Dietary Cannabidiol Supplementation on Growth Performance, Behavior, Blood Profile, Metabolomic Analysis, and Fatty Acid Composition in Rabbits: A Multi-Disciplinary Approach to Improve Welfare and Productivity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Behavioral Analysis
2.3. Blood Biochemistry and Oxidative Status
2.4. Metabolomic Analysis
2.5. Total Lipids and Fatty Acid Profiles of Feedstuff, Meat Cuts, and Livers
2.6. Cholesterol Content of the Loins, Thighs, and Livers
2.7. Oxidative Status of the Loins, Thighs, and Livers
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Characteristics
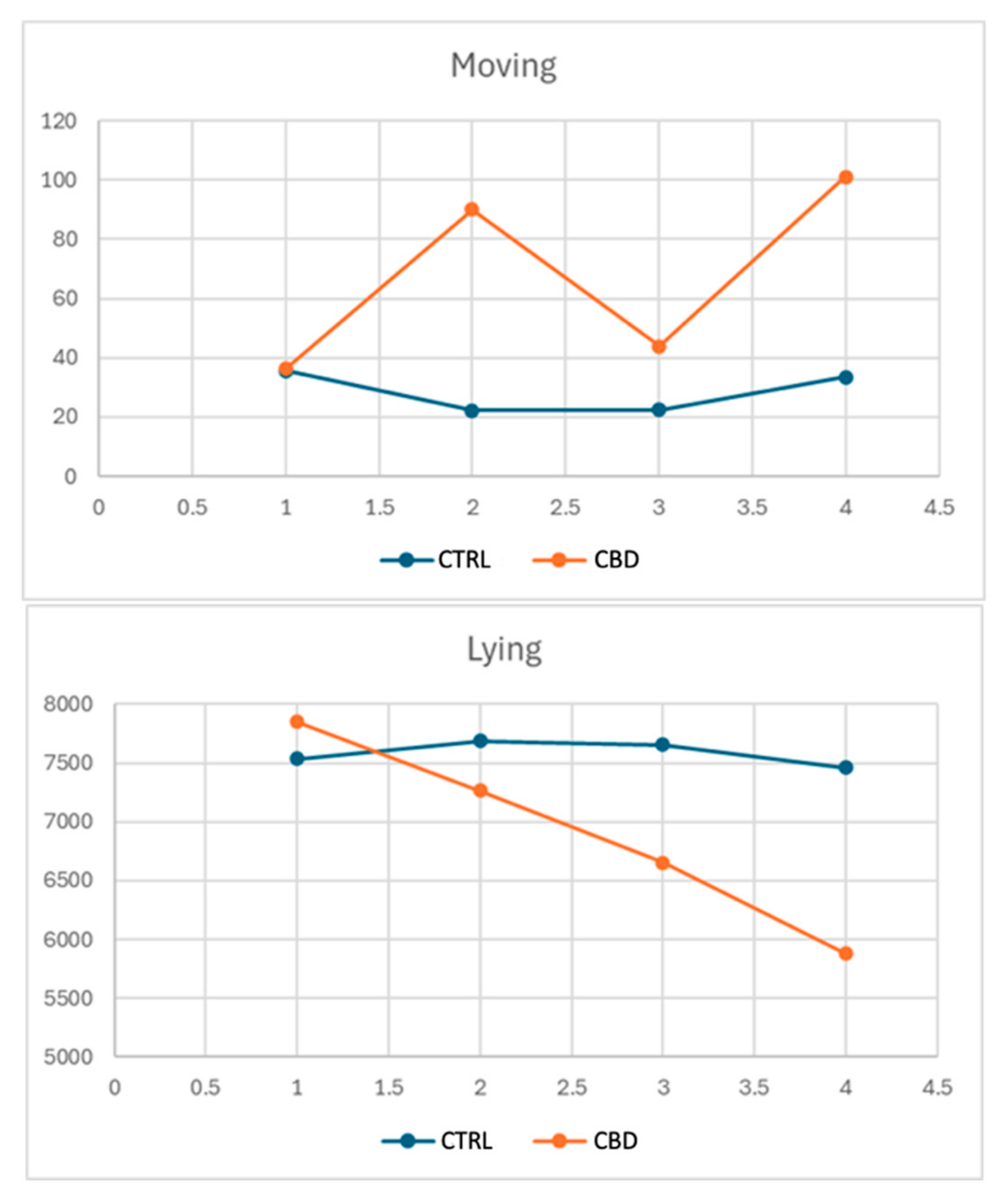
3.2. Behavioral Analysis
3.3. Blood Profiles
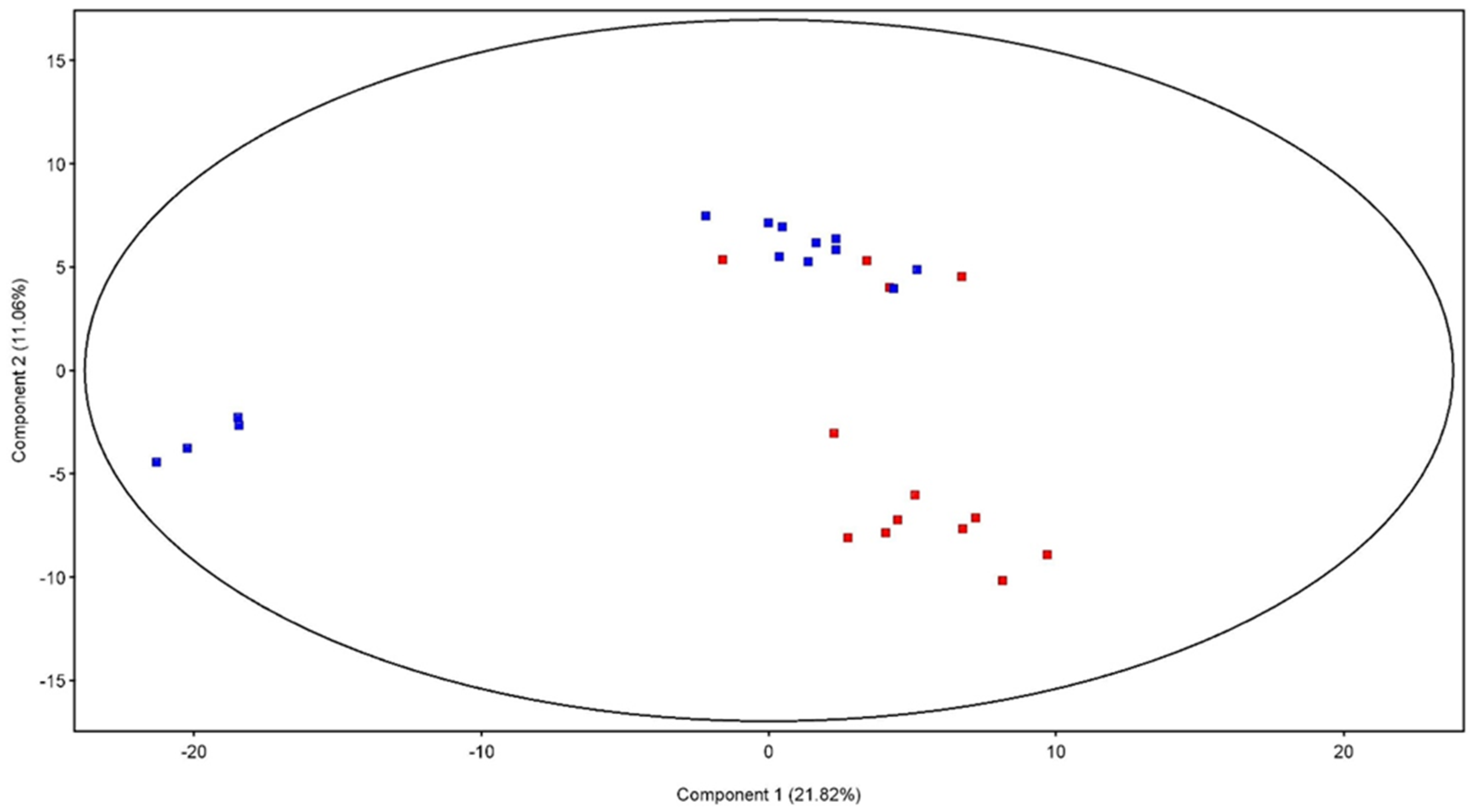
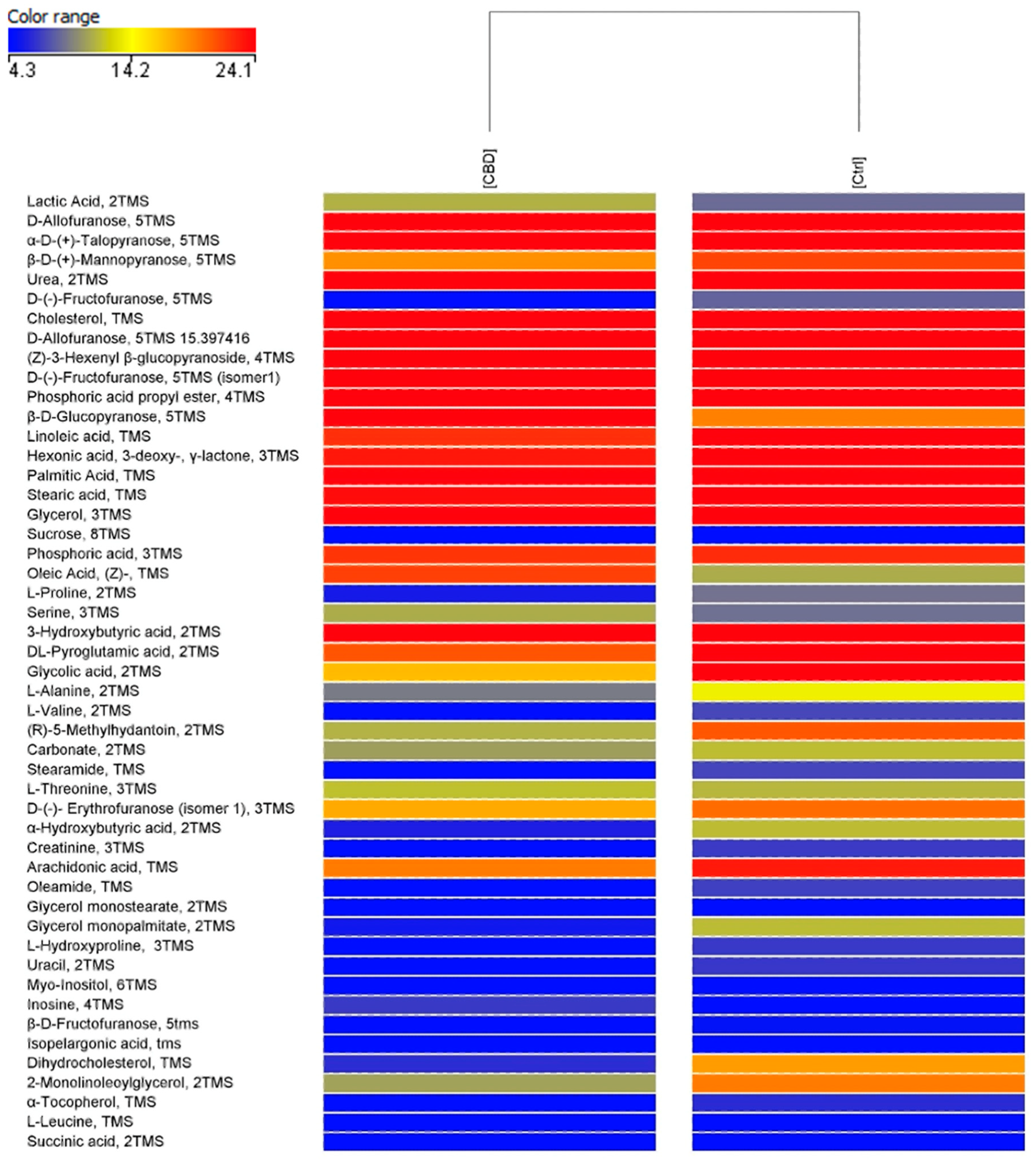
3.4. Metabolomic Analysis
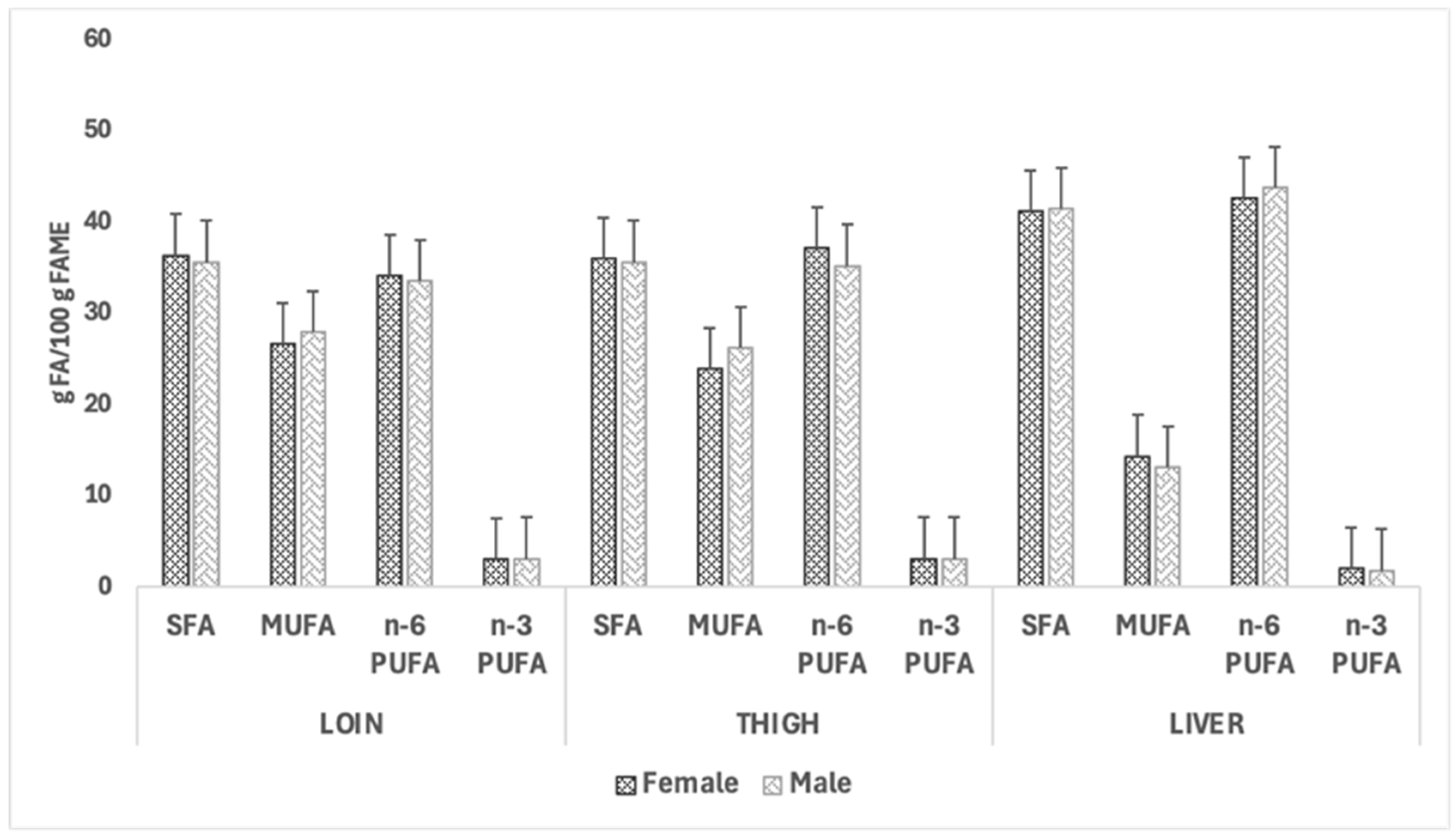
3.5. Total Lipids and Fatty Acid Profile of the Loins, Thighs, and Livers
3.6. Cholesterol of Meat Cuts and Livers
3.7. Oxidative Status of Meat and Livers
4. Discussion
4.1. Growth Performance and Carcass Characteristics
4.2. Metabolomic Analysis
4.3. Blood Profiles
4.4. Behavioral Analysis
4.5. Total Lipids and Fatty Acid Profile of the Loins, Thighs, and Livers
4.6. Lipid Oxidative Status of the Muscles and Livers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukefahr, S.D.; McNitt, J.I.; Cheeke, P.R.; Patton, N.M. Rabbit Production; CABI: Wallingford, UK, 2022. [Google Scholar]
- Kumar, P.; Sharma, N.; Narnoliya, L.K.; Verma, A.K.; Umaraw, P.; Mehta, N.; Sazili, A.Q. Improving Quality and Consumer Acceptance of Rabbit Meat: Prospects and Challenges. Meat Sci. 2024, 219, 109660. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, A.S.; Fayed, A.M.; Basyony, M.M.; Abu Hafsa, S.H.; Mahmoud, A.E. Hematology Profile, Digestive Enzymes, Thyroid Hormones, Productivity, and Nitrogen Balance of Growing Male Rabbits Supplemented with Exogenous Dietary Lysozyme. Anim. Biotechnol. 2023, 34, 3637–3646. [Google Scholar] [CrossRef]
- De Cerqueira Magalhães, L.C.; Costa, R.B.; de Camargo, G.M.F. Consumption of Rabbit Meat in Brazil: Potential and Limitations. Meat Sci. 2022, 191, 108873. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, S.; Bobak, Ł.; Opaliński, S. Hemp in Animal Diets—Cannabidiol. Animals 2022, 12, 2541. [Google Scholar] [CrossRef]
- Papatzimos, G.; Kasapidou, E. Review of hemp components as functional feed and food ingredients: Impact on animal product quality traits and nutritional value. Explor. Foods Foodomics 2024, 2, 626–650. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Halawa, O.I.; Furnish, T.J.; Mark, S.; Wallace, M.S. Role of Cannabinoids in Pain Management. In Essentials of Pain Medicine (Fourth Edition); Benzon, H.T., Raja, S.N., Liu, S.S., Fishman, S.M., Cohen, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 56, pp. 509–520.e2. [Google Scholar] [CrossRef]
- Jang, E.; Kim, H.; Jang, S.; Lee, J.; Baeck, S.; In, S.; Han, E. Concentrations of THC, CBD, and CBN in Commercial Hemp Seeds and Hempseed Oil Sold in Korea. Forensic Sci. Int. 2020, 306, 110064. [Google Scholar] [CrossRef]
