Individual Genomic Loci, Transcript Levels, and Serum Profiles of Immune and Antioxidant Markers Associated with Bacteria-Induced Abortion in Sheep (Ovis aries)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Animals and Study Design
2.3. Blood Sampling and Measurements
2.4. Bacteriological Examination
2.5. Bacterial PCR Detection
2.6. Total RNA Extraction, Reverse Transcription, and Quantitative Real Time PCR
2.7. DNA Sequencing and Polymorphism Detection
2.8. Biochemical Analysis
2.9. Control Procedures and Materials Used
2.10. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Bacterial Prevalence and Identification
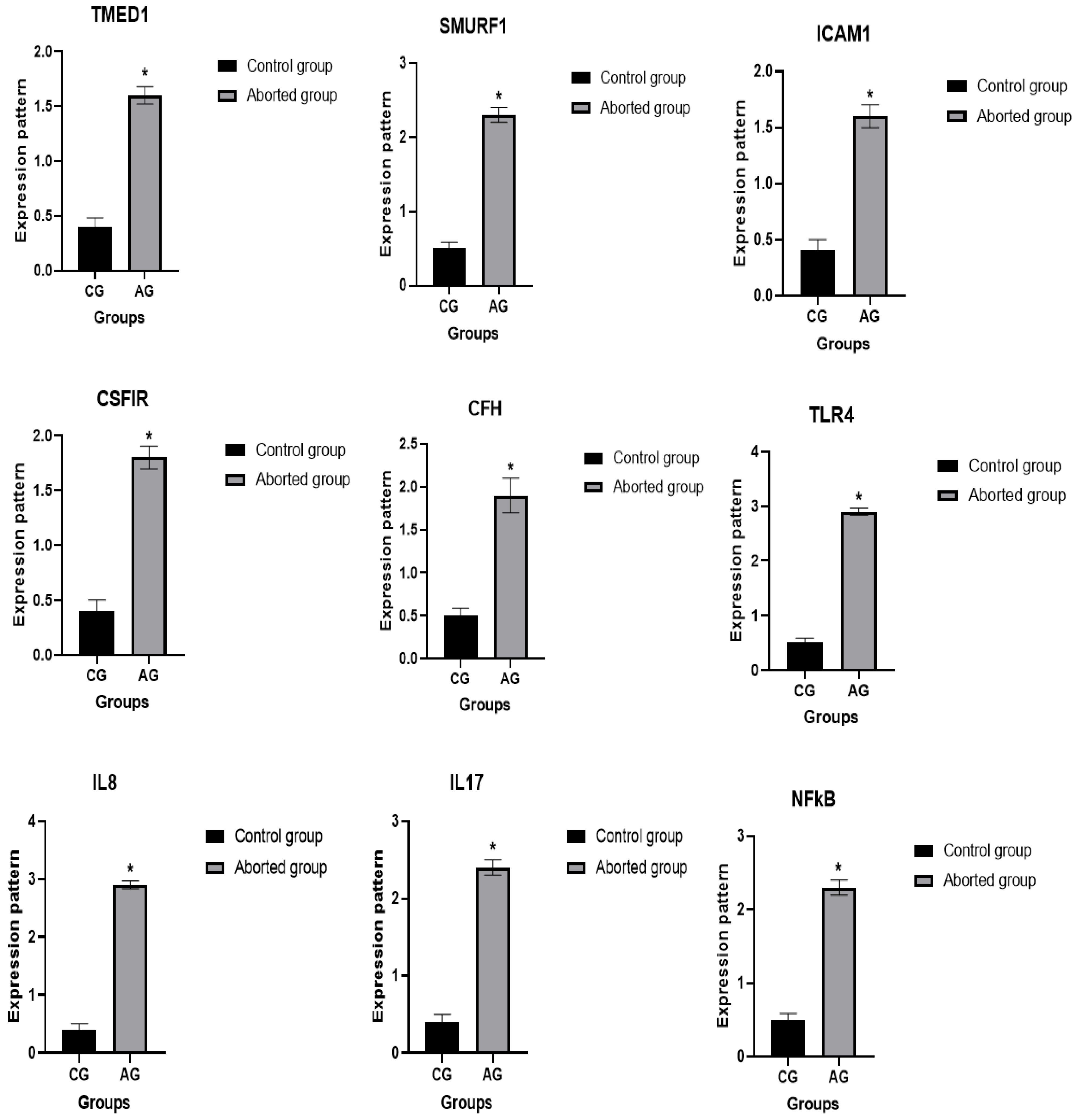
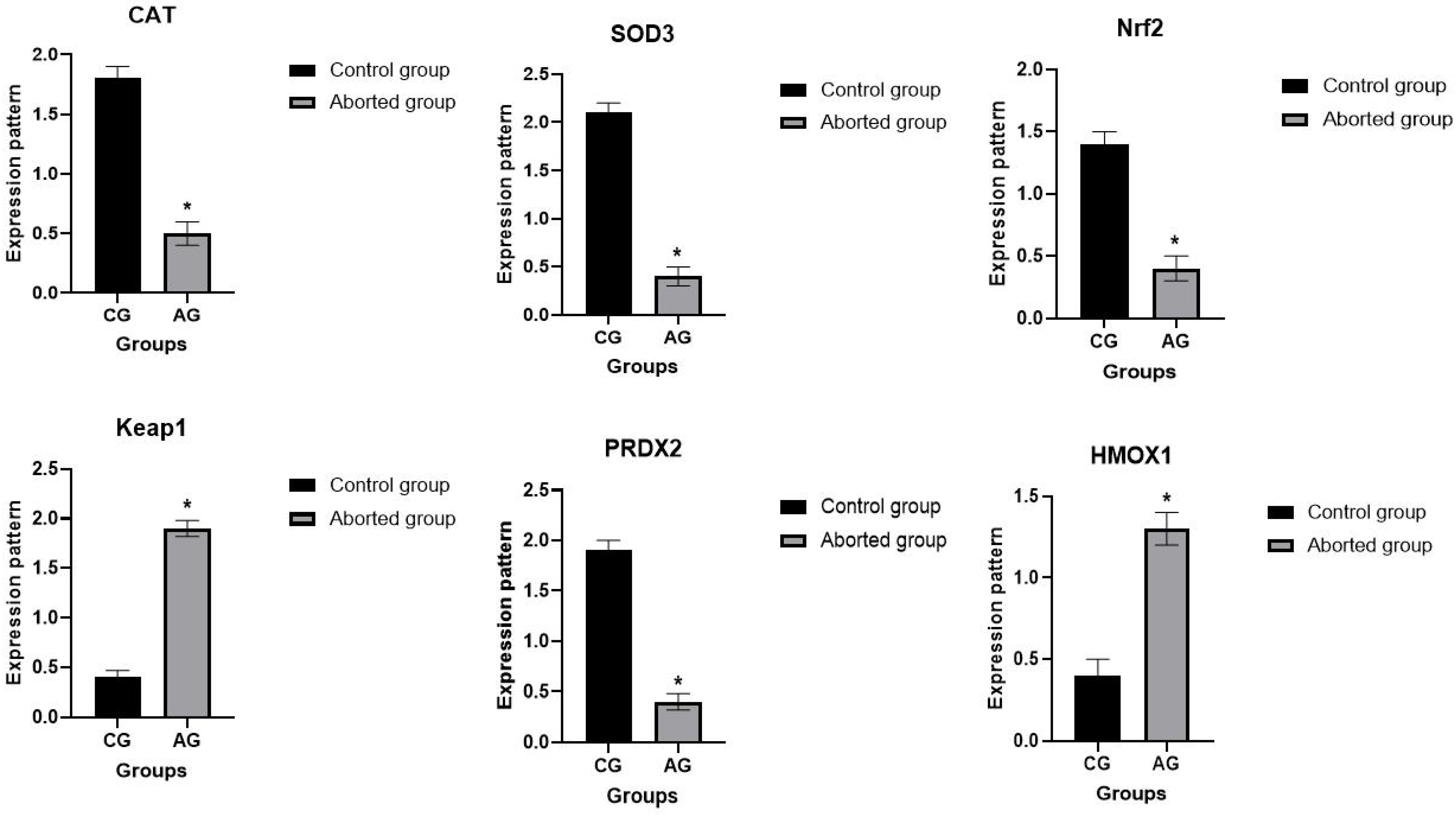
3.3. Patterns for Transcript Levels of Immune and Antioxidant Indicators
3.4. Genetic Polymorphisms of Immune and Antioxidant Genes
3.5. Biochemical Profile of Inflammatory and Antioxidant Markers
4. Discussion
4.1. Clinical Discrimination Between Healthy and Aborted Sheep
4.2. Bacterial Identification in Aborted Sheep
4.3. Immune and Antioxidant Gene Polymorphism Linked with Abortion
4.4. Biochemical Profile Changes Linked with Abortion
4.5. Relevance of Findings to Vaccination Strategies
4.6. Potential for Diagnostic Kit Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- March Desert Research Center. Development of Animal Wealth in Shalateen, Aburamad and Halaieb; First Progress Report; March Desert Research Center: Cairo, Egypt, 1996; Volume 17, p. 7. [Google Scholar]
- Khalil, M.; Khalifa, H.; El-Gabbas, H.; Abdel-Fattah, M. The adaptive responses to water deprivation in local and crossbred sheep. Egypt J. Anim. Prod. 1990, 27, 195–215. [Google Scholar]
- Ali, S.; Zhao, Z.-H.; Zhen, G.; Kang, J.Z.; Yi, P.Z. Reproductive problems in small ruminants (sheep and goats): A substantial economic loss in the world. Large Anim. Rev. 2019, 25, 215–223. [Google Scholar]
- Lokamar, P.N.; Kutwah, M.A.; Atieli, H.; Gumo, S.; Ouma, C. Socio-economic impacts of brucellosis on livestock production and reproduction performance in Koibatek and Marigat regions, Baringo County, Kenya. BMC Vet. Res. 2020, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.; Mamo, G.; Alemu, B.; Desta, H.; Tadesse, B.; Benti, T.; Bahiru, A.; Yimana, M.; Wieland, B. Causes and flock level risk factors of sheep and goat abortion in three agroecology zones in Ethiopia. Front. Vet. Sci. 2021, 8, 615310. [Google Scholar] [CrossRef] [PubMed]
- Vidić, B.; Savić-Jevđenić, S.; Grgić, Ž.; Bugarski, D.; Maljković, M. Infectious abortion in sheep. Biotechnol. Anim. Husb. 2007, 23, 383–389. [Google Scholar] [CrossRef]
- Entrican, G.; Wattegedera, S.; Rocchi, M.; Wheelhouse, N. Pregnancy, indoleamine 2, 3-dioxygenase (IDO) and chlamydial abortion: An unresolved paradox. Vet. Microbiol. 2009, 135, 98–102. [Google Scholar] [CrossRef]
- Khan, H.; Bhakat, M.; Mohanty, T.; Raina, V.; Gupta, A. Effect of non-genetic factors on reproductive disorders in Murrah buffaloes. Buffalo Bull. 2011, 30, 120–147. [Google Scholar]
- Pugh, D. Sheep and Goat Medicine, 2nd ed.; Pugh, D.G., Baird, A.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Available online: https://www.elsevier.com (accessed on 15 May 2025).
- Elhaig, M.M.; Selim, A.; Mandour, A.S.; Schulz, C.; Hoffmann, B. Prevalence and molecular characterization of peste des petits ruminants virus from Ismailia and Suez, Northeastern Egypt, 2014–2016. Small Rumin. Res. 2018, 169, 94–98. [Google Scholar] [CrossRef]
- Tejedor-Junco, M.T.; González-Martín, M.; Corbera, J.A.; Santana, Á.; Hernández, C.N.; Gutiérrez, C. Preliminary evidence of the seroprevalence and risk factors associated with Chlamydia abortus infection in goats on the Canary Islands, Spain. Trop. Anim. Health Prod. 2019, 51, 257–260. [Google Scholar] [CrossRef]
- Dun, K. Ovine abortion—Causes and diagnosis. Livestock 2019, 24, 44–50. [Google Scholar] [CrossRef]
- Clune, T.; Beetson, S.; Besier, S.; Knowles, G.; Paskin, R.; Rawlin, G.; Suter, R.; Jacobson, C. Ovine abortion and stillbirth investigations in Australia. Aust. Vet. J. 2021, 99, 72–78. [Google Scholar] [CrossRef]
- Serhan Serhat, A. Küçük ruminatlarda abortus sorunu ve reprodüktif aşılama programları. Turk. Klin. J. Vet. Sci. Obs. Gynecol-Spec. Top. 2017, 3, 129–136. [Google Scholar]
- Roshan, H.M.; Saadati, D.; Najimi, M. Molecular detection of Brucella melitensis, Coxiella burnetii and Salmonella abortusovis in aborted fetuses of Baluchi sheep in Sistan region, south-eastern Iran. Iran. J. Vet. Res. 2018, 19, 128. [Google Scholar]
- Ashmawy, N.A. Blood metabolic profile and certain hormones concentrations in Egyptian buffalo during different physiological states. Asian J. Anim. Vet. Adv. 2015, 10, 271–280. [Google Scholar] [CrossRef]
