavonol glycosides from Paederia scandens var. mairei. Z. Naturforsch. C J. Biosci. 1990, 45, 1081–1084. [Google Scholar] [CrossRef]
- Chin, Y.-W.; Yoon, K.D.; Ahn, M.-J.; Kim, J. Two new phenylpropanoid glycosides from the aerial parts of Paederia scandens. Bull. Korean Chem. Soc. 2010, 31, 1070–1072. [Google Scholar] [CrossRef]
- Zou, X.; Liang, J.; Ding, L.-S.; Peng, S.-L. Studies on chemical constituents of Paederia scandense. Zhongguo Zhong Yao Za Zhi 2006, 31, 1436–1441. [Google Scholar]
- Bhuyan, B.; Paul, A.; Paul, B.; Dhar, S.S.; Dutta, P. Paederia foetida Linn. promoted biogenic gold and silver nanoparticles: Synthesis, characterization, photocatalytic and in vitro efficacy against clinically isolated pathogens. J. Photochem. Photobiol. B 2017, 173, 210–215. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Kaempferol enhances sperm post-thaw survival by its cryoprotective and antioxidant behavior. Stresses 2023, 3, 687–700. [Google Scholar] [CrossRef]
- Phankhieo, S.; Laoung-on, J.; Quiggins, R.; Sudwan, P. Phytochemical screening and effect of Paederia foetida Linn. leaf extract on sperm viability. In Proceedings of the 7th IASCBC & 46th AAT Annual Conference, Chonburi, Thailand, 14–17 May 2024; pp. 115–118. [Google Scholar]
- Huang, R.T.; Lu, Y.F.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) kurz by high-performance-liquid-chromatography with photodiode-array detection and tandem mass spectrometry. J. Funct. Foods 2015, 12, 498–508. [Google Scholar] [CrossRef]
- Wang, L.; Mei, Q.; Wan, D. Simultaneous determination by HPLC of quercetin and kaempferol in three Sedum medicinal plants harvested in different seasons. J. Chromatogr. Sci. 2014, 52, 334–338. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://iris.who.int/handle/10665/343208 (accessed on 10 November 2024).
- Keller, A.; Maus, M.; Keller, E.; Kerns, K. Deep learning classification method for boar sperm morphology analysis. Andrology 2024, 1–11. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Dang, H.N.T.; Nguyen, T.T.T.; Nguyen, T.V.; Dang, T.C.; Nguyen, Q.H.V.; Le, M.T. Correlations between abnormalities of morphological details and DNA fragmentation in human sperm. Clin. Exp. Reprod. Med. 2022, 49, 40–48. [Google Scholar] [CrossRef]