- Hassan, F.U.; Liu, C.; Mehboob, M.; Bilal, R.M.; Arain, M.A.; Siddique, F.; Lin, Q. Potential of Dietary Hemp and Cannabinoids to Modulate Immune Response to Enhance Health and Performance in Animals: Opportunities and Challenges. Front. Immunol. 2023, 14, 1285052. [Google Scholar] [CrossRef]
- Corsato Alvarenga, I.; Panickar, K.S.; Hess, H.; McGrath, S. Cannabinoids in Animal Health: A Review. Annu. Rev. Anim. Biosci. 2023, 11, 227–246. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Vitale, R.M.; Iannotti, F.A.; Amodeo, P. The (Poly)Pharmacology of Cannabidiol in Neurological and Neuropsychiatric Disorders: Molecular Mechanisms and Targets. Int. J. Mol. Sci. 2021, 22, 4876. [Google Scholar] [CrossRef]
- Yu, C.H.J.; Rupasinghe, H.V. Cannabidiol-Based Natural Health Products for Companion Animals: Recent Advances in the Management of Anxiety, Pain, and Inflammation. Res. Vet. Sci. 2021, 140, 38–46. [Google Scholar] [CrossRef]
- Amato, R.; Pacifico, E.; Lotito, D.; Iervolino, V.; Pierantoni, L.; Cortese, L.; Pero, M.E. Effects of a Cannabinoid-Based Phytocomplex (Pain Relief™) on Chronic Pain in Osteoarthritic Dogs. Animals 2025, 15, 101. [Google Scholar] [CrossRef]
- Zicarelli, F.; Lotito, D.; Iommelli, P.; Amato, R.; Mahayri, T.M.; Musco, N.; Lombardi, P. Hemp Hay (Cannabis sativa L.) in Grazing Goats’ Diet: Effects on Oxidative and Inflammatory Status. Animals 2025, 15, 364. [Google Scholar] [CrossRef]
- Iommelli, P.; Zicarelli, F.; Amato, R.; Musco, N.; Sarubbi, F.; Bailoni, L.; Tudisco, R. The Effects of Hemp Hay (Cannabis sativa L.) in the Diets of Grazing Goats on Milk Production and Fatty Acid Profile. Animals 2024, 14, 2373. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Corroon, J.; Felice, J.F. The Endocannabinoid System and Its Modulation by Cannabidiol (CBD). Altern. Ther. Health Med. 2019, 25, 6–14. [Google Scholar] [PubMed]
- Trocino, A.; Menegon, F.; Zomeño, C.; Pasqualin, D.; Cunial, G.; Xiccato, G.; Pirrone, F.; Bertotto, D.; Bortoletti, M.; Dorigo, F.; et al. A Pilot Study about On-Farm Assessment of Health and Welfare in Rabbits Kept in Different Housing Systems. Front. Vet. Sci. 2022, 9, 936643. [Google Scholar] [CrossRef]
- Formelová, Z.; Chrastinová, Ľ.; Chrenková, M.; Polačiková, M.; Rajský, M.; Bučko, O.; Vizzarri, F. Hempseed Cake in Rabbit Nutrition: Livestock Performances, Quality of Meat, Digestibility of Nutrients and Animal Health Status. Slovak J. Anim. Sci. 2023, 56, 3–15. [Google Scholar] [CrossRef]
- Jacobson, K.J.; Verret, E.G.; Runyan, C.L.; Kinman, L.A.; Owsley, W.F.; Muir, J.P.; Smith, W.B. Hempseed Meal as a Feedstuff in the Diet of Growing Meat Rabbits. Appl. Anim. Sci. 2023, 39, 125–132. [Google Scholar] [CrossRef]
- Baláži, A.; Svoradová, A.; Kováčik, A.; Vašíček, J.; Chrenek, P. The Effects of Adding Hempseed Cake on Sperm Traits, Body Weight, Haematological and Biochemical Parameters in Rabbit Males. Vet. Sci. 2024, 11, 509. [Google Scholar] [CrossRef]
