BRAF Mutation Analysis: A Retrospective Evaluation of 8365 Diagnostic Samples with a Special View on Canine Breeds (2018–2024)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Complete Data Set
3.2. Data Set of the Most Common Breeds
3.3. Non-Terrier Breeds with the Highest and Lowest Proportion of BRAF-Positive Results
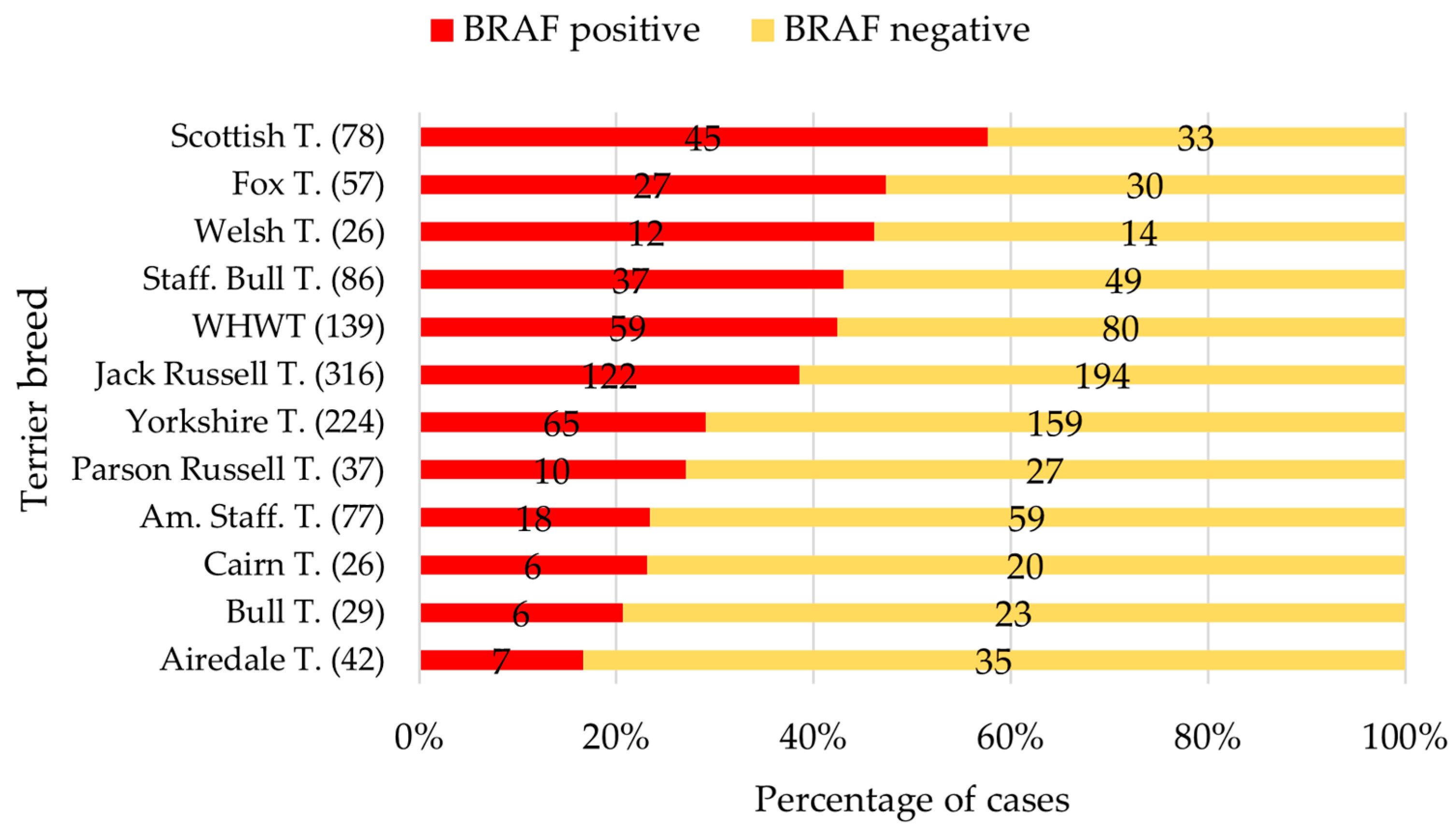
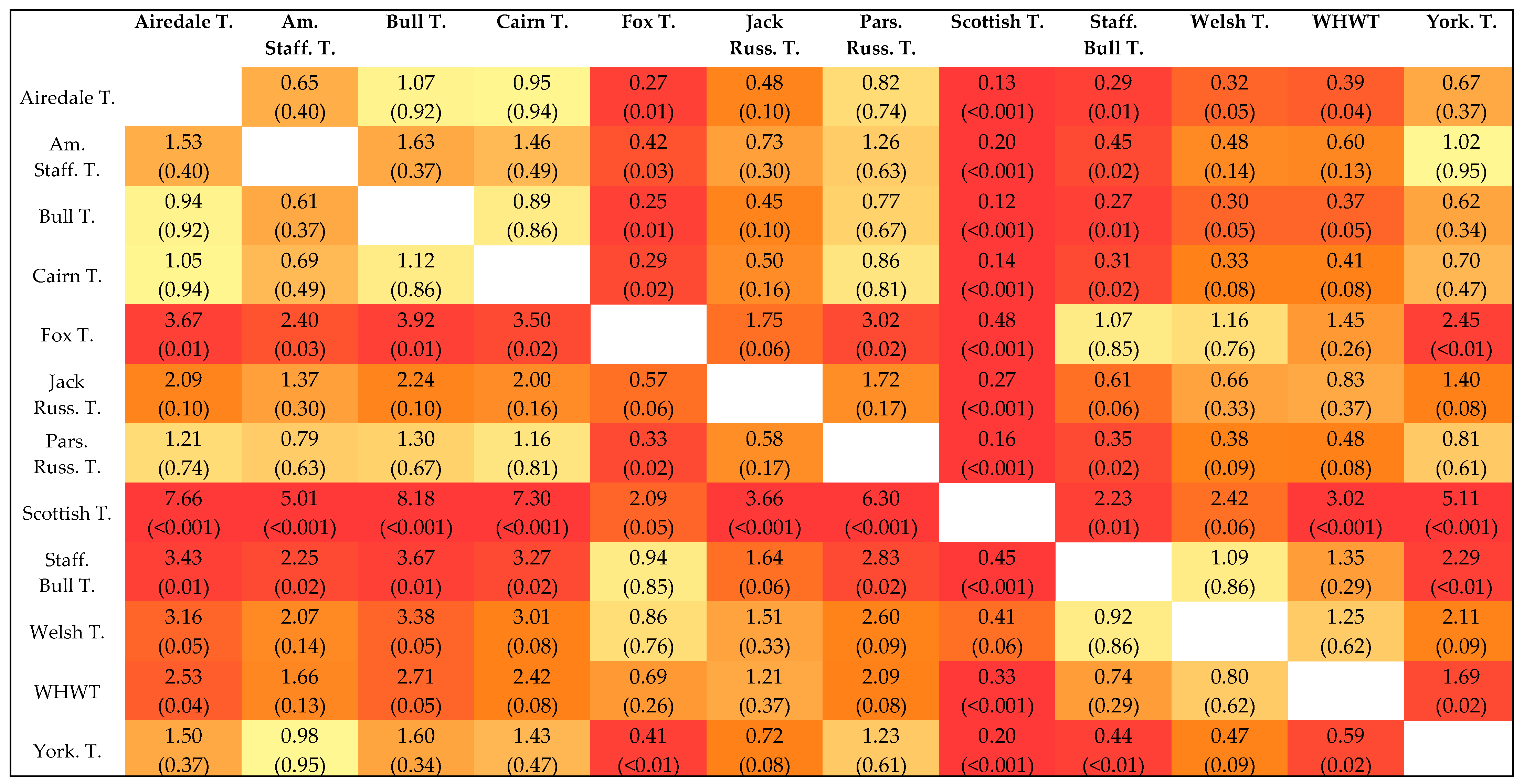
3.4. Data Set of the Terrier Breeds
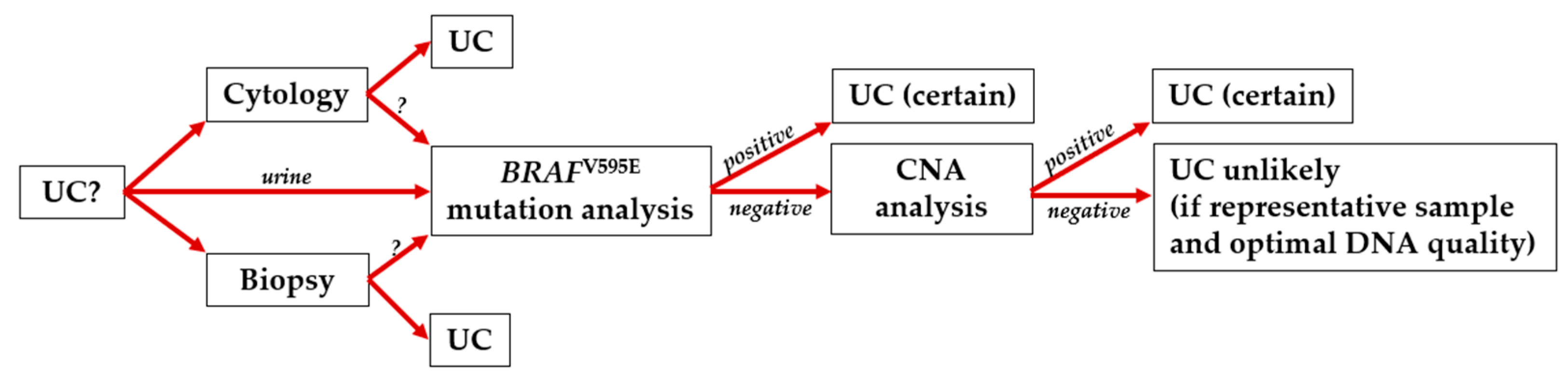
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNA | Copy number alterations |
| ddPCR | Droplet digital PCR |
| FCI | Fédération Cynologique Internationale |
| FFPE | Formalin-fixed, paraffin-embedded |
| CI | Confidence interval |
| MAPK/ERK | Mitogen-activated protein kinase/extracellular regulated kinase |
| MEK | MAPK/ERK kinase |
| OR | Odds ratio |
| p | p-value |
| UC | Urothelial carcinoma |
| WHWT | West Highland White Terrier |
References
- Adams, V.J.; Evans, K.M.; Sampson, J.; Wood, J.L.N. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010, 51, 512–524. [Google Scholar] [CrossRef]