- Asim Faraz, A.F.; Muhammad Younas, M.Y.; Abdul Waheed, A.W.; Muhammad Yaqoob, M.Y.; Kashif Ishaq, K.I. Growth performance and hair mineral status of Marecha (Camelus dromedarius) calves reared under different management systems. Pak. J. Zool. 2019, 51, 503–509. [Google Scholar]
- Bazzano, M.; Giudice, E.; Giannetto, C.; Fazio, F.; Scollo, C.; Piccione, G. The peripartum period influenced the serum macromineral profile in mares. Arch. Anim. Breed. 2016, 59, 65–70. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Abd Ellah, M.R. Oxidant and Antioxidants During the Transition Period in Dairy Cows. J. Adv. Vet. Res. 2016, 6, 130–133. [Google Scholar] [CrossRef]
- Al-Ani, A.K.; Yu, N.A.; Jinyou, Z.; Khudhair, N.; Peng, Z.; Xunwu, Z.; Guixue, Z. Responses of chicken Sertoli cells and fibroblasts after transfection with plasmids pEGFPN3-HNP-1. Pak. Vet. J. 2015, 35, 504–509. [Google Scholar]
- Tóthová, C.; Nagy, O.; Kovác, G. Changes in the concentrations of selected acute phase proteins and variables of energetic profile in dairy cows after parturition. J. Appl. Anim. Res. 2014, 42, 278–283. [Google Scholar] [CrossRef]
- Iliev, P.; Georgieva, T. Acute phase biomarkers of diseases in small ruminants: An overview. Bulg. J. Vet. Med. 2019, 22, 1–12. [Google Scholar] [CrossRef]
- Hadfield, J.M.; Bowdridge, E.C.; Holásková, I.; Elsasser, T.H.; Dailey, R.A. Breed-specific differences in the immune response to lipopolysaccharide in ewes. J. Anim. Sci. 2018, 96, 4220–4228. [Google Scholar] [CrossRef]
- Berry, D.P.; Bermingham, M.L.; Good, M.; More, S.J. Genetics of animal health and disease in cattle. Ir. Vet. J. 2011, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.F.; Brown, V.R.; Olsen, S.C.; Boggiatto, P.; Pedersen, K.; Miller, R.S.; Speidel, S.E.; Smyser, T.J. Loci associated with antibody response in feral swine (Sus scrofa) infected with Brucella suis. Front. Vet. Sci. 2020, 7, 554674. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.N.; Winter, M.G.; Spees, A.M.; Nguyen, K.; Atluri, V.L.; Silva, T.M.; Bäumler, A.J.; Müller, W.; Santos, R.L.; Tsolis, R.M. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 2013, 9, e1003454. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.M.; Abou-Souliman, I.; Reyer, H.; Wimmers, K.; Rabee, A.E. New insights into the genetic predisposition of brucellosis and its effect on the gut and vaginal microbiota in goats. Sci. Rep. 2023, 13, 20086. [Google Scholar] [CrossRef]
- Garry Adams, L.; Schutta, C.J. Natural resistance against brucellosis: A review. Open Vet. Sci. J. 2010, 4, 61–71. [Google Scholar] [CrossRef]
- Ibrahim, S.; Al-Sharif, M.; Younis, F.; Ateya, A.; Abdo, M.; Fericean, L. Analysis of potential genes and economic parameters associated with growth and heat tolerance in sheep (Ovis aries). Animals 2023, 13, 353. [Google Scholar] [CrossRef]
- Nassar, M. Effect of grazing time on productive performance of goat in Halaib–Shalateen region. Egypt. J. Nutr. Feed. 2020, 23, 37–54. [Google Scholar] [CrossRef]
- Badawy, H. Nutritional Studies on Camels Grazing the Natural Ranges of Halaib-Shalateen Triangle Region. Ph.D. Thesis, Cairo University, Cairo, Egypt, 2005. [Google Scholar]
- Allam, S.M.; El-Nasr, H.; El-Gawad, M.; Nassar, M. Performance of local goats maintained on natural ranges and supplementary feeding in Halaib-Shalateen region, Egypt: 1. Does performance through pregnancy and lactation. Egypt J. Nutr. Feed. 2007, 10, 323–347. [Google Scholar]
- Askar, A.R.; Masoud, A.; El-Bordeny, N.E.; Kewan, K.Z.; El-Galil, E.; El Ezz, S.S.A.; Shoukry, M.M. Grazing camels under semi-extensive production system: Selectivity, feed intake capacity, digestion and energy expenditure. BMC Vet Res. 2024, 20, 364. [Google Scholar] [CrossRef]
- Alton, G.G.; Jones, L.M.; Pietz, D.E.; World Health Organization. Laboratory Techniques in Brucellosis; World Health Organization: Geneva, Switzerland, 1975. [Google Scholar]
- Persson, S.; Olsen, K.E. Multiplex PCR for identification of Campylobacter coli and Campylobacter jejuni from pure cultures and directly on stool samples. J. Med. Microbiol. 2005, 54, 1043–1047. [Google Scholar] [CrossRef]
- Quinn, P.; Markey, B.K.; Carter, M.; Donnelly, W.; Leonard, F. Veterinary Microbiology and Microbial Disease; Blackwell science: Bognor Regis, UK, 2002. [Google Scholar]
- Oliveira, S.; Rodenbusch, C.; Ce, M.; Rocha, S.; Canal, C. Evaluation of selective and non-selective enrichment PCR procedures for Salmonella detection. Lett. Appl. Microbiol. 2003, 36, 217–221. [Google Scholar] [CrossRef]
- Kumar, A.; Grover, S.; Batish, V.K. Exploring specific primers targeted against different genes for a multiplex PCR for detection of Listeria monocytogenes. 3 Biotech 2015, 5, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Engelberts, M.F.; Boer, K.R.; Ahmed, N.; Hartskeerl, R.A. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS ONE 2009, 4, e7093. [Google Scholar] [CrossRef] [PubMed]