- Dochez-Arnault, J.; Desdoits-Lethimonier, C.; Matias, I.; Evrard, B.; Lagarrigue, M.; Toupin, M.; Lardenois, A.; Chalmel, F.; Mazaud-Guittot, S.; Dejucq-Rainsford, N.; et al. Expression of the endocannabinoid system and response to cannabinoid components by the human fetal testis. BMC Med. 2023, 21, 219. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Olofinsan, K.A.; Oyedemi, S.O.; Chukwuma, C.I.; Nde, A.L.; Shahidul, I.M. Cannabidiol Improves Glucose Utilization and Modulates Glucose-Induced Dysmetabolic Activities in Isolated Rats’ Peripheral Adipose Tissues. Biomed. Pharmacother. 2022, 149, 112863. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Adli, D.N.; Nugraha, W.S.; Yudhistira, B.; Lavrentev, F.V.; Shityakov, S.; Ibrahim, S.A. Social, Ethical, Environmental, Economic and Technological Aspects of Rabbit Meat Production—A Critical Review. Heliyon 2024, 10, e2963510. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Saxmose Nielsen, S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Michel, V. Health and Welfare of Rabbits Farmed in Different Production Systems. EFSA J. 2020, 18, e05944. [Google Scholar]
- European Commission. National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Working Document on Specific Articles in Directive 2010/63/EU; European Commission: Brussels, Belgium, 2011; pp. 1–12. [Google Scholar]
- Rooney, T.A.; Carpenter, J.W.; KuKanich, B.; Gardhouse, S.M.; Magnin, G.C.; Tully, T.N. Feeding Decreases the Oral Bioavailability of Cannabidiol and Cannabidiolic Acid in Hemp Oil in New Zealand White Rabbits (Oryctolagus cuniculus). Am. J. Vet. Res. 2022, 83, ajvr.22.01.0006. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines (EDQM). European Pharmacopoeia, 6th ed.; European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2008; Chapter 2; pp. 4407–4413. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000; Volume 2. [Google Scholar]
- Fernández-Carmona, J.; Cervera, C.; Blas, E. Prediction of the Energy Value of Rabbit Feeds Varying Widely in Fibre Content. Anim. Feed Sci. Technol. 1996, 64, 61–75. [Google Scholar] [CrossRef]
- Hassan, F.A.; Mahrose, K.M.; Basyony, M.M. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch. Anm. Nutr. 2016, 70, 141–154. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. A Simple Procedure for Rapid Transmethylation of Glycerolipids and Cholesteryl Esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Bovera, F.; Nizza, S.; Baronti, N.; Gasco, L.; Conte, G.; Parisi, G. Quality of Eggs from Lohmann Brown Classic Laying Hens Fed Black Soldier Fly Meal as Substitute for Soya Bean. Animal 2018, 12, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Xiong, Y.L.; Decker, E.A. Inhibition of Protein and Lipid Oxidation in Beef Heart Surimi-Like Material by Antioxidants and Combinations of pH, NaCl, and Buffer Type in the Washing Media. J. Agric. Food Chem. 1996, 44, 119–125. [Google Scholar] [CrossRef]
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Parisi, G. Effect of Mechanical Separation Process on Lipid Oxidation in European Aquacultured Sea Bass, Gilthead Sea Bream, and Rainbow Trout Products. Food Control 2016, 67, 75–81. [Google Scholar] [CrossRef]
- SAS/STAT Software, SAS Institute, Inc.: Cary, NC, USA, 2021.
