Congenital Malformations of the Central Nervous System Caused by Bluetongue Virus Serotype 3 (BTV-3) in Two Calves
Simple Summary
Abstract
1. Introduction
2. Case Description
2.1. Calf 1
2.2. Calf 2
3. Results
3.1. General Clinical Findings (Calf 2)
3.2. Neurological Findings (Calf 2)
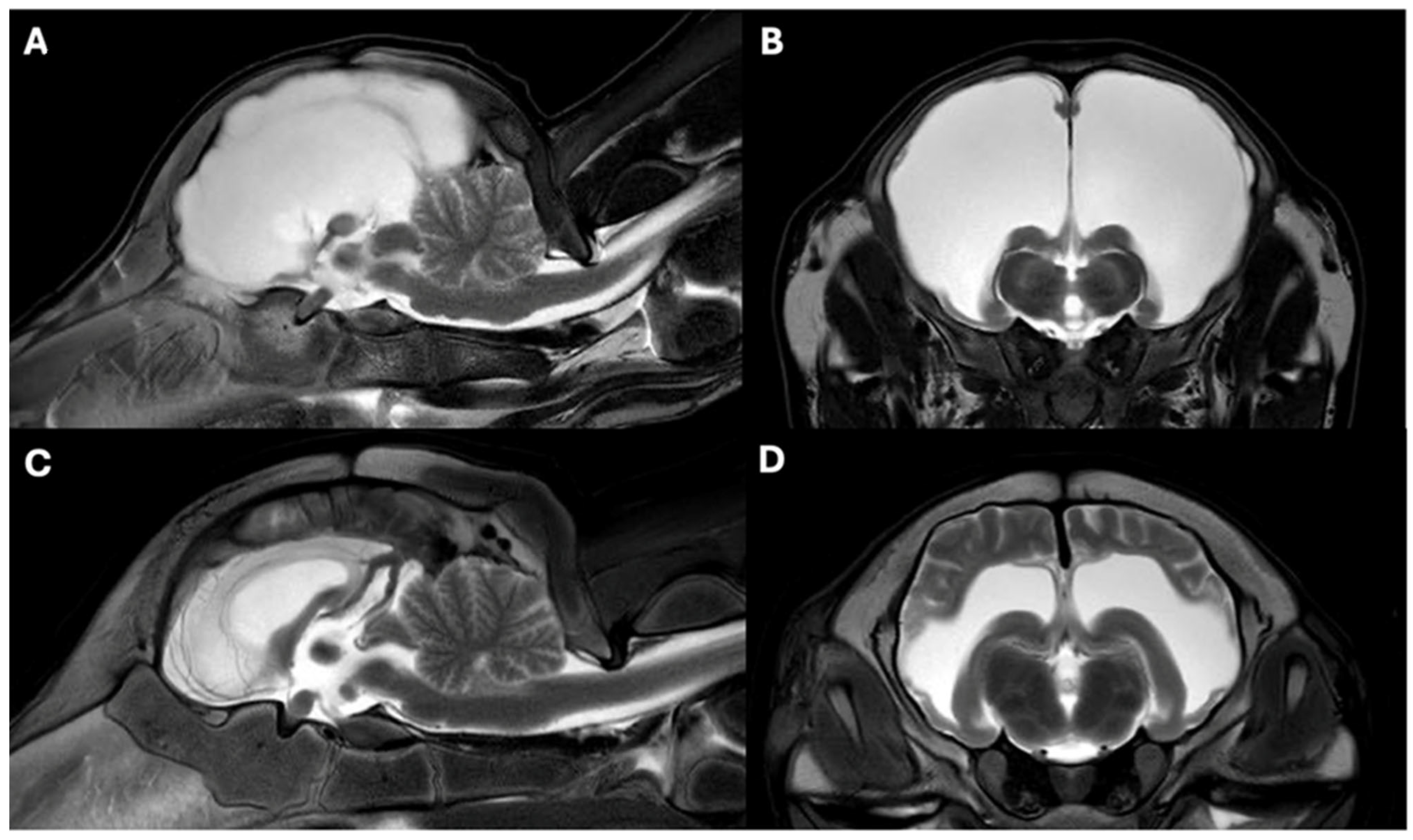
3.3. MRI Findings
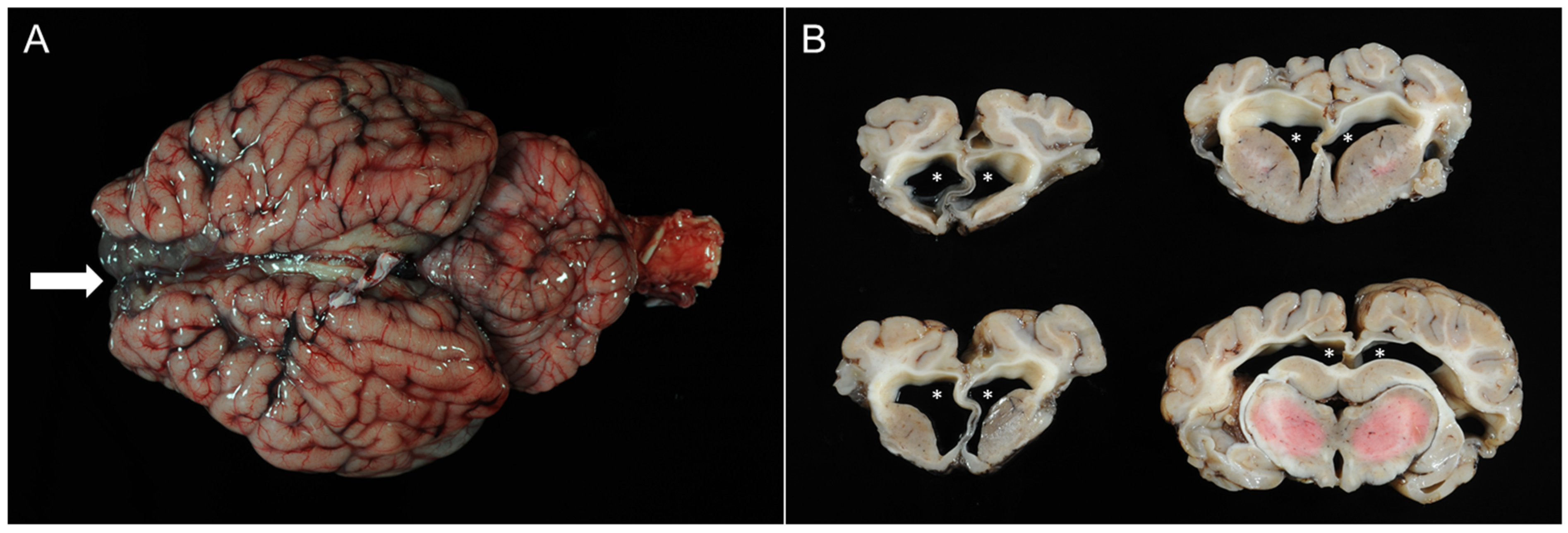
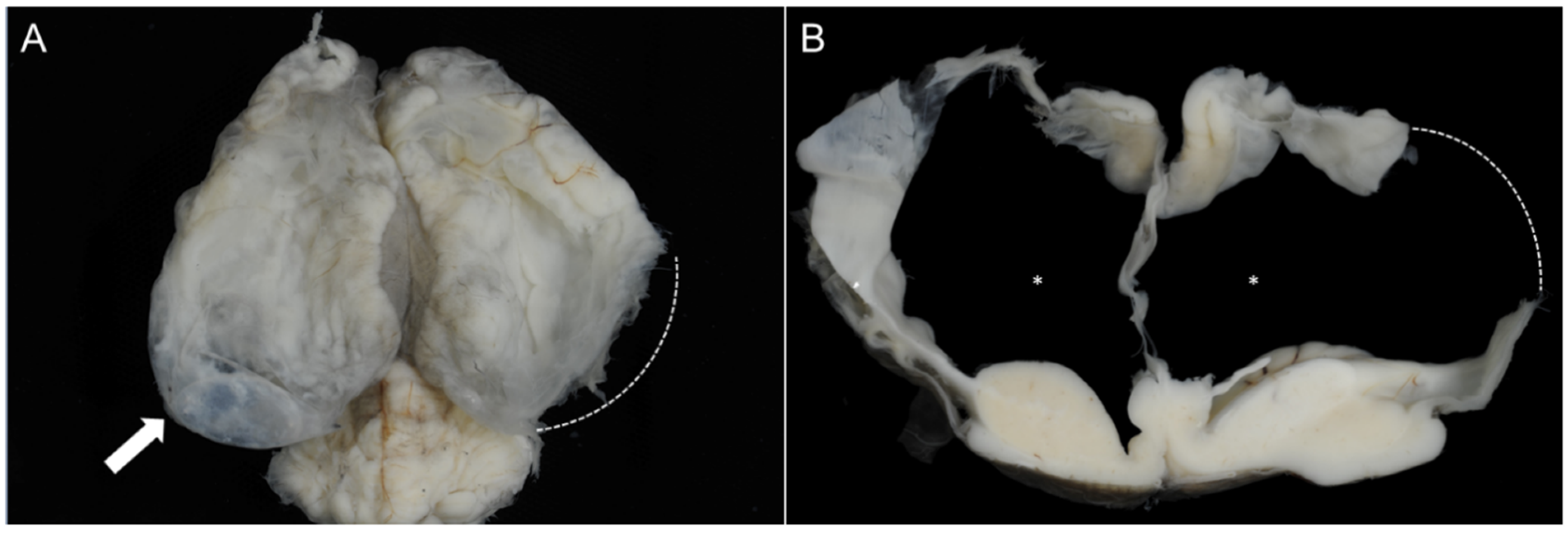
3.4. Pathomorphological Findings
3.4.1. Case 1
3.4.2. Case 2
3.5. Virological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tago, D.; Hammitt, J.K.; Thomas, A.; Raboisson, D. Cost assessment of the movement restriction policy in France during the 2006 bluetongue virus episode (BTV-8). Prev. Vet. Med. 2014, 117, 577–589. [Google Scholar] [CrossRef]
- Gethmann, J.; Probst, C.; Conraths, F.J. Economic Impact of a Bluetongue Serotype 8 Epidemic in Germany. Front. Vet. Sci. 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; Hewicker-Trautwein, M.; Peperkamp, K.; Windsor, P.A. Virus-induced congenital malformations in cattle. Acta Vet. Scand. 2015, 57, 54. [Google Scholar] [CrossRef] [PubMed]
- Vandevelde, M.; Higgins, R.J.; Oevermann, A. Veterinary Neuropathology Essentials of Therapy and Practice; Wiley-Blackwell: Hoboken, NJ, USA, 2012; p. 97. [Google Scholar]
- Leipold, H.W.; Dennis, S.M. Congenital defects of the bovine central nervous system. Vet. Clin. N. Am. Food Anim. Pract. 1987, 3, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Baumgaertner, W.; Gruber Achim, D. Spezielle Pathologie für Die Tiermedizin, 2nd ed.; Thieme: Erlangen, Germany, 2019. [Google Scholar]
- Hewicker-Trautwein, M.; Liess, B.; Trautwein, G. Brain lesions in calves following transplacental infection with bovine-virus diarrhoea virus. Zentralbl. Veterinarmed. B 1995, 42, 65–77. [Google Scholar] [CrossRef]