- Dhein, E.S.; Heikkilä, U.; Oevermann, A.; Blatter, S.; Meier, D.; Hartnack, S.; Guscetti, F. Incidence rates of the most common canine tumors based on data from the Swiss Canine Cancer Registry (2008 to 2020). PLoS ONE 2024, 19, e0302231. [Google Scholar] [CrossRef]
- Norris, A.M.; Laing, E.J.; Valli, V.E.; Withrow, S.J.; Macy, D.W.; Ogilvie, G.K.; Tomlinson, J.; McCaw, D.; Pidgeon, G.; Jacobs, R.M. Canine bladder and urethral tumors: A retrospective study of 115 cases (1980–1985). J. Vet. Int. Med. 1992, 6, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, A.J.; Widmer, W.R.; Knapp, D.W. Canine Transitional Cell Carcinoma. J. Vet. Int. Med. 2003, 17, 136. [Google Scholar] [CrossRef]
- Patrick, D.J.; Fitzgerald, S.D.; Sesterhenn, I.A.; Davis, C.J.; Kiupel, M. Classification of canine urinary bladder urothelial tumours based on the World Health Organization/International Society of Urological Pathology consensus classification. J. Comp. Pathol. 2006, 135, 190–199. [Google Scholar] [CrossRef]
- Valli, V.E.; Norris, A.; Jacobs, R.M.; Laing, E.; Withrow, S.; Macy, D.; Tomlinson, J.; McCaw, D.; Ogilvie, G.K.; Pidgeon, G. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J. Comp. Pathol. 1995, 113, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Glickman, N.W.; DeNicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-occurring canine transitional cell carcinoma of the urinary bladder A relevant model of human invasive bladder cancer. Urol. Oncol. 2000, 5, 47–59. [Google Scholar] [CrossRef]
- Sommer, B.C.; Dhawan, D.; Ratliff, T.L.; Knapp, D.W. Naturally-Occurring Canine Invasive Urothelial Carcinoma: A Model for Emerging Therapies. Bladder Cancer 2018, 4, 149–159. [Google Scholar] [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef]
- Charney, V.A.; Miller, M.A.; Heng, H.G.; Weng, H.Y.; Knapp, D.W. Skeletal Metastasis of Canine Urothelial Carcinoma: Pathologic and Computed Tomographic Features. Vet. Pathol. 2017, 54, 380–386. [Google Scholar] [CrossRef]
- Henry, C.J. Management of transitional cell carcinoma. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 597–613. [Google Scholar] [CrossRef]
- Burgess, K.E.; DeRegis, C.J. Urologic Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 311–323. [Google Scholar] [CrossRef]