- Bricker, B.J.; Halling, S.M. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 1994, 32, 2660–2666. [Google Scholar] [CrossRef]
- Sareyyüpoğlu, B.; Cantekin, Z. Use of a multiplex-polymerase chain reaction for detection of Salmonella and Chlamydophila psittaci from caged birds. Ank. Üniversitesi Vet. Fakültesi Derg. 2009, 56, 269–273. [Google Scholar] [CrossRef]
- Borji, S.; Jamshidi, A.; Khanzadi, S.; Razmyar, J. Detection of Coxiella burnetii and sequencing the IS1111 gene fragment in bulk tank milk of dairy herds. Iran. J. Vet. Sci. Technol. 2014, 6, 21–28. [Google Scholar]
- Darlay, R.; McCarthy, A.; Illot, N.; Smith, J.; Shaw, M.A. Novel polymorphisms in ovine immune response genes and their association with abortion. Anim. Genet. 2011, 42, 535–543. [Google Scholar] [CrossRef]
- Al-Sharif, M.; Abdo, M.; Shabrawy, O.E.; El-Naga, E.M.A.; Fericean, L.; Banatean-Dunea, I.; Ateya, A. Investigating polymorphisms and expression profile of immune, antioxidant, and erythritol-related genes for limiting postparturient endometritis in Holstein cattle. Vet. Sci. 2023, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.A.; El-Kattan, A.M.; Mahmoud, M.A.; Ragab, M. Virulence genes and immunological biomarkers for brucellosis in sheep & goat. J. Anim. Health Prod. 2023, 11, 165–175. Available online: https://www.researchgate.net/publication/370947142_Virulence_genes_and_immunological_biomarkers_for_brucellosis_in_sheep_goat (accessed on 24 July 2025).
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Thaenkham, U.; Chaisiri, K.; Hui En Chan, A. PCR and DNA sequencing: Guidelines for PCR, primer design, and sequencing for molecular systematics and identification. In Molecular Systematics of Parasitic Helminths; Springer Nature: Singapore, 2022; pp. 183–199. [Google Scholar]
- Boesenberg-Smith, K.A.; Pessarakli, M.M.; Wolk, D.M. Assessment of DNA yield and purity: An overlooked detail of PCR troubleshooting. Clin. Microbiol. Newsl. 2012, 34, 1–6. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Holler, L.D. Ruminant abortion diagnostics. Vet. Clin. N. Am.-Food Anim. Pract. 2012, 28, 407. [Google Scholar] [CrossRef]
- Darwish, A.; Ebissy, E.; Ateya, A.; El-Sayed, A. Single nucleotide polymorphisms, gene expression and serum profile of immune and antioxidant markers associated with postpartum disorders susceptibility in Barki sheep. Anim. Biotechnol. 2023, 34, 327–339. [Google Scholar] [CrossRef]
- Celli, J.; Gorvel, J.-P. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 2004, 7, 93–97. [Google Scholar] [CrossRef]
- Singh, S.; Agarwal, G.; Batra, H.; Gupta, V.; Singh, N. Monitoring of Brucella infection associated with reproductive losses using multiple serological tests in organized goat and sheep flocks. Indian J. Anim. Sci. 2000, 70, 2394–3327. [Google Scholar]
- Al-Dabagh, I.; Jasim, B.; Jarjees, M. Seroprevalence of antibodies to toxoplasmosis, brucellosis and chlamydiosis in abortive sheep in Nineveh governorate, Iraq. Iraqi J. Vet. Sci. 2014, 28, 21–25. [Google Scholar] [CrossRef]
- Esmaeili, H.; Shakeri, A.P.; Rad, Z.N.; Arani, E.B.; Villanueva-Saz, S.; Ruiz, H.; Lacasta, D. Causes of abortion in Iranian sheep flocks and associated risk factors. Vet. Res. Commun. 2022, 46, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Leyla, G.; Kadri, G.; Ümran, O. Comparison of polymerase chain reaction and bacteriological culture for the diagnosis of sheep brucellosis using aborted fetus samples. Vet. Microbiol. 2003, 93, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Atwa, E.; Rady, F. Bacteria and fungi associated with abortion in sheep and goat in Menoufiea Governorate. Assiut Vet. Med. J. 2007, 53, 1–23. [Google Scholar]
- Abdelbaset, A.E.; Abushahba, M.F.; Hamed, M.I.; Rawy, M.S. Sero-diagnosis of brucellosis in sheep and humans in Assiut and El-Minya governorates, Egypt. Int. J. Vet. Sci. Med. 2018, 6, S63–S67. [Google Scholar] [CrossRef]
- Derbala, A.; Ghazi, Y. Some investigations on brucella and psoroptes mites infections among Barki sheep flocks. Mansoura Vet. Med. J. 2001, 3, 173–183. [Google Scholar] [CrossRef]
- Shakerian, A.; Deo, P.; Rahimi, E.; Shahjavan, A.-R.; Khamesipour, F. Molecular detection of Brucella melitensis in sheep and goat milk in Iran. Trop. J. Pharm. Res. 2016, 15, 913–918. [Google Scholar] [CrossRef]
- Haif, A.; Khelifi-Ouchene, N.A.; Khelifi, M.; Ouchetati, I.; Zeroual, F.; Ouchene, N. Abortive diseases and their various associated risk factors in small ruminants in Algeria: A systematic review. Trop. Anim. Health Prod. 2021, 53, 520. [Google Scholar] [CrossRef]
- Luna-Martí;nez, J.E.; Mejía-Terán, C. Brucellosis in Mexico: Current status and trends. Vet. Microbiol. 2002, 90, 19–30. [Google Scholar] [CrossRef]
- Anderson, M.L. Infectious causes of bovine abortion during mid-to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef]