- Rodrigues Hoffmann, A.; Welsh, C.J.; Wilcox Varner, P.; de la Concha-Bermejillo, A.; Marchand Ball, J.; Ambrus, A.; Edwards, J.F. Identification of the target cells and sequence of infection during experimental infection of ovine fetuses with Cache Valley virus. J. Virol. 2012, 86, 4793–4800. [Google Scholar] [CrossRef]
- Herder, V.; Hansmann, F.; Wohlsein, P.; Peters, M.; Varela, M.; Palmarini, M.; Baumgartner, W. Immunophenotyping of inflammatory cells associated with Schmallenberg virus infection of the central nervous system of ruminants. PLoS ONE 2013, 8, e62939. [Google Scholar] [CrossRef][Green Version]
- Varela, M.; Schnettler, E.; Caporale, M.; Murgia, C.; Barry, G.; McFarlane, M.; McGregor, E.; Piras, I.M.; Shaw, A.; Lamm, C.; et al. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathog. 2013, 9, e1003133. [Google Scholar] [CrossRef]
- Summers, B.A.; Cummings, J.F.; DeLahunta, A. Veterinary Neuropathology; Mosby: St. Louis, MO, USA, 1995. [Google Scholar]
- Matthijnssens, J.; Attoui, H.; Banyai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Tabachnick, W.J. Culicoides and the global epidemiology of bluetongue virus infection. Vet. Ital. 2004, 40, 144–150. [Google Scholar]
- DeHaven, W.R.; del Valle Molina, J.A.; Evans, B. Bluetongue viruses and trade issues: A North American perspective. Vet. Ital. 2004, 40, 683–687. [Google Scholar]
- Bumbarov, V.; Golender, N.; Jenckel, M.; Wernike, K.; Beer, M.; Khinich, E.; Zalesky, O.; Erster, O. Characterization of bluetongue virus serotype 28. Transbound. Emerg. Dis. 2020, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; Nomikou, K.; Veronesi, E.; Bachanek-Bankowska, K.; Belaganahalli, M.N.; Attoui, H.; Mertens, P.P. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait. PLoS ONE 2011, 6, e26147. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Breard, E.; Sailleau, C.; Jenckel, M.; Viarouge, C.; Vitour, D.; Palmarini, M.; Gallois, M.; Hoper, D.; Hoffmann, B.; et al. Bluetongue virus serotype 27: Detection and characterization of two novel variants in Corsica, France. J. Gen. Virol. 2016, 97, 2073–2083. [Google Scholar] [CrossRef]