- Kaiin, E.M.; Gunawan, M.; Maulana, T. Morphometry and abnormality evaluation of sex-sorted sperm of spotted buffalo (Tedong bonga). Nusant. Biosci. 2017, 9, 175–180. [Google Scholar] [CrossRef]
- Hanson, R.; Reddick, S.; Thuerauf, S.; Webb, K.; Kasimanickam, V.; Kasimanickam, R. Comparison of bull sperm morphology evaluation methods under field conditions. Clin. Theriogenol. 2023, 15, 9425. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Domínguez-Rebolledo, A.E.; Garde, J.J. Sperm flagellum volume determines freezability in red deer spermatozoa. PLoS ONE 2014, 9, e112382. [Google Scholar] [CrossRef]
- El-Naby, S.H.H.; Kandiel, M.M.M.; Mahmoud, K.G.M.; El-Sisy, G.A.; Soliman, W.T.M.; Ahmed, Y.F. Morphological comparison in spermatozoa from Italian and Egyptian buffalo bulls, and their IVF outcome. Adv. Anim. Vet. Sci. 2021, 9, 1449–1455. [Google Scholar]
- Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective effect of chlorogenic acid on human sperm: In Vitro studies and frozen-thawed protocol. Antioxidants 2021, 10, 744. [Google Scholar] [CrossRef]
- Tvrdá, E.; Debacker, M.; Ďuračka, M.; Kováč, J.; Bučko, O. Quercetin and naringenin provide functional and antioxidant protection to stored boar semen. Animals 2020, 10, 1930. [Google Scholar] [CrossRef]
- Liu, Y.; Zhe, W.; Zhang, R.; Peng, Z.; Wang, Y.; Gao, H.; Guo, Z.; Xiao, J. Ultrasonic-assisted extraction of polyphenolic compounds from Paederia scandens (Lour.) Merr. Using deep eutectic solvent: Optimization, identification, and comparison with traditional methods. Ultrason. Sonochem. 2022, 86, 106005. [Google Scholar] [CrossRef]
- Bordoloi, M.; Bordoloi, P.K.; Dutta, P.P.; Singh, V.; Nath, S.; Narzary, B.; Bhuyan, P.D.; Rao, P.G.; Barua, I.C. Studies on some edible herbs: Antioxidant activity, phenolic content, mineral content and antifungal properties. J. Funct. Foods 2016, 23, 220–229. [Google Scholar] [CrossRef]
- Ojha, S.; Raj, A.; Roy, A.; Roy, S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. Leaves and their relation with antioxidant activity. Pharmacogn. J. 2018, 10, 541–547. [Google Scholar] [CrossRef]