- Gentilini, F.; Palgrave, C.J.; Neta, M.; Tornago, R.; Furlanello, T.; McKay, J.S.; Sacchini, F.; Turba, M.E. Validation of a Liquid Biopsy Protocol for Canine BRAFV595E Variant Detection in Dog Urine and Its Evaluation as a Diagnostic Test Complementary to Cytology. Front. Vet. Sci. 2022, 9, 909934. [Google Scholar] [CrossRef]
- Straub, J.; Strittmatter, F.; Karl, A.; Stief, C.G.; Tritschler, S. Ureterorenoscopic biopsy and urinary cytology according to the 2004 WHO classification underestimate tumor grading in upper urinary tract urothelial carcinoma. Urol. Oncol. 2013, 31, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Kehl, A.; Aupperle-Lellbach, H.; de Brot, S.; van der Weyden, L. Review of Molecular Technologies for Investigating Canine Cancer. Animals 2024, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Aupperle-Lellbach, H.; Kehl, A.; de Brot, S.; van der Weyden, L. Clinical Use of Molecular Biomarkers in Canine and Feline Oncology: Current and Future. Vet. Sci. 2024, 11, 199. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. RAS oncogenes: The first 30 years. Nat. Rev. Cancer 2003, 3, 459–465. [Google Scholar] [CrossRef]