- Maan, N.S.; Maan, S.; Belaganahalli, M.N.; Ostlund, E.N.; Johnson, D.J.; Nomikou, K.; Mertens, P.P. Identification and differentiation of the twenty six bluetongue virus serotypes by RT-PCR amplification of the serotype-specific genome segment 2. PLoS ONE 2012, 7, e32601. [Google Scholar] [CrossRef]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Desmecht, D.; Bergh, R.V.; Sartelet, A.; Leclerc, M.; Mignot, C.; Misse, F.; Sudraud, C.; Berthemin, S.; Jolly, S.; Mousset, B.; et al. Evidence for transplacental transmission of the current wild-type strain of bluetongue virus serotype 8 in cattle. Vet. Rec. 2008, 163, 50–52. [Google Scholar] [CrossRef]
- Mahrt, C.R.; Osburn, B.I. Experimental bluetongue virus infection of sheep; effect of vaccination: Pathologic, immunofluorescent, and ultrastructural studies. Am. J. Vet. Res. 1986, 47, 1198–1203. [Google Scholar] [CrossRef]
- Darpel, K.E.; Batten, C.A.; Veronesi, E.; Williamson, S.; Anderson, P.; Dennison, M.; Clifford, S.; Smith, C.; Philips, L.; Bidewell, C.; et al. Transplacental transmission of bluetongue virus 8 in cattle, UK. Emerg. Infect. Dis. 2009, 15, 2025–2028. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Nunamaker, R.A.; Katz, J.B.; Sawyer, M.M.; Akita, G.Y.; Osburn, B.I.; Tabachnick, W.J. Detection of bluetongue virus in the blood of inoculated calves: Comparison of virus isolation, PCR assay, and in vitro feeding of Culicoides variipennis. Arch. Virol. 1994, 136, 1–8. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Osburn, B.I. Bluetongue virus-induced hydranencephaly in cattle. Vet. Pathol. 1983, 20, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Mosenfechtel, S.; Jacobsen, B.; Beineke, A.; Wohlsein, P. Bluetongue virus serotype 8 (BTV-8)-associated brain malformations in two calves. Dtsch. Tierarztl. Wochenschr. 2008, 115, 298–303. [Google Scholar] [PubMed]