- Horakova, L.; Šťastník, O.; Pavlata, L.; Mrkvicova, E. The use of hemp herb in diet for growing rabbits. In Proceedings of the MendelNet, Brno, Czech Republic, 11 November 2020; pp. 102–106. [Google Scholar]
- Aguilar-Roblero, R.; González-Mariscal, G. Behavioral, Neuroendocrine and Physiological Indicators of the Circadian Biology of Male and Female Rabbits. Eur. J. Neurosci. 2020, 51, 429–453. [Google Scholar] [CrossRef]
- Setiaji, A.; Sutopo, S.; Kurnianto, E. Growth Analysis in Rabbit Using Gompertz Non-Linear Model. J. Indones. Trop. Anim. Agric. 2013, 38, 92–97. [Google Scholar] [CrossRef]
- Krebs, G.L.; De Rosa, D.W.; White, D.M.; Blake, B.L.; Dods, K.C.; May, C.D.; Tai, Z.X.; Clayton, E.H.; Lynch, E.E. Intake, Nutrient Digestibility, Rumen Parameters, Growth Rate, Carcase Characteristics and Cannabinoid Residues of Sheep Fed Pelleted Rations Containing Hemp (Cannabis sativa L.) Stubble. Transl. Anim. Sci. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Khamhan, S.; Tathong, T.; Phoemchalard, C. The Effects of Fresh Hemp Leaf Supplementation (Cannabis sativa) on the Physiological and Carcass Characteristics and Meat Quality in Transported Goats. Animals 2023, 13, 3881. [Google Scholar] [CrossRef]
- Yusuf, M.; Yusup, M.; Pramudya, R.D.; Fauzi, A.Y.; Rizky, A. Enhancing User Login Efficiency via Single Sign-On Integration in Internal Quality Assurance System (espmi). Int. Trans. Artif. Intell. 2024, 2, 164–172. [Google Scholar] [CrossRef]
- Bhatt, H.K.; Song, D.; Musgrave, G.; Rao, P.S.S. Cannabinoid-Induced Changes in the Immune System: The Role of microRNAs. Int. Immunopharmacol. 2021, 98, 107832. [Google Scholar] [CrossRef]
- Nasr, M.A.; Abd-Elhamid, T.; Hussein, M.A. Growth Performance, Carcass Characteristics, Meat Quality and Muscle Amino-Acid Profile of Different Rabbit’s Breeds and Their Crosses. Meat Sci. 2017, 134, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Nealon, N.J.; Summers, S.; Quimby, J.; Winston, J.A. Untargeted Metabolomic Profiling of Serum from Client-Owned Cats with Early and Late-Stage Chronic Kidney Disease. Sci. Rep. 2024, 14, 4755. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of Cholesterol Homeostasis in Health and Diseases: From Mechanisms to Targeted Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Wang, Y.F.; Lee, G.L.; Huang, Y.H.; Kuo, C.C. sn-1,2-Diacylglycerols Protect against Lethal Endotoxemia by Controlling Systemic Inflammation. Immunobiology 2016, 221, 1309–1318. [Google Scholar] [CrossRef]
- Tortoriello, G.; Beiersdorf, J.; Romani, S.; Williams, G.; Cameron, G.A.; Mackie, K.; Harkany, T. Genetic Manipulation of sn-1-Diacylglycerol Lipase and CB1 Cannabinoid Receptor Gain-of-Function Uncover Neuronal 2-Linoleoyl Glycerol Signaling in Drosophila melanogaster. Cannabis Cannabinoid Res. 2021, 6, 119–136. [Google Scholar] [CrossRef]
- Morris, E.M.; Kitts-Morgan, S.E.; Spangler, D.M.; Ogunade, I.M.; McLeod, K.R.; Harmon, D.L. Alteration of the Canine Metabolome after a 3-Week Supplementation of Cannabidiol (CBD) Containing Treats: An Exploratory Study of Healthy Animals. Front. Vet. Sci. 2021, 8, 685606. [Google Scholar] [CrossRef] [PubMed]
- Musco, N.; Lombardi, P.; Addeo, N.F.; Secci, G.; Parisi, G.; Pero, M.E.; Piccolo, G.; Nizza, A.; Bovera, F. Mirrors Can Affect Growth Rate, Blood Profile, Carcass and Meat Traits and Caecal Microbial Activity of Rabbits Reared in a “Small Group” Free-Range System. Animals 2019, 9, 639. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Oyedemi, S.O.; Oyenihi, O.R.; Ibeji, C.U.; Islam, M.S. Cannabis sativa L. (var. Indica) Exhibits Hepatoprotective Effects by Modulating Hepatic Lipid Profile and Mitigating Gluconeogenesis and Cholinergic Dysfunction in Oxidative Hepatic Injury. Front. Pharmacol. 2021, 12, 705402. [Google Scholar]
- Li, Z.; He, H.; Chen, M.; Ni, M.; Guo, C.; Wan, Z.; Xu, H. Novel Mechanism of Clostridium butyricum Alleviated Coprophagy Prevention-Induced Intestinal Inflammation in Rabbit. Int. Immunopharmacol. 2024, 130, 111773. [Google Scholar] [CrossRef]
- Cattaneo, L.; Lopreiato, V.; Piccioli-Cappelli, F.; Trevisi, E.; Minuti, A. Plasma albumin-to-globulin ratio before dry-off as a possible index of inflammatory status and performance in the subsequent lactation in dairy cows. J. Dairy Sci. 2021, 104, 8228–8242. [Google Scholar] [CrossRef]
- Peres, F.F.; Lima, A.C.; Hallak, J.E.C.; Crippa, J.A.; Silva, R.H.; Abílio, V.C. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front. Pharmacol. 2018, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Addeo, N.F.; Vozzo, S.; Secci, G.; Mastellone, V.; Piccolo, G.; Lombardi, P.; Parisi, G.; Asiry, K.A.; Attia, Y.A.; Bovera, F. Different combinations of butchery and vegetable wastes on growth performance, chemical-nutritional characteristics and oxidative status of black soldier fly growing larvae. Animals 2021, 11, 3515. [Google Scholar] [CrossRef]
- Della Rocca, G.; Di Salvo, A. Hemp in veterinary medicine: From feed to drug. Front. Vet. Sci. 2020, 7, 387. [Google Scholar] [CrossRef]
- Sacchettino, L.; Gatta, C.; Maruccio, L.; Boncompagni, C.; Napolitano, F.; Avallone, L.; d’Angelo, D. Combining cannabis and melatonin treatment with a rehabilitation program improved symptoms in a dog with compulsive disoder: A case report. Res Vet. Sci. 2023, 160, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Hassan, R.A.; Addeo, N.F.; Bovera, F.; Alhotan, R.A.; Al-Qurashi, A.D.; Basiouni, S. Effects of spirulina Spirulina platensis and/or allium Allium sativum on antioxidant status, immune response, gut morphology, and intestinal lactobacilli and coliforms of heat-stressed broiler chicken. Vet. Sci. 2023, 10, 678. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Vari, C.E.; Rusz, C.-M.; Tero-Vescan, A.; Pușcaș, A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants 2023, 12, 485. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Overall, K.L. Manual of Clinical Behavioral Medicine for Dogs and Cats; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of cannabis Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [CrossRef]