- Kumari, K.; Adhikari, P.; Pandey, A.; Samant, S.S.; Lal, M.; Pande, V. Influence of solvent polarity on phytochemicals, antioxidants, and antimicrobial properties of Delphinium denudatum: A medicinal herb from sainj valley, himachal pradesh, India. Bioactivities 2024, 2, 30–40. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, Y.; Zhu, Q.-F.; Song, M.; Xiong, S.; Manyande, A.; Du, H. The mechanism of chlorogenic acid inhibits lipid oxidation: An investigation using multi-spectroscopic methods and molecular docking. Food Chem. 2020, 333, 127528. [Google Scholar] [CrossRef]
- Auta, T.; Hassan, A.T. Reproductive toxicity of aqueous wood-ash extract of Azadirachta indica (neem) on male albino mice. Asian Pac. J. Reprod. 2016, 5, 111–115. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Sengupta, P.; Pinggera, G.-M.; Calogero, A.E.; Agarwal, A. Oxidative stress affects sperm health and fertility—Time to apply facts learned at the bench to help the patient: Lessons for busy clinicians. Reprod. Med. Biol. 2024, 23, e12598. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]

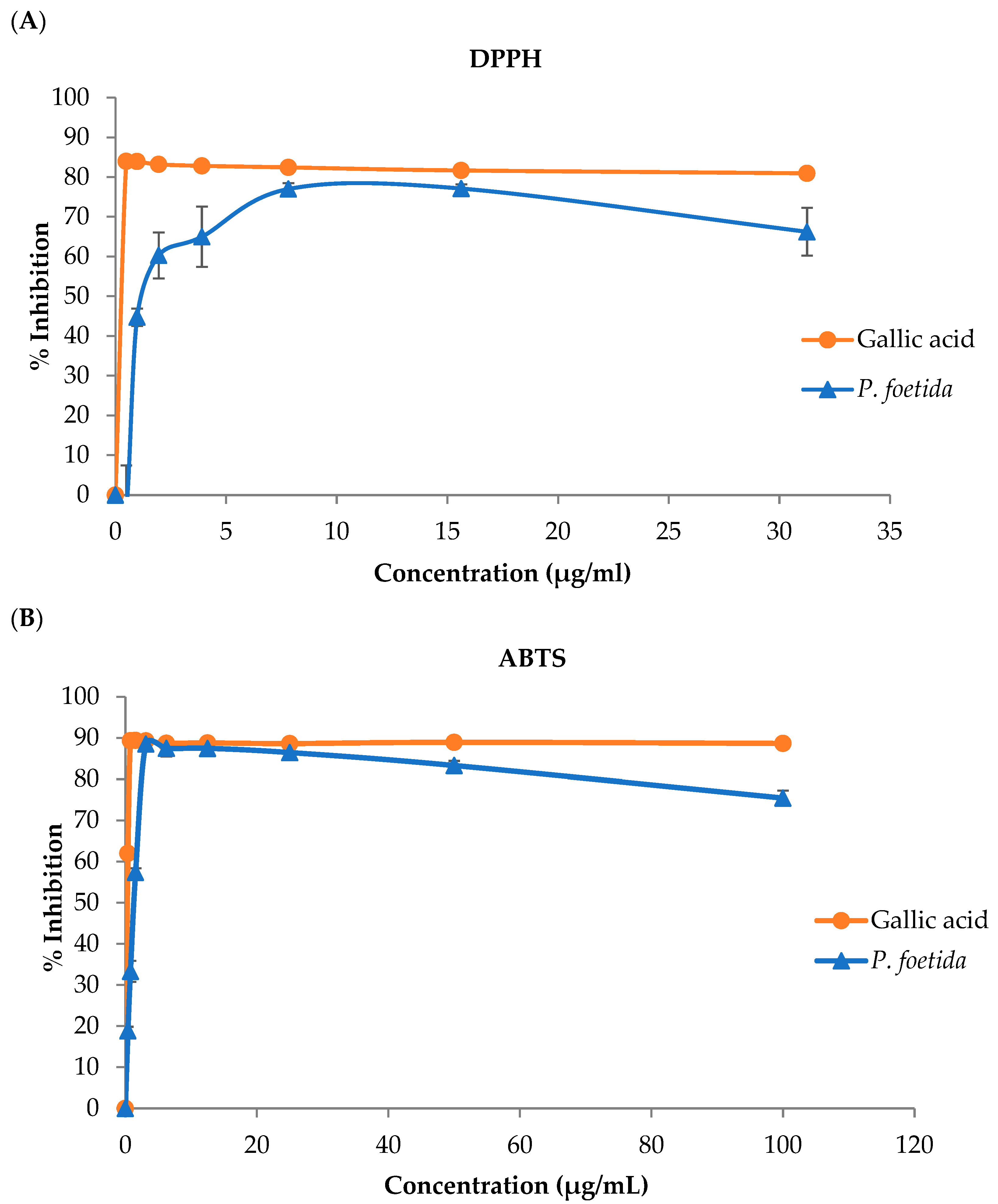

| Samples | IC50 (µg/mL) | |
|---|---|---|
| DPPH | ABTS | |
| Gallic acid | 0.29 ± 0.01 a | 0.39 ± 0.01 a |
| P. foetida | 1.43 ± 0.08 b | 1.31 ± 0.03 b |
| Groups | Concentrations (mg/mL) | Number of Motile Sperm | Number of Immotile Sperm | ||
|---|---|---|---|---|---|
| Progressive | Non-Progressive | Circle | |||
| Control | 0 mg/mL | 25.00 ± 19.47 a | 41.33 ± 7.37 ab | 0.33 ± 0.58 a | 133.67 ± 12.10 a |
| P. foetida | 0.1375 mg/mL | 0.00 ± 0.00 b | 44.00 ± 6.56 ab | 0.00 ± 0.00 a | 156.00 ± 6.56 bc |
| 0.275 mg/mL | 0.00 ± 0.00 b | 53.33 ± 8.02 b | 0.00 ± 0.00 a | 146.67 ± 8.02 ab | |
| 0.55 mg/mL | 0.00 ± 0.00 b | 55.33 ± 4.93 b | 0.00 ± 0.00 a | 144.67 ± 4.93 ab | |
| 1.10 mg/mL | 0.00 ± 0.00 b | 42.67 ± 1.53 ab | 0.00 ± 0.00 a | 157.33 ± 1.53 bc | |
| 2.20 mg/mL | 0.00 ± 0.00 b | 29.33 ± 9.87 a | 0.00 ± 0.00 a | 170.67 ± 9.87 c | |
| Groups | Concentrations (mg/mL) | Number of Viable Sperm | Number of Dead Sperm | ||
|---|---|---|---|---|---|
| Intact | Detached | Intact | Detached | ||
| Control | 0 mg/mL | 65.00 ± 12.77 | 32.00 ± 16.64 | 3.33 ± 4.16 | 100.33 ± 9.07 |