- Derdour, S.-Y.; Hafsi, F.; Azzag, N.; Tennah, S.; Laamari, A.; China, B.; Ghalmi, F. Prevalence of the main infectious causes of abortion in dairy cattle in Algeria. J. Vet. Res. 2017, 61, 337–343. [Google Scholar] [CrossRef]
- Ayoub, M.; Abu-Rawash, A.; Ibrahim, S.; Aragan, A. Hygienic studies on microbial causes of abortion in sheep. Damanhour J. Vet. Sci. 2020, 5, 11–13. [Google Scholar] [CrossRef]
- Farag, H.E.; Abdallah, M.; Nossair, M.A. Epidemiological studies on some infectious diseases causing abortion in sheep. Alex. J. Vet. Sci. 2021, 68, 54–61. [Google Scholar] [CrossRef]
- Rabah, I.; Nossair, M.; Abdou, E.; Elkamshishi, M.; Khalifa, E. Serological and Molecular Epidemiological Study on Brucellosis in Camels and Human in Matrouh Province. Damanhour J. Vet. Sci. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Musallam, I.I.; Abo-Shehada, M.N.; Guitian, J. Knowledge, attitudes, and practices associated with brucellosis in livestock owners in Jordan. Am. J. Trop. Med. Hyg. 2015, 93, 1148. [Google Scholar] [CrossRef]
- Ashenafi, F.; Teshale, S.; Ejeta, G.; Fikru, R.; Laikemariam, Y. Distribution of brucellosis among small ruminants in the pastoral region of Afar, eastern Ethiopia. Rev. Sci. Et. Tech. 2007, 26, 731. [Google Scholar] [CrossRef]
- Varga, J. Occurrence and significance of different Campylobacter types in domestic animals in Hungary. Deut. Tierarztl. Wochenschr. 1990, 97, 317–321. [Google Scholar]
- Li, X.; Tang, H.; Xu, Z.; Tang, H.; Fan, Z.; Jiao, X.; Huang, J. Prevalence and characteristics of Campylobacter from the genital tract of primates and ruminants in Eastern China. Transbound. Emerg. Dis. 2022, 69, e1892–e1898. [Google Scholar] [CrossRef]
- Runge, M.; Binder, A.; Schotte, U.; Ganter, M. Investigations concerning the prevalence of Coxiella burnetii and Chlamydia abortus in sheep in correlation with management systems and abortion rate in Lower Saxony in 2004. Berl. Und Münchener Tierärztliche Wochenschr. 2012, 125, 10–15. [Google Scholar]
- Plagemann, O. The most frequent infectious causes of abortion in sheep in north Bavaria with special reference to Chlamydia and Salmonella infections. Tierarztl. Prax. 1989, 17, 145–148. [Google Scholar] [PubMed]
- Yeni, D.K. Molecular diagnosis of neglected infectious agents of heep and attle abortions: The prevalences of Coxiella burnetii, Francisella tularensis and Chlamydophila abortus at a glance. Ank. Üniversitesi Vet. Fakültesi Derg. 2022, 69, 425–430. [Google Scholar] [CrossRef]
- Arif, E.D.; Saeed, N.M.; Rachid, S.K. Isolation and Identification of Chlamydia abortus from Aborted Ewes in Sulaimani Province, Northern Iraq. Pol. J. Microbiol. 2020, 69, 1–7. [Google Scholar] [CrossRef]
- Aras, Z.; Sayın, Z.; Gölen, G. Investigation of Chlamydophila abortus in abortion of cattle by PCR. Eurasian J. Vet. Sci. 2017, 33, 77–80. [Google Scholar] [CrossRef]
- Rekiki, A.; Thabti, F.; Dlissi, I.; Russo, P.; Sanchis, R.; Pepin, M.; Rodolakis, A.; Hammami, S. Seroprevalence survey of major infectious abortive diseases in small ruminants in Tunisia.Revue Méd. Veterinary 2005, 156, 395–401. [Google Scholar]
- Livingstone, M.; Wheelhouse, N.; Maley, S.W.; Longbottom, D. Molecular detection of Chlamydophila abortus in post-abortion sheep at oestrus and subsequent lambing. Vet. Microbiol. 2009, 135, 134–141. [Google Scholar] [CrossRef]
- Elandalousi, R.B.; Ghram, A.; Maaroufi, A.; Mnif, W.; Maaroufi, A.; Mnif, W. Séroprévalence des maladies abortives zoonotiques chez les ruminants au nord de la Tunisie. Res. Fr 2015, 2, 1419. Available online: https://www.researchgate.net/publication/280043878_Seroprevalence_des_maladies_abortives_zoonotiques_chez_les_ruminants_au_nord_de_la_Tunisie (accessed on 24 July 2025).
- Mamlouk, A.; Guesmi, K.; Ouertani, I.; Kalthoum, S.; Selmi, R.; Aicha, E.B.; Mohamed, B.B.H.; Gharbi, R.; Lachtar, M.; Dhaouadi, A. Seroprevalence and associated risk factors of Chlamydia abortus infection in ewes in Tunisia. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101500. [Google Scholar] [CrossRef]
- Song, Y.; Sun, L.; Yang, H.; Hua, G.; Guo, A.; Yang, L. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphism is associated with abortion in Chinese Holstein cows. Afr. J. Biotechnol. 2011, 10, 13999–14004. [Google Scholar] [CrossRef]
- Barton, N.H. Mutation and the evolution of recombination. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1281–1294. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kala, D.; Dhiman, G.; Yadav, V.; Krokhotin, A.; Dokholyan, N.V. Predicting the functional consequences of non-synonymous single nucleotide polymorphisms in IL8 gene. Sci. Rep. 2017, 7, 6525. [Google Scholar] [CrossRef]
- Al-Sharif, M.; Ateya, A. New insights on coding mutations and mRNA levels of candidate genes associated with diarrhea susceptibility in baladi goat. Agriculture 2023, 13, 143. [Google Scholar] [CrossRef]