- Peiretti, P.G. Effects of dietary fatty acids on lipid traits in the muscle and perirenal fat of growing rabbits fed mixed diets. Animals 2012, 2, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Simonová, M.P.; Chrastinová, Ľ.; Chrenková, M.; Formelová, Z.; Kandričáková, A.; Bino, E.; Lauková, A. Benefits of enterocin M and sage combination on the physico-chemical traits, fatty acid, amino acid, and mineral content of rabbit meat. Probiotics Antimicrob. Proteins 2020, 12, 1235–1245. [Google Scholar] [CrossRef]
- Miteva, D.; Velikov, K.; Ivanova, S.; Dimov, K. Production of rabbit meat with functional properties. AgroLife Sci. J. 2020, 9, 221–228. [Google Scholar]
- Ostapczuk, K.; Apori, S.O.; Estrada, G.; Tian, F. Hemp Growth Factors and Extraction Methods Effect on Antimicrobial Activity of Hemp Seed Oil: A Systematic Review. Separations 2021, 8, 183. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Valli, E.; Toschi, T.G. Quality Indexes and Composition of 13 Commercial Hemp Seed Oils. J. Food Compos. Anal. 2023, 117, 105112. [Google Scholar] [CrossRef]
- Kim, O.Y.; Song, J. Important Roles of Linoleic Acid and α-linolenic acid in Regulating Cognitive Impairment and Neuropsychiatric Issues in Metabolic-Related Dementia. Life Sci. 2024, 337, 122356. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Collodel, G. Expression of genes and localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef]
- Zubiri-Gaitán, A.; Blasco, A.; Ccalta, R.; Satué, K.; Hernández, P. Intramuscular Fat Selection in Rabbits Modifies the Fatty Acid Composition of Muscle and Liver Tissues. Animals 2022, 12, 893. [Google Scholar] [CrossRef]
- Jackson, K.H.; Harris, W.S.; Belury, M.A.; Kris-Etherton, P.M.; Calder, P.C. Beneficial Effects of Linoleic Acid on Cardiometabolic Health: An Update. Lipids Health Dis. 2024, 23, 296. [Google Scholar] [CrossRef]
- Chernukha, I.; Kotenkova, E.; Pchelkina, V.; Ilyin, N.; Utyanov, D.; Kasimova, T.; Fedulova, L. Pork Fat and Meat: A Balance between Consumer Expectations and Nutrient Composition of Four Pig Breeds. Foods 2023, 12, 690. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annual Review of Pharmacology and Toxicology 2020, 60, 637–659. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef]
- Gál, R.; Zapletal, D.; Jakešová, P.; Straková, E. Proximate Chemical Composition, Amino Acids Profile and Minerals Content of Meat Depending on Carcass Part, Sire Genotype and Sex of Meat Rabbits. Animals 2022, 12, 1537. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Xiaoyi, S.; Fuchang, L. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr. Res. 2017, 61, 1348866. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Youssef, A.A.A.; Sweeney, C.; Cai, C.; Dudhipala, N.; Majumdar, S. Cannabidiol loaded topical ophthalmic nanoemulsion lowers intraocular pressure in normotensive Dutch-belted rabbits. Pharmaceutics 2022, 14, 2585. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Rathinasabapathy, T.; Wagner, C.; Metzger, B.; Carlisle, C.; Panda, C.; Le Brun-Blashka, S.; Troup, J.P.; Varadharaj, S. Endocannabinoid System and Its Regulation by Polyunsaturated Fatty Acids and Full Spectrum Hemp Oils. Int. J. Mol. Sci. 2021, 22, 5479. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the endocannabinoid system in the adipose tissue with focus on energy metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef]
- Bielawiec, P.; Dziemitko, S.; Konstantynowicz-Nowicka, K.; Chabowski, A.; Dzięcioł, J.; Harasim-Symbor, E. Cannabidiol improves muscular lipid profile by affecting the expression of fatty acid transporters and inhibiting de novo lipogenesis. Sci. Rep. 2023, 13, 3694. [Google Scholar]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Cannabidiol (CBD) and D9-tetrahydrocannabivarin (THCV) are nonpsychoactive phytocannabinoids affecting lipid and glucose metabolism in animal models. This study set out to examine the effects of these compounds in patients with type 2 diabetes. Diabetes Care 2016, 39, 1–10. [Google Scholar]
- He, M.; Shi, J.; Xu, Y.J.; Liu, Y. Cannabidiol (CBD) inhibits foam cell formation via regulating cholesterol homeostasis and lipid metabolism. Mol. Nutr. Food Res. 2024, 68, 2400154. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. Effect of Multiple Freeze–Thaw Cycles on Protein and Lipid Oxidation in Rabbit Meat. Int. J. Food Sci. Technol. 2021, 56, 3004–3015. [Google Scholar] [CrossRef]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed]
- Schuelert, N.; McDougall, J.J. The Abnormal Cannabidiol Analogue O-1602 Reduces Nociception in a Rat Model of Acute Arthritis via the Putative Cannabinoid Receptor GPR55. Neurosci. Lett. 2011, 500, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]

| Total cannabidiol (CBD + CBA) | 10.35% |
| Cannabidiol, CBD | 9.42% |
| Cannabidiolic acid, CBA | 1.06% |
| Total tetrahydrocannabinol (THC + THCA) | 0.19% |
| D9-Tetrahydrocannabinol | 0.19% |
| Tetrahydrocannabinolic acid | ND |
| D8-Tetrahydrocannabidinol | ND |
| Total cannabigerol (CBG + CBGA) | 0.36% |
| Cannabigerol | 0.24% |
| Cannabierolic acid | 0.11% |
| Cannabinol | 0.04% |
| Cannabichromene | 0.03% |
| Cannabidivarin | 0.07% |
| Cannabidivarinic acid | ND |
| Basal Diet 1 | Wafer 2 | |
|---|---|---|
| Crude protein, % AF | 15.6 | 11.8 |
| Ether extract, % AF | 3.2 | 1.5 |
| Crude fiber, % AF | 19.9 | 19.8 |
| Ashes, % AF | 6.6 | 8.4 |
| Ca, % AF | 0.34 | - |
| P, % AF | 0.46 | 0.13 |
| Na, % AF | 0.22 | 0.05 |
| Lysine, % AF | 0.66 | - |
| Methionine, % AF | 0.23 | - |
| DE, MJ/kg | 11.6 | 10.9 |
| Fatty Acid | Mean Value |
|---|---|
| C6:0 | 0.03 |
| C8:0 | 41.83 |
| C10:0 | 39.78 |
| C12:0 | 0.15 |
| C14:0 | 0.01 |
| C16:0 iso | 0.04 |
| C16:0 | 1.20 |
| C16:1n-7 | 0.01 |
| C18:0 | 0.44 |
| C18:1n-9 | 1.57 |
| C18:1n-7 | 0.15 |
| C18:2n-6 | 9.76 |
| C18:3n-6 | 0.85 |
| C18:3-n3 | 3.58 |
| C18:4n-3 | 0.28 |
| C20:0 | 0.13 |
| C20:1n-9 | 0.05 |
| C22:0 | 0.05 |
| C22:5n-3 | 0.06 |
| SFA | 83.67 |
| MUFA | 1.79 |
| n-6 PUFA | 10.62 |
| n-3 PUFA | 3.92 |
| Fatty Acid | Mean Value |
|---|---|
| C10:0 | 0.16 |
| C12:0 | 0.18 |
| C14:0 | 0.53 |
| C14:1n-5 | 0.02 |
| iso-C15:0 | 0.05 |
| anteiso-C15:0 | 0.02 |
| C15:0 | 0.14 |
| iso-C16:0 | 0.01 |
| C16:0 | 19.53 |
| C16:1n-9 | 0.14 |
| C16:1n-7 | 0.42 |
| C17:0 | 0.15 |
| C17:1 | 0.01 |
| C18:0 | 3.90 |
| C18:1n-9 | 26.71 |
| C18:1n-7 | 1.17 |
| C18:2n-6 | 39.84 |
| C18:3n-6 | 0.10 |
| C18:3n-3 | 4.89 |
| C20:0 | 0.34 |
| C20:1n-11 | 0.03 |
| C20:1n-9 | 0.38 |
| C20:1n-7 | 0.01 |
| C20:2n-6 | 0.05 |
| C20:3n-6 | 0.02 |
| C20:4n-6 | 0.21 |
| C22:0 | 0.32 |