- Wouda, W.; Roumen, M.P.; Peperkamp, N.H.; Vos, J.H.; van Garderen, E.; Muskens, J. Hydranencephaly in calves following the bluetongue serotype 8 epidemic in the Netherlands. Vet. Rec. 2008, 162, 422–423. [Google Scholar] [CrossRef]
- Vercauteren, G.; Miry, C.; Vandenbussche, F.; Ducatelle, R.; Van der Heyden, S.; Vandemeulebroucke, E.; De Leeuw, I.; Deprez, P.; Chiers, K.; De Clercq, K. Bluetongue virus serotype 8-associated congenital hydranencephaly in calves. Transbound. Emerg. Dis. 2008, 55, 293–298. [Google Scholar] [CrossRef]
- Williamson, S.M.; Scholes, S.F.E.; Welchman, D.; Dennison, M.; Batten, C.A.; Williams, D.L.; Mertens, P.P.; Mellor, P.S.; Darpel, K.E. Bluetongue virus serotype 8-associated hydranencephaly in two calves in south-eastern England. Vet. Rec. 2010, 167, 216–218. [Google Scholar] [CrossRef]
- Dal Pozzo, F.; De Clercq, K.; Guyot, H.; Vandemeulebroucke, E.; Sarradin, P.; Vandenbussche, F.; Thiry, E.; Saegerman, C. Experimental reproduction of bluetongue virus serotype 8 clinical disease in calves. Vet. Microbiol. 2009, 136, 352–358. [Google Scholar] [CrossRef]
- Saegerman, C.; Berkvens, D.; Mellor, P.S. Bluetongue epidemiology in the European Union. Emerg. Infect. Dis. 2008, 14, 539–544. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Conley, A.J.; Kennedy, P.C. Bluetongue and equine viral arteritis viruses as models of virus-induced fetal injury and abortion. Anim. Reprod. Sci. 2000, 60–61, 643–651. [Google Scholar] [CrossRef]
- Osburn, B.I.; Drost, M.; Stabenfeldt, G.H. Response of fetal adrenal cortex to congenital infections. Am. J. Obstet. Gynecol. 1972, 114, 622–627. [Google Scholar] [CrossRef]
- Osburn, B.I. The impact of bluetongue virus on reproduction. Comp. Immunol. Microbiol. Infect. Dis. 1994, 17, 189–196. [Google Scholar] [CrossRef]