- Ateya, A.; Safhi, F.A.; El-Emam, H.; Marawan, M.A.; Fayed, H.; Kadah, A.; Mamdouh, M.; Hizam, M.M.; Al-Ghadi, M.Q.; Abdo, M. Combining Nucleotide Sequence Variants and Transcript Levels of Immune and Antioxidant Markers for Selection and Improvement of Mastitis Resistance in Dromedary Camels. Agriculture 2023, 13, 1909. [Google Scholar] [CrossRef]
- Safhi, F.A.; Ateya, A. New Insights into Polymorphisms in Candidate Genes Associated with Incidence of Postparturient Endometritis in Ossimi Sheep (Ovis aries). Agriculture 2023, 13, 2273. [Google Scholar] [CrossRef]
- Fujita, M.; Into, T.; Yasuda, M.; Okusawa, T.; Hamahira, S.; Kuroki, Y.; Eto, A.; Nisizawa, T.; Morita, M.; Shibata, K.-i. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J. Immunol. 2003, 171, 3675–3683. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Katz, Y.; Schlesinger, M.; Carmi, R.; Shalev, H.; Haider, N.; Beck, G.; Sheffield, V.C.; Landau, D. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 1999, 65, 1538–1546. [Google Scholar] [CrossRef]
- Pastor-Cantizano, N.; Montesinos, J.C.; Bernat-Silvestre, C.; Marcote, M.J.; Aniento, F. p24 family proteins: Key players in the regulation of trafficking along the secretory pathway. Protoplasma 2016, 253, 967–985. [Google Scholar] [CrossRef]
- Van de Stolpe, A.; Van der Saag, P. Intercellular adhesion molecule-1. J. Mol. Med. 1996, 74, 13–33. [Google Scholar] [CrossRef]
- Zhu, H.; Kavsak, P.; Abdollah, S.; Wrana, J.L.; Thomsen, G.H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 1999, 400, 687–693. [Google Scholar] [CrossRef]
- Meyers, M.J.; Pelc, M.; Kamtekar, S.; Day, J.; Poda, G.I.; Hall, M.K.; Michener, M.L.; Reitz, B.A.; Mathis, K.J.; Pierce, B.S.; et al. Structure-based drug design enables conversion of a DFG-in binding CSF-1R kinase inhibitor to a DFG-out binding mode. Bioorg. Med. Chem. Lett. 2010, 20, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Suzuki, T.; Takahashi, Y.; Hishinuma, E.; Saigusa, D.; Yamamoto, M. Geldanamycin-derived HSP90 inhibitors are synthetic lethal with NRF2. Mol. Cell. Biol. 2020, 40, e00377-20. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Takahashi, M.; Keicho, K.; Takahashi, H.; Ogawa, H.; Schulte, R.; Okano, A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology 2000, 54, 137–145. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Seiler, F.; Schilderman, P.A.; Nehls, P.; Bruch, J.; Schins, R.P.; Borm, P.J. Neutrophils cause oxidative DNA damage in alveolar epithelial cells. Free. Radic. Biol. Med. 1999, 27, 234–240. [Google Scholar] [CrossRef]
- Mordak, R.; Stewart, P.A. Periparturient stress and immune suppression as a potential cause of retained placenta in highly productive dairy cows: Examples of prevention. Acta Vet. Scand. 2015, 57, 84. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Raghupathy, R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Sahele, M.; Sori, T.; Guyassa, C.; Garoma, A. Seroprevalence and associated risk factors for chlamydiosis, coxiellosis and brucellosis in sheep and goats in Borana pastoral area, southern Ethiopia. BMC Vet. Res. 2020, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Simplício, K.; Rocha, T.; Sanchez, D.; Cotrim, F.; Silva, P.; Fagliari, J. Serum concentrations of acute phase proteins in goats and ewes with naturally acquired Staphylococcus aureus mastitis. Arq. Bras. Med. Veterinária E Zootec. 2017, 69, 285–292. [Google Scholar] [CrossRef]
- El-Deeb, W.M. Clinicobiochemical investigations of gangrenous mastitis in does: Immunological responses and oxidative stress biomarkers. J. Zhejiang Univ. Sci. B 2013, 14, 33–39. [Google Scholar] [CrossRef]
- Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Mohanty, T.; Sejian, V.; Kumar, N.; Sreela, L.; Prakash, M.A.; Mooventhan, P.; Anantharaj, A.; et al. Potential of acute phase proteins as predictor of postpartum uterine infections during transition period and its regulatory mechanism in dairy cattle. Vet. World 2016, 9, 91. [Google Scholar] [CrossRef]
- Hansen, V.L.; Faber, L.S.; Salehpoor, A.A.; Miller, R.D. A pronounced uterine pro-inflammatory response at parturition is an ancient feature in mammals. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171694. [Google Scholar] [CrossRef]
- Kaya, S.; Öğün, M.; Özen, H.; Kuru, M.; Şahin, L.; Kükürt, A.; Kaçar, C. The impact of endometritis on specific oxidative stress parameters in cows. J. Hell. Vet. Med. Soc. 2017, 68, 231–236. [Google Scholar] [CrossRef]
- Mondal, S.; Minj, A.; Pathak, M.; Singh, D.; Varshney, V. Importance of hormonal changes during the periparturition period in black Bengal goats. Int. J. Clin. Exp. Physiol. 2014, 1, 20. [Google Scholar] [CrossRef]
- Ali, A.-F.; Abdelwahab, M.G. Interleukin-1β, tumor necrosis factor-α, and oxidative stress biomarkers in cows with acute Brucella abortus infection. Comp. Clin. Pathol. 2021, 30, 311–315. [Google Scholar] [CrossRef]