- Baccarini, M. Second nature: Biological functions of the Raf-1 “kinase”. FEBS Lett. 2005, 579, 3271–3277. [Google Scholar] [CrossRef]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Esteban-Villarrubia, J.; Soto-Castillo, J.J.; Pozas, J.; San Román-Gil, M.; Orejana-Martín, I.; Torres-Jiménez, J.; Carrato, A.; Alonso-Gordoa, T.; Molina-Cerrillo, J. Tyrosine Kinase Receptors in Oncology. Int. J. Mol. Sci. 2020, 21, 8529. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.; Rapp, U.R. Isotype-specific functions of Raf kinases. Exp. Cell Res. 1999, 253, 34–46. [Google Scholar] [CrossRef]
- Crews, C.M.; Alessandrini, A.; Erikson, R.L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 1992, 258, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010, 399, 313–317. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous Mutation to Human BRAF V600E Is Common in Naturally Occurring Canine Bladder Cancer--Evidence for a Relevant Model System and Urine-Based Diagnostic Test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF Mutations in Canine Cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of BRAF Mutation in Urine DNA as a Molecular Diagnostic for Canine Urothelial and Prostatic Carcinoma. PLoS ONE 2015, 10, e0144170. [Google Scholar] [CrossRef]
- Pantke, P.; Diekmann, C.; Hettig, M.; Aupperle-Lellbach, H. Erste klinische Erhebungen zur Überlebensrate von Hunden mit Übergangszellkarzinom und BRAF-Mutation V595E. Kleintierpraxis 2019, 64, 680–686. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Kehl, A.; Merz, S.; Grassinger, J.; Hohloch, C.; Pantke, P. Die BRAF-Mutation V595E im Übergangszellkarzinom—Untersuchungen zur Rassedisposition bei Terriern. Kleintiermedizin 2019, 1, 30–33. [Google Scholar]
- Aupperle-Lellbach, H.; Grassinger, J.; Hohloch, C.; Kehl, A.; Pantke, P. Diagnostische Aussagekraft der BRAF-Mutation V595E in Urinproben, Ausstrichen und Bioptaten beim kaninen Übergangszellkarzinom. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2018, 46, 289–295. [Google Scholar] [CrossRef]
- Wayne, R.K.; Leonard, J.; Vilá, C. Genetic analysis of dog domestication. In Documenting Domestication: New Genetic and Archaeological Paradigms; University of California Press: Berkeley, CA, USA, 2006; pp. 279–293. [Google Scholar]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE 2014, 9, e90501. [Google Scholar] [CrossRef]