- Maclachlan NJ, O.B. Induced brain lesions in calves infected with bluetongue virus. Vet. Rec. 2008, 162, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, A.S.; Anderson, G.A.; Phillips, D.L.; Osburn, B.I. Infection of bovine fetuses at 120 days’ gestation with virulent and avirulent strains of bluetongue virus serotype 11. Comp. Immunol. Microbiol. Infect. Dis. 1992, 15, 53–63. [Google Scholar] [CrossRef] [PubMed]
- FLI. FAQ Blauzungenkrankheit; Friedrich-Loeffler-Institut: Greifswald, Germany, 2024. [Google Scholar]
- van den Brink, K.M.J.A.; Santman-Berends, I.M.G.A.; Harkema, L.; Scherpenzeel, C.G.M.; Dijkstra, E.; Bisschop, P.I.H.; Peterson, K.; van de Burgwal, N.S.; Waldeck, H.W.F.; Dijkstra, T.; et al. Bluetongue virus serotype 3 in ruminants in The Netherlands: Clinical signs, seroprevalence and pathological findings. Vet. Rec. 2024, 195, e4533. [Google Scholar] [CrossRef]
- Richards, W.P.; Crenshaw, G.L.; Bushnell, R.B. Hydranencephaly of calves associated with natural bluetongue virus infection. Cornell Vet. 1971, 61, 336–348. [Google Scholar]
- Flanagan, M.; Wilson, A.J.; Trueman, K.F.; Shepherd, M.A. Bluetongue virus serotype 20 infection in pregnant Merino sheep. Aust. Vet. J. 1982, 59, 18–20. [Google Scholar] [CrossRef]
- Parsonson, I.M.; Luedke, A.J.; Barber, T.L.; Walton, T.E. Bluetongue virus infection in pregnant ewes. Am. J. Vet. Res. 1994, 55, 666–669. [Google Scholar] [CrossRef]
- De Clercq, K.; De Leeuw, I.; Verheyden, B.; Vandemeulebroucke, E.; Vanbinst, T.; Herr, C.; Meroc, E.; Bertels, G.; Steurbaut, N.; Miry, C.; et al. Transplacental infection and apparently immunotolerance induced by a wild-type bluetongue virus serotype 8 natural infection. Transbound. Emerg. Dis. 2008, 55, 352–359. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.; van Wuijckhuise, L.; Vellema, P.; van Rijn, P.A. Vertical transmission of bluetongue virus serotype 8 virus in Dutch dairy herds in 2007. Vet. Microbiol. 2010, 141, 31–35. [Google Scholar] [CrossRef][Green Version]
- MacLachlan, N.J.; Osburn, B.I.; Ghalib, H.W.; Stott, J.L. Bluetongue virus-induced encephalopathy in fetal cattle. Vet. Pathol. 1985, 22, 415–417. [Google Scholar] [CrossRef]