- Corsetti, P.P.; de Almeida, L.A.; Carvalho, N.B.; Azevedo, V.; Silva, T.M.; Teixeira, H.C.; Faria, A.C.; Oliveira, S.C. Lack of endogenous IL-10 enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS ONE 2013, 8, e74729. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci 1997, 2, d12–d26. [Google Scholar] [PubMed]
- Hashem, M.A.; El-Mandrawy, S.A.; El-Diasty, M.M.; Zidan, A.Z. Hematological, biochemical and immunological studies on brucellosis in cows and ewes in Dakahlia and Damietta Governorates, Egypt. Zagazig Vet. J. 2020, 48, 23–35. [Google Scholar] [CrossRef]
- Kataria, N.; Kataria, A.; Maan, R.; Gahlot, A. Evaluation of oxidative stress in brucella infected cows. J. Stress Physiol. Biochem. 2010, 6, 19–25. [Google Scholar]
- Hamdy, M.; Khoudair, R.; Ibrahim, M.; Shalby, N.; El-Shafei, A.; Abo El-Maaty, A.; Abd El Hameed, A.; Mohamed, R. Acute Phase Proteins (APP) and Minerals Levels Associated with Brucellosis in Camels. Anim. Health Res. J. 2019, 7, 732–741. [Google Scholar]
- Shalby, N.; El-Maaty, A.A.; Ali, A.; Elgioushy, M. Acute phase biomarkers, oxidants, antioxidants, and trace minerals of mobile sheep flocks naturally infected with brucellosis. Bulg. J. Vet. Med. 2021, 24, 559–573. [Google Scholar] [CrossRef]
- Kachuee, R.; Moeini, M.; Souri, M. The effect of dietary organic and inorganic selenium supplementation on serum Se, Cu, Fe and Zn status during the late pregnancy in Merghoz goats and their kids. Small Rumin. Res. 2013, 110, 20–27. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Rana, N.; Laudadio, V. Effect of vitamin E in heat-stressed poultry. World’s Poult. Sci. J. 2011, 67, 469–478. [Google Scholar] [CrossRef]
- Kandemir, O.; Eskandari, G.; Camdeviren, H.; Sahin, E.; Kaya, A.; Atik, U. Plasma malondialdehyde and nitrate levels in patients with brucellosis. MEU Tıp Fak Derg 2002, 3, 405–409. [Google Scholar]
- Manat, T.D.; Chaudhary, S.S.; Singh, V.K.; Patel, S.B.; Tyagi, K.K. Oxidative stress profile during postpartum period in Surti goats. Indian J. Anim. Res. 2017, 51, 837–840. [Google Scholar] [CrossRef]
- Binelli, M.; Subramaniam, P.; Diaz, T.; Johnson, G.A.; Hansen, T.R.; Badinga, L.; Thatcher, W.W. Bovine interferon-τ stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biol. Reprod. 2001, 64, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Cai, J.; Jiang, Q.; Loor, J.J.; Deng, G.; Li, X.; Yang, J. Interferon-tau protects bovine endometrial epithelial cells against inflammatory injury by regulating the PI3K/AKT/β-catenin/FoxO1 signaling axis. J. Dairy Sci. 2024, 107, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Sobel, D.O.; Ahvazi, B.; Amjad, F.; Mitnaul, L.; Pontzer, C.; Sobel, D.O.; Ahvazi, B.; Amjad, F.; Mitnaul, L.; Pontzer, C. Interferon-tau inhibits the development of diabetes in NOD mice. Autoimmunity 2008, 41, 543–553. [Google Scholar] [CrossRef]
- Talukder, A.K.; Rashid, M.B.; Yousef, M.S.; Kusama, K.; Shimizu, T.; Shimada, M.; Suarez, S.S.; Imakawa, K.; Miyamoto, A. Oviduct epithelium induces interferon-tau in bovine Day-4 embryos, which generates an anti-inflammatory response in immune cells. Sci. Rep. 2018, 8, 7850. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.B.; Talukder, A.K.; Kusama, K.; Haneda, S.; Takedomi, T.; Yoshino, H.; Moriyasu, S.; Matsui, M.; Shimada, M.; Imakawa, K. Evidence that interferon-tau secreted from Day-7 embryo in vivo generates anti-inflammatory immune response in the bovine uterus. Biochem. Biophys. Res. Commun. 2018, 500, 879–884. [Google Scholar] [CrossRef]
- Chaouat, G.; Assal Meliani, A.; Martal, J.; Raghupathy, R.; Elliott, J.; Elliot, J.; Mosmann, T.; Wegmann, T. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J. Immunol. 1995, 154, 4261–4268. [Google Scholar] [CrossRef]
- Graber, J.J.; Dhib-Jalbut, S. Interferons. Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff Michael, J., Daroff Robert, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Grimberg, A.; Cohen, P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J. Cell. Physiol. 2000, 183, 1–9. [Google Scholar] [CrossRef]
- Han, V.; Carter, A. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta 2000, 21, 289–305. [Google Scholar] [CrossRef]
- Ravelich, S.R.; Breier, B.H.; Reddy, S.; Keelan, J.A.; Wells, D.N.; Peterson, A.J.; Lee, R.S. Insulin-like growth factor-I and binding proteins 1, 2, and 3 in bovine nuclear transfer pregnancies. Biol. Reprod. 2004, 70, 430–438. [Google Scholar] [CrossRef]
- Crossey, P.A.; Pillai, C.C.; Miell, J.P. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J. Clin. Investig. 2002, 110, 411–418. [Google Scholar] [CrossRef][Green Version]
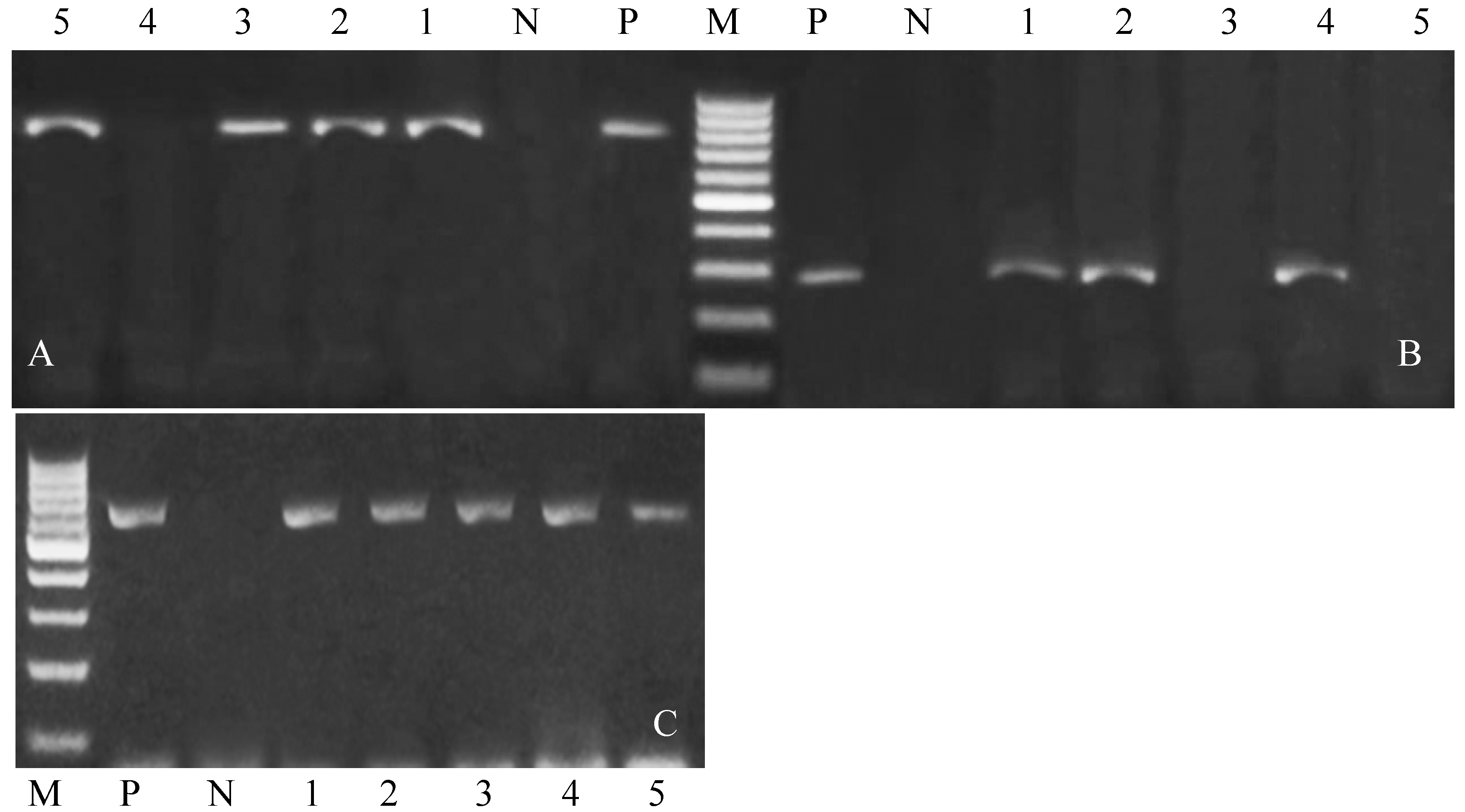
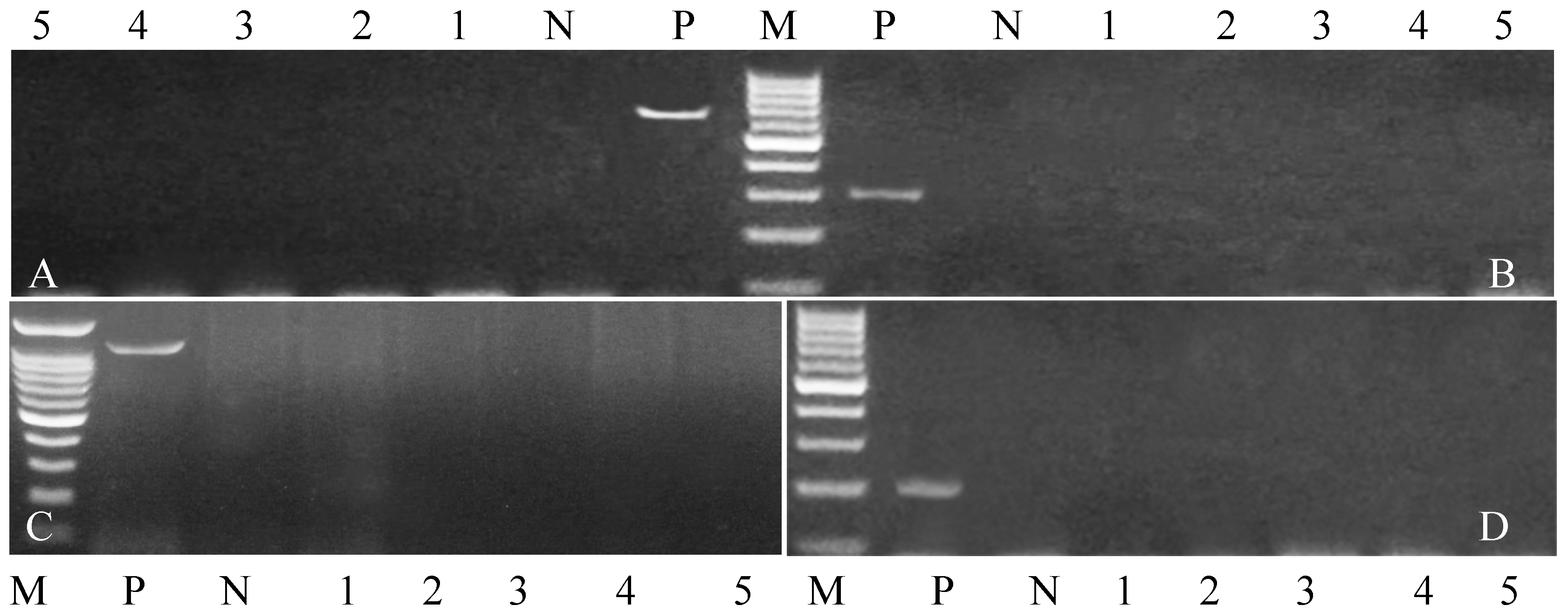