| C22:1n-11 | 0.10 |
| C22:1n-9 | 0.23 |
| C22:4n-6 | 0.05 |
| C22:5n-3 | 0.03 |
| C24:0 | 0.27 |
| SFA | 25.61 |
| MUFA | 29.22 |
| n-6 PUFA | 40.26 |
| n-3 PUFA | 4.91 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Females | Males | Group | Sex | Interaction | ||
| LW 65d, g | 1727.1 | 1707.1 | 1625.7 b | 1808.6 a | 0.95 | 0.03 | 0.38 | 91.6 |
| LW 92d, g | 2637.1 b | 2731.4 a | 2608.6 b | 2760.0 a | 0.05 | 0.03 | 0.62 | 101.3 |
| BWG 65–70d, g | 35.71 b | 48.27 a | 40.29 b | 43.71 a | 0.01 | 0.04 | 0.49 | 3.96 |
| BWG 86–92d, g/d | 38.98 B | 44.08 A | 46.33 A | 36.74 B | <0.01 | <0.01 | 0.51 | 3.26 |
| BWG 65–92d, g/d | 33.70 B | 37.94 A | 36.40 | 35.23 | <0.01 | 0.12 | 0.28 | 2.75 |
| FI 65–70d, g/d | 126.6 B | 145.4 A | 135.1 | 136.9 | <0.01 | 0.63 | 0.38 | 10.0 |
| FI 71–78d, g/d | 153.7 | 153.9 | 145.3 B | 162.3 A | 0.54 | <0.01 | 0.79 | 11.3 |
| FI 79–85d, g/d | 170.7 A | 157.1 B | 147.6 B | 180.2 A | <0.01 | <0.01 | 0.61 | 13.5 |
| FI 86–92d, g/d | 186.8 | 179.2 | 169.3 B | 196.8 A | 0.11 | <0.01 | 0.74 | 12.63 |
| FI 65–92d, g/d | 161.9 | 161.4 | 151.8 B | 171.5 A | 0.45 | <0.01 | 0.57 | 11.9 |
| FCR 65–70d, g/g | 3.54 A | 3.02 B | 3.35 | 3.13 | <0.01 | 0.07 | 0.70 | 0.26 |
| FCR 71–78d, g/g | 4.30 | 4.14 | 4.13 | 4.30 | 0.15 | 0.012 | 0.80 | 0.32 |
| FCR 79–85d, g/g | 5.51 | 5.27 | 5.01 B | 5.77 A | 0.06 | <0.01 | 0.47 | 0.39 |
| FCR 86–92d, g/g | 4.79 A | 4.06 B | 3.65 B | 5.35 A | <0.01 | <0.01 | 0.82 | 0.30 |
| FCR 65–92d, g/g | 4.80 A | 4.25 B | 4.17 B | 4.86 A | <0.01 | <0.01 | 0.63 | 0.32 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Females | Males | Group | Sex | Interaction | ||
| LW, g | 2617.1 | 2668.3 | 2585.2 b | 2700.2 a | 0.06 | 0.03 | 0.79 | 165.4 |
| Skin, % LW | 14.23 | 15.31 | 13.72 | 15.81 | 0.19 | 0.06 | 0.27 | 1.05 |
| Full GUT, % LW | 17.65 | 16.69 | 17.86 a | 16.49 b | 0.72 | 0.04 | 0.36 | 1.39 |
| Empty GUT, % LW | 8.80 | 8.44 | 8.76 | 8.49 | 0.42 | 0.51 | 0.24 | 0.87 |
| Warm Carcass, g | 1604.3 a | 1702.9 b | 1581.4 a | 1725.8 b | 0.03 | 0.02 | 0.75 | 109.1 |
| Carcass Yield, % | 61.30 B | 63.82 A | 61.17 B | 63.91 A | <0.01 | <0.01 | 0.16 | 1.65 |
| Spleen, % HC | 4.25 B | 6.70 A | 5.41 | 5.54 | <0.01 | 0.59 | 0.20 | 0.23 |
| Urinary + genitals, % LW | 0.66 | 0.62 | 0.64 | 0.63 | 0.74 | 0.95 | 0.49 | 0.04 |
| pH LD1h | 7.11 | 6.86 | 6.97 | 7.06 | 0.11 | 0.80 | 0.95 | 0.33 |
| pH BF1h | 6.91 | 6.75 | 6.71 | 6.96 | 0.56 | 0.30 | 0.44 | 0.40 |
| Chilled carcass, g | 1577.1 | 1688.6 | 1591.4 | 1674.3 | 0.07 | 0.08 | 0.91 | 98.6 |
| CC Yield, % | 60.26 B | 63.28 A | 61.56 | 62.00 | <0.01 | 0.63 | 0.84 | 0.47 |
| pH LD24h | 5.87 A | 5.76 B | 5.81 | 5.83 | <0.01 | 0.94 | 0.31 | 0.045 |
| pH BF24h | 5.93 | 5.89 | 5.91 | 5.92 | 0.28 | 0.95 | 0.43 | 0.048 |
| Reference carcass, g | 1029.6 b | 1116.5 a | 1022.1 b | 1124.0 a | 0.03 | 0.03 | 0.70 | 84.93 |
| Head, % RC | 14.33 | 13.04 | 14.02 | 14.15 | 0.24 | 0.63 | 0.35 | 1.06 |
| Liver, % RC | 8.47 A | 6.91 B | 7.55 | 7.82 | <0.01 | 0.92 | 0.20 | 0.43 |
| Kidney, % RC | 1.71 | 1.60 | 1.66 | 1.66 | 0.22 | 0.85 | 0.46 | 0.14 |
| Lungs, % RC | 1.43 | 1.43 | 1.41 | 1.45 | 0.96 | 0.66 | 0.59 | 0.12 |
| Heart, % RC | 1.29 | 1.33 | 1.26 | 1.36 | 0.31 | 0.28 | 0.23 | 0.09 |
| Inguinal fat % RC | 0.19 A | 0.15 B | 0.18 | 0.16 | 0.01 | 0.45 | 0.84 | 0.01 |
| Perirenal fat, % RC | 2.39 | 2.40 | 2.55 a | 2.23 b | 0.96 | 0.02 | 0.76 | 0.16 |
| Scapular fat, % RC | 0.49 | 0.49 | 0.50 | 0.47 | 0.97 | 0.80 | 0.94 | 0.03 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Grooming | 974.0 | 1064.5 | 1131.8 | 906.2 | 0.8024 | 0.3719 | 0.5942 | 482.9 |
| Moving | 35.74 | 36.23 | 29.73 | 42.27 | 0.8330 | 0.0706 | 0.0306 | 11.66 |
| Setting | 610.7 | 816.1 | 740.2 | 686.6 | 0.2162 | 0.6184 | 0.7890 | 305.03 |
| Lying | 7534.6 | 7850.8 | 7393.8 | 7891.7 | 0.4336 | 0.3243 | 0.8155 | 799.4 |
| Look around | 4.27 | 5.79 | 6.24 | 3.84 | 0.4786 | 0.1932 | 0.1917 | 2.99 |
| Gnawing | 3.90 | 3.57 | 4.74 | 2.73 | 0.9890 | 0.5115 | 0.9167 | 5.49 |
| Stretching | 28.99 | 24.03 | 23.44 | 29.57 | 0.4394 | 0.4241 | 0.7208 | 15.51 |
| Sniffing | 121.6 | 120.9 | 129.4 | 112.2 | 0.9574 | 0.3360 | 0.9717 | 31.86 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Grooming | 1074.6 | 1155.8 | 1244.3 | 986.1 | 0.7375 | 0.3150 | 0.5688 | 440.67 |
| Moving | 22.29 b | 90.09 a | 39.91 | 72.46 | 0.0192 | 0.3440 | 0.2839 | 10.31 |
| Setting | 784.5 | 564.6 | 698.8 | 650.4 | 0.1640 | 0.6088 | 0.9097 | 285.4 |
| Lying | 7689.1 | 7267.1 | 7212.5 | 7743.7 | 0.4552 | 0.3154 | 0.8271 | 842.3 |
| Look around | 0.46 B | 11.80 A | 10.86 a | 1.40 b | 0.0011 | 0.0450 | 0.4079 | 2.86 |
| Gnawing | 1.51 | 4.43 | 4.53 | 1.40 | 0.4817 | 0.4378 | 0.9361 | 6.38 |
| Stretching | 24.97 | 35.31 | 30.49 | 29.80 | 0.2846 | 0.9321 | 0.5799 | 17.13 |
| Sniffing | 115.5 | 187.8 | 163.97 | 139.29 | 0.1487 | 0.7506 | 0.8829 | 83.05 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Grooming | 1019.2 b | 1820.5 a | 1635.5 | 1204.1 | 0.0498 | 0.3619 | 0.9746 | 626.95 |
| Moving | 33.40 | 101.03 | 54.23 | 80.14 | 0.0927 | 0.3759 | 0.4383 | 72.58 |
| Setting | 923.7 | 967.9 | 946.8 | 944.7 | 0.8791 | 0.9884 | 0.6739 | 531.5 |
| Lying | 7458.2 | 5875.6 | 6340.3 | 6993.5 | 0.0850 | 0.5956 | 0.8224 | 1472.8 |
| Look around | 11.20 | 37.06 | 24.11 | 24.14 | 0.0772 | 0.7825 | 0.8891 | 24.82 |
| Gnawing | 6.11 | 14.03 | 13.91 | 6.23 | 0.5397 | 0.5559 | 0.7101 | 20.29 |
| Stretching | 27.31 b | 44.11 a | 38.71 | 32.74 | 0.0101 | 0.4915 | 0.2946 | 9.58 |
| Sniffing | 94.0 | 107.6 | 82.91 | 118.7 | 0.6736 | 0.4037 | 0.0928 | 81.78 |
| Group | Sex | p-Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Glu, mg/dL | 107.9 | 97.34 | 99.30 | 105.9 | 0.1381 | 0.4062 | 0.1219 | 11.19 |
| Chol, mg/dL | 66.94 | 57.88 | 64.64 | 59.19 | 0.0853 | 0.1689 | 0.5971 | 9.87 |
| Try, mg/dL | 82.50 A | 72.33 B | 78.63 | 76.20 | 0.0217 | 0.3398 | 0.8062 | 7.32 |
| TP, g/L | 7.20 | 7.29 | 7.29 | 7.19 | 0.8676 | 0.8077 | 0.0735 | 0.70 |
| Alb, g/L | 3.00 | 2.87 | 2.84 | 3.03 | 0.5011 | 0.3042 | 0.3546 | 0.29 |
| Glob, g/d | 4.20 | 4.41 | 4.45 | 4.16 | 0.5946 | 0.4235 | 0.0163 | 0.5853 |
| Alb/Glob | 0.71 a | 0.65 b | 0.64 b | 0.73 a | 0.0221 | 0.0296 | 0.7859 | 0.059 |
| Urea, mg/dL | 30.43 | 29.14 | 31.14 | 28.43 | 0.6801 | 0.4791 | 0.6089 | 7.45 |
| Crea, mg/dL | 0.877 | 0.867 | 0.94 | 0.81 | 0.8217 | 0.3088 | 0.3684 | 0.24 |