| P. foetida | 0.1375 mg/mL | 66.00 ± 4.58 | 33.67 ± 7.51 | 2.67 ± 3.06 | 100.33 ± 12.01 |
| 0.275 mg/mL | 61.67 ± 4.04 | 42.00 ± 9.52 | 2.33 ± 1.53 | 96.33 ± 8.50 | |
| 0.55 mg/mL | 59.67 ± 6.81 | 38.33 ± 4.04 | 4.00 ± 3.61 | 102.00 ± 6.56 | |
| 1.10 mg/mL | 52.33 ± 11.93 | 38.00 ± 9.85 | 3.00 ± 1.00 | 109.67 ± 9.81 | |
| 2.20 mg/mL | 48.33 ± 5.69 | 19.33 ± 10.02 | 9.67 ± 5.51 | 122.67 ± 11.50 | |
| Groups | Concentrations (mg/mL) | Number of Normal Sperm | Number of Abnormal Sperm | ||
|---|---|---|---|---|---|
| Head Only | Head and Tail | Tail Only | |||
| Control | 0 mg/mL | 39.00 ± 16.52 a | 51.00 ± 7.21 a | 59.33 ± 24.79 a | 50.67 ± 10.02 ab |
| P. foetida | 0.1375 mg/mL | 19.33 ± 14.01 ab | 17.00 ± 1.73 c | 78.33 ± 26.35 ab | 85.33 ± 12.86 c |
| 0.275 mg/mL | 16.00 ± 4.36 ab | 29.00 ± 5.00 bc | 86.00 ± 5.57 abc | 69.00 ± 7.00 bc | |
| 0.55 mg/mL | 22.33 ± 2.89 ab | 32.67 ± 6.35 b | 92.33 ± 4.16 abc | 52.67 ± 4.93 ab | |
| 1.10 mg/mL | 10.00 ± 4.58 b | 30.67 ± 2.08 bc | 125.67± 4.04 c | 33.67 ± 2.52 a | |
| 2.20 mg/mL | 10.33 ± 5.51 b | 40.67 ± 7.02 ab | 120.67 ± 11.24 bc | 28.33 ± 11.93 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phankhieo, S.; Laoung-on, J.; Quiggins, R.; Nuchniyom, P.; Sudwan, P. Antioxidant Activity of Paederia foetida Linn. Leaf Extract and Its Effect on Bovine Sperm Quality. Vet. Sci. 2025, 12, 775. https://doi.org/10.3390/vetsci12080775
Phankhieo S, Laoung-on J, Quiggins R, Nuchniyom P, Sudwan P. Antioxidant Activity of Paederia foetida Linn. Leaf Extract and Its Effect on Bovine Sperm Quality. Veterinary Sciences. 2025; 12(8):775. https://doi.org/10.3390/vetsci12080775
Chicago/Turabian StylePhankhieo, Sasitorn, Jiraporn Laoung-on, Ranida Quiggins, Pimchanok Nuchniyom, and Paiwan Sudwan. 2025. "Antioxidant Activity of Paederia foetida Linn. Leaf Extract and Its Effect on Bovine Sperm Quality" Veterinary Sciences 12, no. 8: 775. https://doi.org/10.3390/vetsci12080775
APA StylePhankhieo, S., Laoung-on, J., Quiggins, R., Nuchniyom, P., & Sudwan, P. (2025). Antioxidant Activity of Paederia foetida Linn. Leaf Extract and Its Effect on Bovine Sperm Quality. Veterinary Sciences, 12(8), 775. https://doi.org/10.3390/vetsci12080775