| Target Gene | Primers Sequences | Amplified Segment (bp) | Primary Denaturation | Amplification (35 Cycles) | Final Extension | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Secondary Denaturation | Annealing | Extension | ||||||
| Salmonella invA | GTGAAATTATCGCCACGTTCGGGCAA | 284 | 94 °C 5 min | 94 °C 30s | 55 °C 30 s | 72 °C 30 s | 72 °C 7 min. | [38] |
| TCATCGCACCGTCAAAGGAACC | ||||||||
| L. monocytogenes 16S rRNA | ggA CCg ggg CTA ATA CCg AAT gAT AA | 1200 | 94 °C 5 min | 94 °C 30 s | 60 °C 40 s | 72 °C 1 min. | 72 °C 10 min. | [39] |
| TTC ATg TAg gCg AgT TgC AgC CTA | ||||||||
| Campylobacter 23S rRNA | TATACCGGTAAGGAGTGCTGGAG | 650 | 94 °C 5 min | 94 °C 30 s | 55 °C 40 s | 72 °C 45 s | 72 °C 10 min. | [40] |
| ATCAATTAACCTTCGAGCACCG | ||||||||
| Leptospira secY | GCGATTCAGTTTAATCCTGC | 202 | 95 °C 5 min | 94 °C 30 s | 54 °C 30 s | 72 °C 30 s | 72 °C 7 min. | [41] |
| GAGTTAGAGCTCAAATCTA | ||||||||
| Brucella IS711 | GGC-GTG-TCT-GCA-TTC-AAC-G | 839 | 95°C 5 min | 94 °C 30 s | 55 °C 40 s | 72 °C 50 s | 72 °C 10 min. | [42] |
| GGC-TTG-TCT-GCA-TTC-AAG-G | ||||||||
| Chlamydia psittaci pmp | ATGAAACATCCAGTCTACTGG | 300 | 94 °C 5 min | 94 °C 30 s | 50 °C 30 s | 72 °C 30 s | 72 °C 7 min. | [43] |
| TTGTGTAGTAATATTATCAAA | ||||||||
| Coxiella burneti ISIIII | TAT GTA TCC ACC GTA GCC AGT C | 687 | 94 °C 5 min | Five cycles at 94 °C for 30 s, 66 to 61 °C (the temperature was decreased by 1 °C between consecutive steps) for 1 min, and 72 °C for 1 min. These cycles were followed by 35 cycles consisting of 94 °C for 30 s, 61 °C for 30 s, and 72 °C for 1 min. | 72 °C 7 min | [44] | ||
| CCC AAC AAC ACC TCC TTA TTC | ||||||||
| Investigated Marker | Primer | Product Size (bp) | Annealing Temperature (°C) | GenBank Isolate |
|---|---|---|---|---|
| TLR4 | F5′-ATGATGGCGCGTGCCCGCCG-3 R5′-GTCTCCACGGCCACCAGCTTC-3′ | 407 | 58 | NM_001135930.1 |
| IL-8 | F5′-CGAGAAGTCCTCTGGGACAGC-3 R5′-CATGGATCTTGCTTCTCAGCTC-3′ | 389 | 60 | X78306.1 |
| IL-17 | F5′-ATCTACAGTGAACTGGAAGGAG-3′ R5′-CGAAGGACCAGGATCTCTTGCT-3′ | 389 | 60 | XM_004018887.6 |
| NF-kB | F5′-ATCCACCTGCACGCACACAGC-3′ R5′-GCTGTCATAGATGGCGTCCGAC-3′ | 411 | 60 | XM_060416845.1 |
| CFH | F5′-GGGCCTCCTCCACCAATAGAC-3′ R5′-CTTTCCTTCCCGACAAGTTGT-3′ | 339 | 58 | EU888587.1 |
| TMED1 | F5′-AGCACTGGCTGGCTTGCAGGT-3′ R5′-GTGACTGTTCTGGCAAGAACAC-3′ | 408 | 58 | XM_060415483.1 |
| ICAM | F5′-TGAGAGTGAACTGCAGTATC-3′ R5′-CTCGGAGCAGCACCATGGAGA-3′ | 324 | 58 | AF110984.1 |
| SMURF1 | F5′-AAGATCCGTCTGACAGTATTA-3′ R5′-CCTGCAGTCCACCACAGAGCCG-3′ | 420 | 60 | XM_027961801.3 |
| CSFIR | F5′-GTGTCTGAGAATCCGGCTCTCT-3′ R5′-TCTCCAGGCTCAGTGCAGCGGT-3′ | 335 | 58 | XM_027970397.2 |
| SOD3 | F5′-ATCCGCGACATGCACGCCAAG-3′ R5′-CCAGACCTGGCCATCTCGCAC-3′ | 381 | 60 | XM_027970902.2 |
| CAT | F5′-CTGATGTCCTGACCACTGGCGC-3′ R5′-CATGTCCGGATCCTTCAGGTG-3′ | 473 | 58 | XM_060400055.1 |
| Nrf2 | F5′-CCGCTGCTCCTCTGCTCAAGA-3′ R5′-CAGCTCATGCTCCTTCTGTCGT-3′ | 416 | 58 | OR900054.1 |
| Keap1 | F5′-GCTCGGTGCGCGGGTGGCAC-3′ R5′-TGGCTGAGCCGCAGTTCATTCA-3′ | 360 | 60 | XM_027969637.3 |
| PRDX2 | F5′-ATGGCCTGCGGCAAGGCGCAC-3′ R5′-TCATCTTCCTTCAGCACGCCAT-3′ | 365 | 58 | NM_001166200.1 |
| HMOX1 | F5′-CAGAGGAGCTGCACCGCCGGG-3′ R5′-ACAGCTGGATGTTGAGCAGGA-3′ | 402 | 60 | OR900057.1 |
| ß. actin | F5′-AATTCCATCATGAAGTGTGAC-3′ R5′-GATCTTGATCTTCATCGTGCT-3′ | 150 | 58 | KU365062.1 |
| Microorganisms | Sites of Isolation | Total Isolates (n = 111) | ||||||
|---|---|---|---|---|---|---|---|---|
| Aborted Fetuses (n = 37) | Vaginal Swab (n = 37) | Placenta Swab (n = 37) | ||||||
| Bacterial isolates | No. | % | No | % | No | % | No | % |
| Brucella melitensis | 5 | 13.5 | 3 | 8.1 | 4 | 10.8 | 12 | 10.8 |
| Salmonella | 4 | 10.8 | 1 | 2.7 | 3 | 8.1 | 8 | 7.2 |
| Campylobacter sp. | 3 | 8.1 | 1 | 2.7 | 2 | 5.4 | 6 | 5.4 |
| Listeria monocytogens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coxiella burnetii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlamydia psittaci | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total bacterial isolates | 12 | 32.4 | 5 | 13.5 | 9 | 24.3 | 26 | 23.4 |
| Microorganisms | Total Isolates | Sites of Isolation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stomach Content | Liver | Spleen | Lungs | |||||||
| Bacterial isolates | No | % | No | % | No | % | No | % | No | % |
| Brucella melitensis | 5 | 13.5 | 5 | 100 | 4 | 80 | 4 | 80 | 3 | 60 |
| Salmonella | 4 | 10.8 | 4 | 100 | 3 | 75 | 3 | 75 | 2 | 50 |
| Campylobacter sp. | 3 | 8 | 3 | 100 | 2 | 66.6 | 2 | 66.6 | 1 | 33.3 |
| Listeria monocytogens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coxiella burnetii | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chlamydia psittaci | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total bacterial isolates | 12 | 32.4 | 12 | 100 | 9 | 75 | 9 | 75 | 6 | 50 |
| Gene | SNP | Healthy n = 43 | Aborted n = 37 | Total n = 80 | Chi-Square Value X2 | p-Value | Kind of Inherited Change | Amino Acid Order and Sort |
|---|---|---|---|---|---|---|---|---|