| Ast, U/L | 25.16 | 28.14 | 28.54 | 24.76 | 0.5301 | 0.3926 | 0.0408 | 7.10 |
| Alt, U/L | 65.36 | 61.73 | 63.06 | 64.03 | 0.2101 | 0.8654 | 0.3005 | 4.92 |
| Ldh, U/L | 460.29 | 468.57 | 437.0 | 491.86 | 0.7235 | 0.2346 | 0.0148 | 83.76 |
| CPK, U/L | 1260.7 | 1324.3 | 1312.7 | 1272.3 | 0.6247 | 0.7898 | 0.0857 | 216.44 |
| dROMS UCARR | 103.7 A | 69.71 B | 88.71 | 84.74 | 0.0022 | 0.3215 | 0.5430 | 16.05 |
| BAP, umol/L | 1636.7 B | 2543.3 A | 2241.9 | 1938.1 | 0.0030 | 0.4503 | 0.9940 | 419.18 |
| Compound | Regulation CBD vs. CTRL |
|---|---|
| D-(-)-Fructofuranose 5TMS (isomer 1) | ↓ |
| Dihydrocholesterol, TMS | ↓ |
| 2-Monolinoleoylglycerol, 2TMS | ↓ |
| Group | Sex | p Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Total lipids | 2.13 | 1.26 | 1.52 | 1.87 | 0.135 | 0.529 | 0.694 | 1.004 |
| Fatty acids | ||||||||
| C10:0 | 0.12 a | 0.05 b | 0.07 | 0.10 | 0.024 | 0.205 | 0.069 | 0.047 |
| C12:0 | 0.17 a | 0.09 b | 0.11 | 0.15 | 0.019 | 0.198 | 0.119 | 0.052 |
| Iso C14:0 | 0.03 | 0.03 | 0.03 | 0.03 | 0.675 | 0.224 | 0.115 | 0.011 |
| C14:0 | 1.61 | 1.55 | 1.48 | 1.68 | 0.846 | 0.478 | 0.896 | 0.494 |
| C14:1n-5 | 0.05 | 0.04 | 0.04 | 0.05 | 0.535 | 0.421 | 0.599 | 0.03 |
| Iso C15:0 | 0.05 | 0.05 | 0.04 | 0.05 | 0.806 | 0.591 | 0.527 | 0.015 |
| Anteiso C15:0 | 0.12 | 0.12 | 0.12 | 0.12 | 0.933 | 0.677 | 0.227 | 0.018 |
| C15:0 | 0.58 | 0.57 | 0.59 | 0.57 | 0.819 | 0.421 | 0.844 | 0.046 |
| Iso C16:0 | 0.16 | 0.15 | 0.16 | 0.15 | 0.568 | 0.384 | 0.754 | 0.029 |
| C16:0 | 23.81 | 24.60 | 24.07 | 24.35 | 0.273 | 0.689 | 0.272 | 1.257 |
| C16:1n-9 | 0.38 | 0.36 | 0.37 | 0.37 | 0.372 | 0.919 | 0.919 | 0.044 |
| C16:1n-7 | 1.37 | 1.36 | 1.12 | 1.60 | 0.978 | 0.147 | 0.828 | 0.564 |
| C17:0 | 0.65 | 0.66 | 0.70 a | 0.61 b | 0.851 | 0.033 | 0.620 | 0.068 |
| C18:0 | 7.77 | 8.35 | 8.61 | 7.51 | 0.415 | 0.140 | 0.829 | 1.269 |
| C18:1n-9 | 23.87 | 23.43 | 23.28 | 24.01 | 0.720 | 0.553 | 0.579 | 2.215 |
| C18:1n-7 | 1.46 | 1.53 | 1.48 | 1.50 | 0.599 | 0.874 | 0.642 | 0.219 |
| C18:2n-6 | 27.97 | 27.29 | 27.48 | 27.78 | 0.444 | 0.729 | 0.221 | 1.568 |
| C18:3n-6 | 0.08 | 0.11 | 0.11 | 0.08 | 0.243 | 0.243 | 0.929 | 0.034 |
| C18:3n-3 | 2.45 | 2.35 | 2.30 | 2.49 | 0.806 | 0.644 | 0.544 | 0.743 |
| C20:0 | 0.14 | 0.14 | 0.15 | 0.12 | 0.925 | 0.130 | 0.887 | 0.032 |
| C20:1n-9 | 0.22 | 0.23 | 0.22 | 0.23 | 0.724 | 0.442 | 0.506 | 0.042 |
| C20:2n-6 | 0.19 | 0.19 | 0.17 b | 0.21 a | 0.624 | 0.015 | 0.099 | 0.031 |
| C20:3n-6 | 0.32 | 0.37 | 0.34 | 0.35 | 0.577 | 0.852 | 0.510 | 0.145 |
| C20:4n-6 | 4.47 | 4.43 | 4.86 | 4.04 | 0.974 | 0.590 | 0.635 | 2.723 |
| C20:3n-3 | 0.05 | 0.04 | 0.04 | 0.05 | 0.375 | 0.375 | 0.283 | 0.015 |
| C20:4n-3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.717 | 0.717 | 0.124 | 0.008 |
| C20:5n-3 | 0.09 | 0.10 | 0.10 | 0.09 | 0.692 | 0.692 | 0.494 | 0.051 |
| C22:0 | 0.05 | 0.06 | 0.05 | 0.05 | 0.338 | 0.766 | 0.898 | 0.017 |
| C22:1n-9 | 0.05 | 0.05 | 0.05 | 0.04 | 0.716 | 0.347 | 0.102 | 0.023 |
| C22:4n-6 | 0.80 | 0.82 | 0.84 | 0.78 | 0.934 | 0.799 | 0.577 | 0.455 |
| C22:5n-6 | 0.27 | 0.27 | 0.29 | 0.25 | 0.913 | 0.631 | 0.775 | 0.144 |
| C22:5n-3 | 0.44 | 0.42 | 0.47 | 0.40 | 0.864 | 0.563 | 0.578 | 0.263 |
| C24:0 | 0.11 | 0.12 | 0.12 | 0.10 | 0.747 | 0.665 | 0.088 | 0.054 |
| C22:6n-3 | 0.09 | 0.04 | 0.09 | 0.04 | 0.223 | 0.223 | 0.815 | 0.064 |
| C24:1n-9 | 0.03 | 0.04 | 0.04 | 0.03 | 0.820 | 0.460 | 0.820 | 0.023 |
| SFA | 35.34 | 36.53 | 36.30 | 35.58 | 0.085 | 0.275 | 0.177 | 1.156 |
| MUFA | 27.43 | 27.03 | 26.61 | 27.85 | 0.778 | 0.391 | 0.651 | 2.567 |
| n-6 PUFA | 34.10 | 33.48 | 34.08 | 33.50 | 0.711 | 0.732 | 0.937 | 3.027 |
| n-3 PUFA | 3.12 | 2.96 | 3.01 | 3.07 | 0.493 | 0.807 | 0.571 | 0.428 |
| n-6 PUFA + n-3 PUFA | 37.22 | 36.40 | 37.10 | 36.60 | 0.620 | 0.796 | 0.865 | 2.858 |
| n-6/n-3 | 11.25 | 11.40 | 11.6 | 11.1 | 0.906 | 0.713 | 0.717 | 2.281 |
| Group | Sex | p Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Total lipids | 1.56 | 1.62 | 1.54 | 1.64 | 0.725 | 0.604 | 0.867 | 0.331 |
| Fatty acids | ||||||||
| C10:0 | 0.11 | 0.07 | 0.06 | 0.12 | 0.114 | 0.060 | 0.067 | 0.048 |
| C12:0 | 0.16 | 0.11 | 0.10 | 0.16 | 0.105 | 0.087 | 0.152 | 0.054 |
| Iso C14:0 | 0.02 | 0.03 | 0.02 | 0.02 | 0.231 | 0.574 | 0.910 | 0.007 |
| C14:0 | 1.39 | 1.48 | 1.30 | 1.58 | 0.674 | 0.218 | 0.823 | 0.389 |
| C14:1n-5 | 0.07 | 0.05 | 0.05 | 0.08 | 0.368 | 0.239 | 0.516 | 0.040 |
| Iso C15:0 | 0.05 | 0.05 | 0.05 | 0.05 | 0.444 | 0.912 | 0.740 | 0.014 |
| Anteiso C15:0 | 0.12 | 0.11 | 0.12 | 0.11 | 0.760 | 0.760 | 0.544 | 0.019 |
| C15:0 | 0.55 | 0.56 | 0.56 | 0.55 | 0.769 | 0.560 | 0.826 | 0.041 |
| Iso C16:0 | 0.14 | 0.14 | 0.15 | 0.14 | 0.884 | 0.470 | 0.942 | 0.021 |
| C16:0 | 23.34 | 23.95 | 23.40 | 23.90 | 0.388 | 0.480 | 0.315 | 1.259 |
| C16:1n-9 | 0.39 | 0.38 | 0.38 | 0.39 | 0.728 | 0.944 | 0.351 | 0.065 |
| C16:1n-7 | 1.63 | 1.44 | 1.23 | 1.84 | 0.581 | 0.089 | 0.428 | 0.602 |
| C17:0 | 0.63 | 0.65 | 0.69 a | 0.59 b | 0.522 | 0.017 | 0.522 | 0.062 |
| C18:0 | 8.63 | 8.67 | 9.24 a | 8.06 b | 0.937 | 0.042 | 0.663 | 0.930 |
| C18:1n-9 | 20.81 | 21.67 | 20.37 | 22.11 | 0.525 | 0.215 | 0.639 | 2.420 |
| C18:1n-7 | 1.55 | 1.47 | 1.51 | 1.51 | 0.296 | 0.950 | 0.886 | 0.131 |
| C18:2n-6 | 28.53 | 28.90 | 29.91 | 28.52 | 0.628 | 0.601 | 0.437 | 1.357 |
| C18:3n-6 | 0.10 | 0.10 | 0.11 | 0.09 | 0.803 | 0.292 | 0.427 | 0.027 |
| C18:3n-3 | 2.16 | 2.33 | 2.14 | 2.35 | 0.525 | 0.406 | 0.702 | 0.465 |
| C20:0 | 0.13 | 0.13 | 0.14 | 0.12 | 0.904 | 0.094 | 0.500 | 0.019 |
| C20:1n-9 | 0.20 | 0.22 | 0.19 | 0.23 | 0.387 | 0.074 | 0.348 | 0.038 |
| C20:2n-6 | 0.24 | 0.26 | 0.21 B | 0.28 A | 0.376 | 0.006 | 0.150 | 0.037 |
| C20:3n-6 | 0.54 | 0.44 | 0.50 | 0.48 | 0.191 | 0.830 | 0.919 | 0.133 |
| C20:4n-6 | 5.80 | 4.62 | 5.91 | 4.50 | 0.319 | 0.232 | 0.739 | 2.056 |
| C20:3n-3 | 0.05 | 0.06 | 0.05 | 0.06 | 0.616 | 0.292 | 0.973 | 0.022 |
| C20:4n-3 | 0.01 | 0.01 | 0.01 | 0.01 | 0.467 | 0.467 | 0.467 | 0.008 |
| C20:5n-3 | 0.10 | 0.09 | 0.10 | 0.08 | 0.359 | 0.434 | 0.842 | 0.031 |
| C22:0 | 0.06 | 0.05 | 0.06 | 0.05 | 0.579 | 0.116 | 0.278 | 0.016 |
| C22:1n-9 | 0.04 a | 0.03 b | 0.04 | 0.03 | 0.048 | 0.330 | 0.842 | 0.008 |
| C22:4n-6 | 1.10 | 0.88 | 1.05 | 0.93 | 0.285 | 0.543 | 0.671 | 0.361 |
| C22:5n-6 | 0.42 | 0.31 | 0.40 | 0.34 | 0.324 | 0.113 | 0.093 | 0.821 |