- Zanella, G.; Durand, B.; Sellal, E.; Breard, E.; Sailleau, C.; Zientara, S.; Batten, C.A.; Mathevet, P.; Audeval, C. Bluetongue virus serotype 8: Abortion and transplacental transmission in cattle in the Burgundy region, France, 2008–2009. Theriogenology 2012, 77, 65–72. [Google Scholar] [CrossRef]
- Hoffmann, B.; Scheuch, M.; Hoper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel orthobunyavirus in Cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef]
- Garigliany, M.M.; Bayrou, C.; Kleijnen, D.; Cassart, D.; Desmecht, D. Schmallenberg virus in domestic cattle, Belgium, 2012. Emerg. Infect. Dis. 2012, 18, 1512–1514. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duc, P.D.; Reeh, S.; Pöpperl, P.; Schreiner, T.; Gundling, N.; Beineke, A.; Wohlsein, P.; Hoedemaker, M. Congenital Malformations of the Central Nervous System Caused by Bluetongue Virus Serotype 3 (BTV-3) in Two Calves. Vet. Sci. 2025, 12, 728. https://doi.org/10.3390/vetsci12080728
Duc PD, Reeh S, Pöpperl P, Schreiner T, Gundling N, Beineke A, Wohlsein P, Hoedemaker M. Congenital Malformations of the Central Nervous System Caused by Bluetongue Virus Serotype 3 (BTV-3) in Two Calves. Veterinary Sciences. 2025; 12(8):728. https://doi.org/10.3390/vetsci12080728
Chicago/Turabian StyleDuc, Phuong Do, Solveig Reeh, Pauline Pöpperl, Tom Schreiner, Natascha Gundling, Andreas Beineke, Peter Wohlsein, and Martina Hoedemaker. 2025. "Congenital Malformations of the Central Nervous System Caused by Bluetongue Virus Serotype 3 (BTV-3) in Two Calves" Veterinary Sciences 12, no. 8: 728. https://doi.org/10.3390/vetsci12080728
APA StyleDuc, P. D., Reeh, S., Pöpperl, P., Schreiner, T., Gundling, N., Beineke, A., Wohlsein, P., & Hoedemaker, M. (2025). Congenital Malformations of the Central Nervous System Caused by Bluetongue Virus Serotype 3 (BTV-3) in Two Calves. Veterinary Sciences, 12(8), 728. https://doi.org/10.3390/vetsci12080728