| TLR4 | A90C | 25/43 | -/37 | 25/80 | 31.2 | 0.001 | Synonymous | 30 V |
| G297A | 31/43 | -/37 | 31/80 | 43.5 | 0.001 | Synonymous | 99 Q | |
| IL-8 | C129T | 18/43 | -/37 | 18/80 | 19.9 | 0.001 | Synonymous | 43 L |
| C238T | -/43 | 21/37 | 21/80 | 33 | 0.001 | Synonymous | 80 L | |
| IL-17 | G90A | 26/43 | -/37 | 26/80 | 33.1 | 0.001 | Synonymous | 30 V |
| G156A | -/43 | 28/37 | 28/80 | 50 | 0.001 | Synonymous | 52 Q | |
| G214A | -/43 | 22/37 | 22/80 | 35.2 | 0.001 | Non-synonymous | 72 D to N | |
| NF-kB | A264G | 23/43 | -/37 | 23/80 | 27.7 | 0.001 | Synonymous | 88 K |
| CFH | C58T | 36/43 | -/37 | 36/80 | 56.3 | 0.001 | Non-synonymous | 20 P to S |
| A250G | -/43 | 22/37 | 22/80 | 35.2 | 0.001 | Non-synonymous | 84 T to A | |
| TMED1 | T337C | 26/43 | -/37 | 26/80 | 33.1 | 0.001 | Non-synonymous | 113 W to L |
| ICAM | C88T | 19/43 | -/37 | 19/80 | 21.4 | 0.001 | Synonymous | 30 P to S |
| SMURF1 | T120G | 31/43 | -/37 | 31/80 | 43.5 | 0.001 | Synonymous | 40 T |
| T198C | -/43 | 28/37 | 28/80 | 50 | 0.001 | Synonymous | 66 S | |
| C369T | -/43 | 22/37 | 22/80 | 35.2 | 0.001 | Synonymous | 123 S | |
| CSFIR | T52C | -/43 | 16/37 | 16/80 | 23.2 | 0.001 | Non-synonymous | 18 Y to H |
| A132G | 32/43 | -/37 | 32/80 | 45.8 | 0.001 | Synonymous | 44 P |
| Gene | SNP | Healthy n = 43 | Aborted n = 37 | Total n = 80 | Chi-Square Value X2 | p-Value | Kind of Inherited Change | Amino Acid Order and Sort |
|---|---|---|---|---|---|---|---|---|
| SOD3 | C147G | -/43 | 22/37 | 22/80 | 35.2 | 0.001 | Non-synonymous | 49 S to R |
| CAT | C155T | 19/43 | -/37 | 19/80 | 21.4 | 0.001 | Non-synonymous | 52 T to M |
| Nrf2 | C179T | -/43 | 24/37 | 24/80 | 39.8 | 0.001 | Non-synonymous | 60 T to I |
| Keap1 | C114T | -/43 | 15/37 | 15/80 | 21.4 | 0.001 | Synonymous | 38 G |
| G268A | 29/43 | -/37 | 29/80 | 39.1 | 0.001 | Non-synonymous | 90 A to T | |
| PRDX2 | T237G | -/43 | 22/37 | 22/80 | 33 | 0.001 | Synonymous | 79 S |
| HMOX1 | T99C | 31/43 | -/37 | 31/80 | 43.5 | 0.001 | Synonymous | 33 S |
| C284T | 19/43 | -/37 | 19/80 | 21.4 | 0.001 | Non-synonymous | 95 A to V |
| Predicted Group Membership | Total | |||
|---|---|---|---|---|
| Healthy | Abortion | |||
| Count | Healthy | 37 | 0 | 100 |
| Diseased | 0 | 43 | 100 | |
| % | Healthy | 37 | 0.0 | 100.0 |
| Diseased | 0.0 | 43 | 100.0 | |
| Parameters | Control Group | Abortion Group | p-Value |
|---|---|---|---|
| IL-1α (Pg/mL) | 7.1 ± 0.8 | 34.9 ± 4.2 * | 0.003 |
| IL-1β (Pg/mL) | 9.3 ± 1.8 | 68.1 ± 6 * | 0.001 |
| IL-6 (Pg/mL) | 2.9 ± 0.4 | 21.2 ± 2 * | 0.001 |
| TNF-α (Pg/mL) | 5 ± 0.4 | 8.1 ± 0.6 * | 0.02 |
| IL-10 (Pg/mL) | 29.8 ± 3.1 | 9.8 ± 1.1 * | 0.004 |
| (IFN-τ) (ng/mL) | 4.7 ± 1.3 | 0.7 ± 0.1 * | 0.04 |
| Parameters | Control Group | Abortion Group | p-Value |
|---|---|---|---|
| MDA (nmol/mL) | 6.8 ± 0.8 | 17.3 ± 1.5 * | 0.004 |
| NO (μmol/L) | 7.4 ± 0.7 | 17.1 ± 1.4 * | 0.004 |
| CAT (U/L) | 37.8 ± 2.6 | 18.8 ± 1.3 * | 0.003 |
| GPx (U/mL) | 60.5 ± 3.5 | 40 ± 0.5 * | 0.004 |
| GSH (mg/dL) | 42.6 ± 2.6 | 26 ± 0.5 * | 0.003 |
| Progesterone (ng/mL) | 1.53 ± 0.2 | 0.33 ± 0.1 * | 0.007 |
| IGFBP1 (ng/mL) | 50 ± 2.8 | 63.3 ± 2 * | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eissa, A.; Elsayed, A.A.; Tahoun, A.; M. El-Kattan, A.; Wassif, I.M.; Hafez, A.A.; Mohamed, R.; Ateya, A.I.; Alharbi, H.M.; Alwutayd, K.M.; et al. Individual Genomic Loci, Transcript Levels, and Serum Profiles of Immune and Antioxidant Markers Associated with Bacteria-Induced Abortion in Sheep (Ovis aries). Vet. Sci. 2025, 12, 719. https://doi.org/10.3390/vetsci12080719
Eissa A, Elsayed AA, Tahoun A, M. El-Kattan A, Wassif IM, Hafez AA, Mohamed R, Ateya AI, Alharbi HM, Alwutayd KM, et al. Individual Genomic Loci, Transcript Levels, and Serum Profiles of Immune and Antioxidant Markers Associated with Bacteria-Induced Abortion in Sheep (Ovis aries). Veterinary Sciences. 2025; 12(8):719. https://doi.org/10.3390/vetsci12080719
Chicago/Turabian StyleEissa, Attia, Ahmed A. Elsayed, Amin Tahoun, Adel M. El-Kattan, Islam M. Wassif, Amani A. Hafez, Ragab Mohamed, Ahmed I. Ateya, Hanan M. Alharbi, Khairiah M. Alwutayd, and et al. 2025. "Individual Genomic Loci, Transcript Levels, and Serum Profiles of Immune and Antioxidant Markers Associated with Bacteria-Induced Abortion in Sheep (Ovis aries)" Veterinary Sciences 12, no. 8: 719. https://doi.org/10.3390/vetsci12080719
APA StyleEissa, A., Elsayed, A. A., Tahoun, A., M. El-Kattan, A., Wassif, I. M., Hafez, A. A., Mohamed, R., Ateya, A. I., Alharbi, H. M., Alwutayd, K. M., Ammari, A. A., Babaker, M. A., Alghamdi, M. A., Bohn, T., AL-Farga, A., & M. Aljawdah, H. (2025). Individual Genomic Loci, Transcript Levels, and Serum Profiles of Immune and Antioxidant Markers Associated with Bacteria-Induced Abortion in Sheep (Ovis aries). Veterinary Sciences, 12(8), 719. https://doi.org/10.3390/vetsci12080719