| C22:5n-3 | 0.64 | 0.50 | 0.67 | 0.47 | 0.365 | 0.225 | 0.450 | 0.284 |
| C24:0 | 0.12 | 0.10 | 0.10 | 0.12 | 0.270 | 0.182 | 0.674 | 0.023 |
| C22:6n-3 | 0.11 | 0.09 | 0.12 | 0.08 | 0.390 | 0.200 | 0.182 | 0.049 |
| C24:1n-9 | 0.04 | 0.04 | 0.05 | 0.03 | 0.939 | 0.201 | 0.285 | 0.029 |
| SFA | 35.46 | 36.12 | 36.00 | 35.58 | 0.307 | 0.509 | 0.152 | 1.135 |
| MUFA | 24.73 | 25.30 | 23.82 | 26.21 | 0.719 | 0.154 | 0.562 | 2.867 |
| n-6 PUFA | 36.74 | 35.50 | 37.10 | 35.14 | 0.492 | 0.283 | 0.961 | 3.194 |
| n-3 PUFA | 3.08 | 3.08 | 3.08 | 3.07 | 0.996 | 0.914 | 0.667 | 0.265 |
| n-6 PUFA + n-3 PUFA | 39.80 | 38.60 | 40.20 | 38.20 | 0.489 | 0.315 | 0.989 | 3.179 |
| n-6/n-3 | 12.04 | 11.58 | 12.14 | 11.48 | 0.581 | 0.433 | 0.746 | 1.496 |
| Group | Sex | p Values | RMSE | |||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Total lipids | 2.95 | 3.56 | 3.27 | 3.24 | 0.951 | 0.104 | 0.602 | 0.625 |
| Fatty acids | ||||||||
| C10:0 | 0.02 | 0.01 | 0.02 | 0.01 | 0.256 | 0.260 | 0.563 | 0.005 |
| C12:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.592 | 0.592 | 0.128 | 0.004 |
| Iso C14:0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.869 | 0.017 | 0.159 | 0.005 |
| C14:0 | 0.31 | 0.34 | 0.36 | 0.30 | 0.319 | 0.563 | 0.737 | 0.110 |
| C14:1n-5 | 0.01 | 0.01 | 0.01 | 0.01 | 0.070 | 0.778 | 0.113 | 0.005 |
| Iso C15:0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.588 | 0.588 | 0.473 | 0.008 |
| anteiso C15:0 | 0.04 | 0.04 | 0.04 | 0.04 | 0.806 | 0.591 | 0.591 | 0.015 |
| C15:0 | 0.38 | 0.38 | 0.40 | 0.36 | 0.156 | 0.868 | 0.659 | 0.054 |
| Iso C16:0 | 0.08 | 0.07 | 0.09 | 0.07 | 0.123 | 0.722 | 0.830 | 0.021 |
| C16:0 | 17.72 | 19.16 | 18.13 | 18.74 | 0.394 | 0.061 | 0.556 | 1.268 |
| C16:1n-9 | 0.26 | 0.28 | 0.29 | 0.25 | 0.223 | 0.554 | 0.703 | 0.063 |
| C16:1n-7 | 0.34 | 0.49 | 0.415 b | 0.418 a | 0.962 | 0.050 | 0.962 | 0.128 |
| C17:0 | 1.03 | 1.01 | 1.05 | 0.99 | 0.260 | 0.809 | 0.983 | 0.103 |
| C18:0 | 20.87 | 19.96 | 20.49 | 20.35 | 0.826 | 0.174 | 0.834 | 1.155 |
| C18:1n-9 | 10.85 | 12.20 | 12.12 | 10.83 | 0.316 | 0.260 | 0.828 | 2.083 |
| C18:1n-7 | 1.05 | 1.07 | 1.08 | 1.04 | 0.704 | 0.735 | 0.312 | 0.144 |
| C18:2n-6 | 31.36 | 30.94 | 30.58 | 31.72 | 0.057 | 0.445 | 0.377 | 0.979 |
| C18:3n-6 | 0.14 | 0.12 | 0.14 | 0.12 | 0.352 | 0.534 | 0.605 | 0.042 |
| C18:3n-3 | 1.18 | 1.25 | 1.28 | 1.15 | 0.249 | 0.539 | 0.635 | 0.189 |
| C20:0 | 0.13 | 0.13 | 0.14 | 0.13 | 0.475 | 0.743 | 0.050 | 0.011 |
| C20:1n-9 | 0.18 | 0.18 | 0.16 | 0.20 | 0.339 | 0.895 | 0.529 | 0.057 |
| C20:2n-6 | 0.57 a | 0.53 b | 0.41 | 0.68 | 0.037 | 0.727 | 0.231 | 0.211 |
| C20:3n-6 | 0.86 | 0.76 | 0.72 | 0.90 | 0.220 | 0.514 | 0.584 | 0.263 |
| C20:4n-6 | 9.56 | 8.46 | 9.25 | 8.78 | 0.609 | 0.244 | 0.780 | 1.647 |
| C20:3n-3 | 0.06 | 0.04 | 0.05 | 0.06 | 0.310 | 0.105 | 0.645 | 0.026 |
| C20:4n-3 | 0.01 | 0.02 | 0.02 | 0.01 | 0.829 | 0.293 | 1.000 | 0.007 |
| C20:5n-3 | 0.06 | 0.06 | 0.07 | 0.06 | 0.339 | 0.889 | 0.385 | 0.016 |
| C22:0 | 0.20 | 0.17 | 0.18 | 0.19 | 0.358 | 0.095 | 0.851 | 0.028 |
| C22:1n-9 | 0.03 | 0.04 | 0.04 | 0.04 | 0.813 | 0.120 | 0.212 | 0.013 |
| C22:4n-6 | 0.99 | 0.85 | 0.89 | 0.95 | 0.644 | 0.256 | 0.543 | 0.220 |
| C22:5n-6 | 0.59 | 0.51 | 0.55 | 0.54 | 0.894 | 0.362 | 0.556 | 0.147 |
| C22:5n-3 | 0.42 | 0.34 | 0.40 | 0.36 | 0.384 | 0.149 | 1.000 | 0.085 |
| C24:0 | 0.19 | 0.17 | 0.19 | 0.18 | 0.715 | 0.519 | 0.692 | 0.005 |
| C22:6n-3 | 0.21 | 0.16 | 0.21 | 0.15 | 0.067 | 0.117 | 0.697 | 0.054 |
| C24:1n-9 | 0.26 | 0.18 | 0.21 | 0.23 | 0.808 | 0.151 | 0.339 | 0.090 |
| SFA | 41.02 | 41.51 | 41.13 | 41.40 | 0.452 | 0.608 | 0.730 | 1.176 |
| MUFA | 12.97 | 14.45 | 14.31 | 13.11 | 0.254 | 0.349 | 0.767 | 2.267 |
| n-6 PUFA | 44.07 | 42.17 | 42.54 | 43.69 | 0.134 | 0.344 | 0.642 | 2.156 |
| n-3 PUFA | 1.94 | 1.86 | 2.01 | 1.79 | 0.481 | 0.07 | 0.743 | 0.199 |
| n-6 PUFA + n-3 PUFA | 46.00 | 44.00 | 44.6 | 45.5 | 0.132 | 0.332 | 0.632 | 2.233 |
| n-3/n-6 | 0.04 | 0.04 | 0.047 a | 0.041 b | 0.023 | 0.953 | 0.993 | 0.004 |
| n-6/n-3 | 22.87 | 23.04 | 21.17 b | 24.74 a | 0.025 | 0.903 | 0.924 | 2.500 |
| Meat Portion/Organ | Group | Sex | p-Values | RMSE | ||||
|---|---|---|---|---|---|---|---|---|
| CTRL | CBD | Female | Male | Group | Sex | Interaction | ||
| Loin | 41.253 | 36.238 | 37.049 | 40.442 | 0.240 | 0.418 | 0.077 | 7.436 |
| Thigh | 48.153 | 50.925 | 50.046 | 49.032 | 0.301 | 0.698 | 0.770 | 4.709 |
| Liver | 352.72 | 416.872 | 388.902 | 380.691 | 0.205 | 0.865 | 0.639 | 87.642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addeo, N.F.; Iervolino, V.; Amato, R.; Lanzieri, M.; Lotito, D.; Tignani, M.V.; Staropoli, A.; Damiano, S.; Lombardi, P.; Vinale, F.; et al. Dietary Cannabidiol Supplementation on Growth Performance, Behavior, Blood Profile, Metabolomic Analysis, and Fatty Acid Composition in Rabbits: A Multi-Disciplinary Approach to Improve Welfare and Productivity. Vet. Sci. 2025, 12, 759. https://doi.org/10.3390/vetsci12080759
Addeo NF, Iervolino V, Amato R, Lanzieri M, Lotito D, Tignani MV, Staropoli A, Damiano S, Lombardi P, Vinale F, et al. Dietary Cannabidiol Supplementation on Growth Performance, Behavior, Blood Profile, Metabolomic Analysis, and Fatty Acid Composition in Rabbits: A Multi-Disciplinary Approach to Improve Welfare and Productivity. Veterinary Sciences. 2025; 12(8):759. https://doi.org/10.3390/vetsci12080759
Chicago/Turabian StyleAddeo, Nicola Francesco, Valeria Iervolino, Ruggero Amato, Mariarosaria Lanzieri, Daria Lotito, Maria Vittoria Tignani, Alessia Staropoli, Sara Damiano, Pietro Lombardi, Francesco Vinale, and et al. 2025. "Dietary Cannabidiol Supplementation on Growth Performance, Behavior, Blood Profile, Metabolomic Analysis, and Fatty Acid Composition in Rabbits: A Multi-Disciplinary Approach to Improve Welfare and Productivity" Veterinary Sciences 12, no. 8: 759. https://doi.org/10.3390/vetsci12080759
APA StyleAddeo, N. F., Iervolino, V., Amato, R., Lanzieri, M., Lotito, D., Tignani, M. V., Staropoli, A., Damiano, S., Lombardi, P., Vinale, F., Parisi, G., Bovera, F., Musco, N., & Mastellone, V. (2025). Dietary Cannabidiol Supplementation on Growth Performance, Behavior, Blood Profile, Metabolomic Analysis, and Fatty Acid Composition in Rabbits: A Multi-Disciplinary Approach to Improve Welfare and Productivity. Veterinary Sciences, 12(8), 759. https://doi.org/10.3390/vetsci12080759