- Lewis, T.W.; Wiles, B.M.; Llewellyn-Zaidi, A.M.; Evans, K.M.; O’Neill, D.G. Longevity and mortality in Kennel Club registered dog breeds in the UK in 2014. Canine Genet. Epidemiol. 2018, 5, 10. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of owned dogs in England. Vet. J. 2013, 198, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.G.; Raghunath, S.; Williams, C.; Motsinger-Reif, A.A.; Cullen, J.M.; Liu, T.; Albertson, D.; Ruvolo, M.; Bergstrom Lucas, A.; Jin, J.; et al. Canine urothelial carcinoma: Genomically aberrant and comparatively relevant. Chromosome Res. 2015, 23, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of Copy Number Imbalance in Canine Urothelial Carcinoma with Droplet Digital Polymerase Chain Reaction. Vet. Pathol. 2016, 53, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.K.; Takahashi, N.; Kato, S.; Hashimoto, Y.; Goto-Koshino, Y.; Uchida, K. Diagnostic challenge in veterinary pathology: Detection of BRAFV595E mutation in a dog with follicular cystitis and flat urothelial lesion with atypia. Vet. Pathol. 2024, 61, 335–338. [Google Scholar] [CrossRef]
- Gollob, J.A.; Wilhelm, S.; Carter, C.; Kelley, S.L. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 2006, 33, 392–406. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858. [Google Scholar] [CrossRef]
- Cronise, K.E.; Hernandez, B.G.; Gustafson, D.L.; Duval, D.L. Identifying the ErbB/MAPK Signaling Cascade as a Therapeutic Target in Canine Bladder Cancer. Mol. Pharmacol. 2019, 96, 36–46. [Google Scholar] [CrossRef]
- Jung, H.; Bae, K.; Lee, J.Y.; Kim, J.-H.; Han, H.-J.; Yoon, H.-Y.; Yoon, K.-A. Establishment of Canine Transitional Cell Carcinoma Cell Lines Harboring BRAF V595E Mutation as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 9151. [Google Scholar] [CrossRef]
- Foskett, A.; Manley, C.; Naramore, R.; Gordon, I.K.; Stewart, B.M.; Khanna, C. Tolerability of oral sorafenib in pet dogs with a diagnosis of cancer. Vet. Med. Res. Rep. 2017, 8, 97–102. [Google Scholar] [CrossRef]
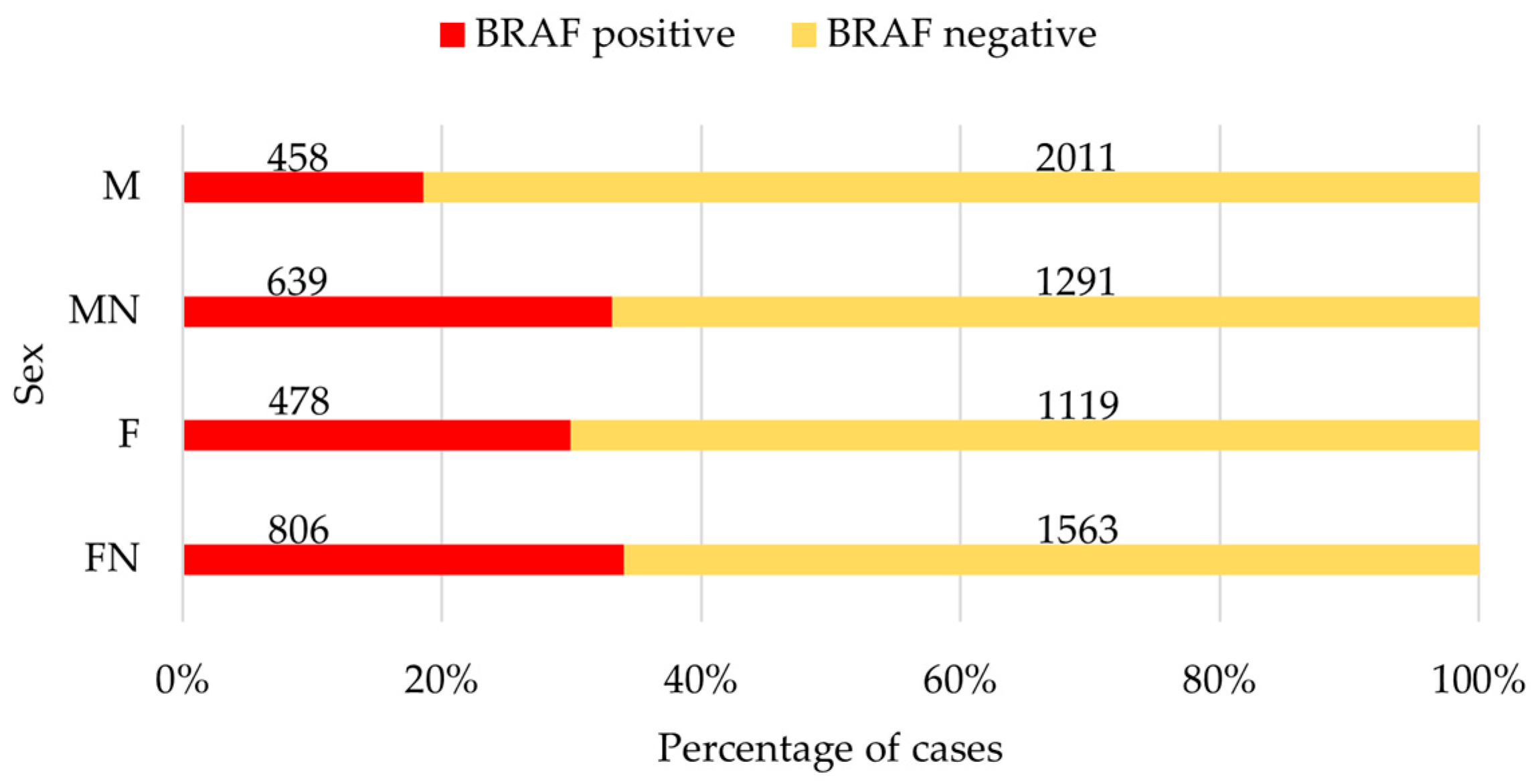
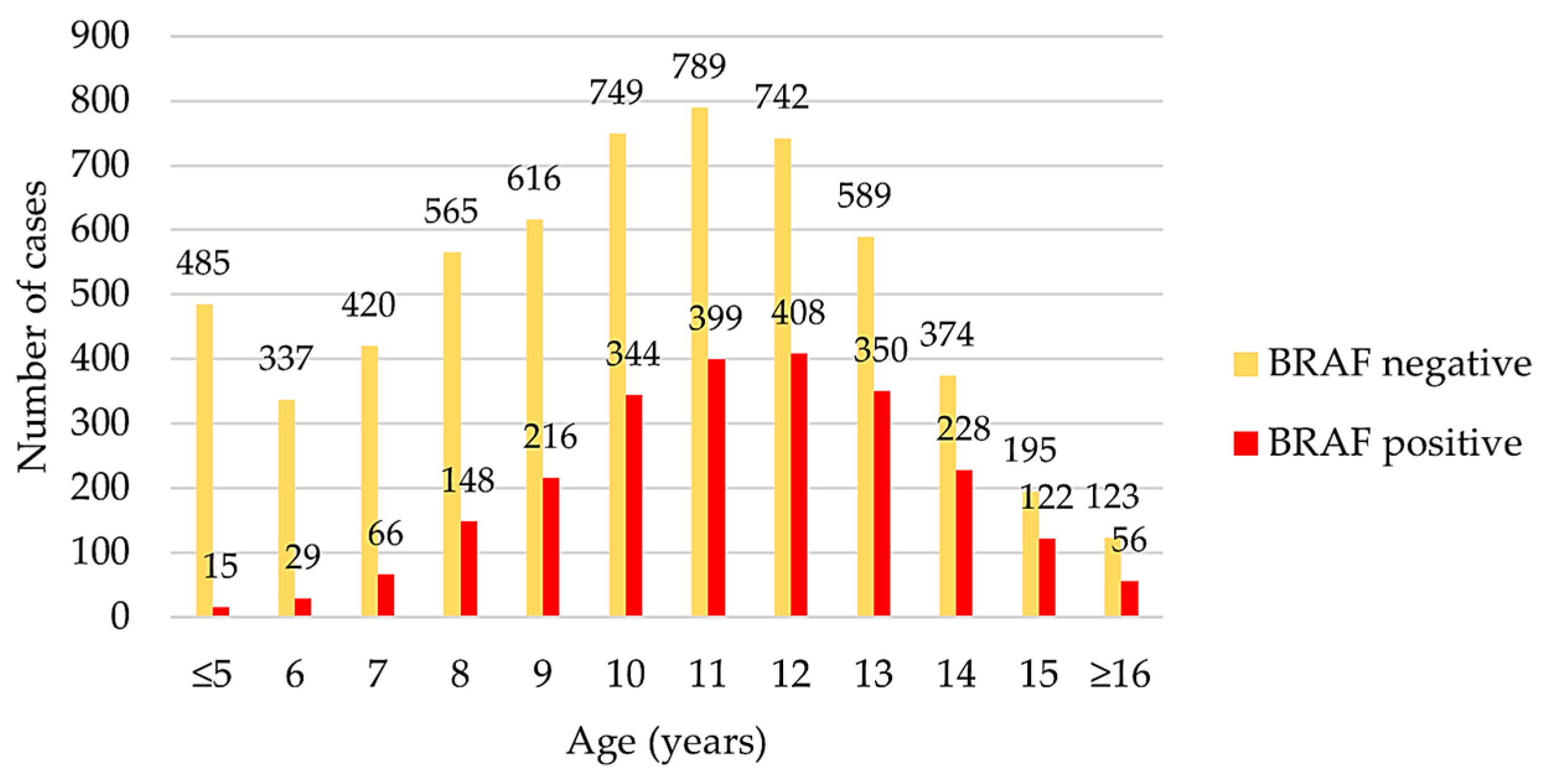
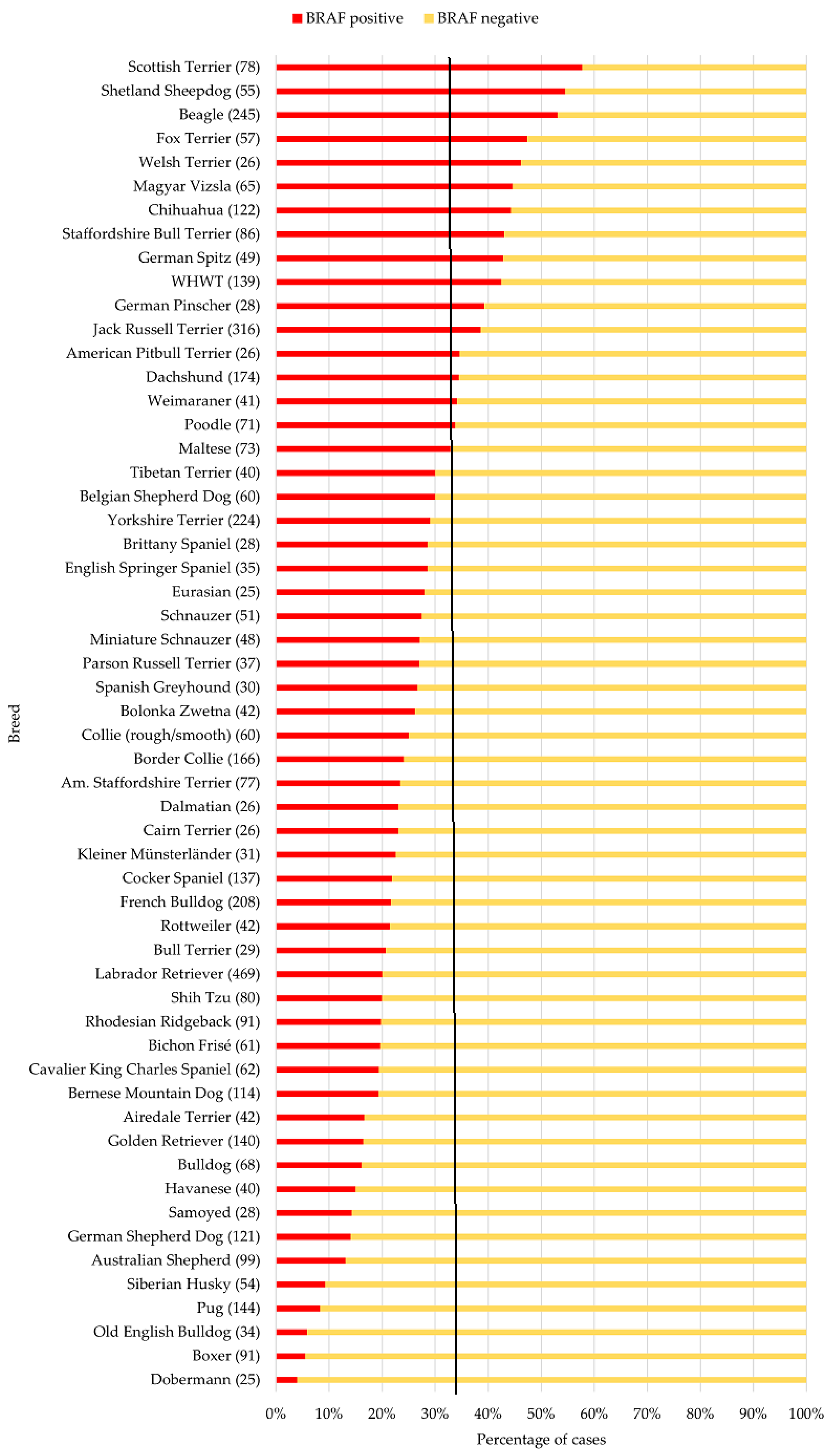
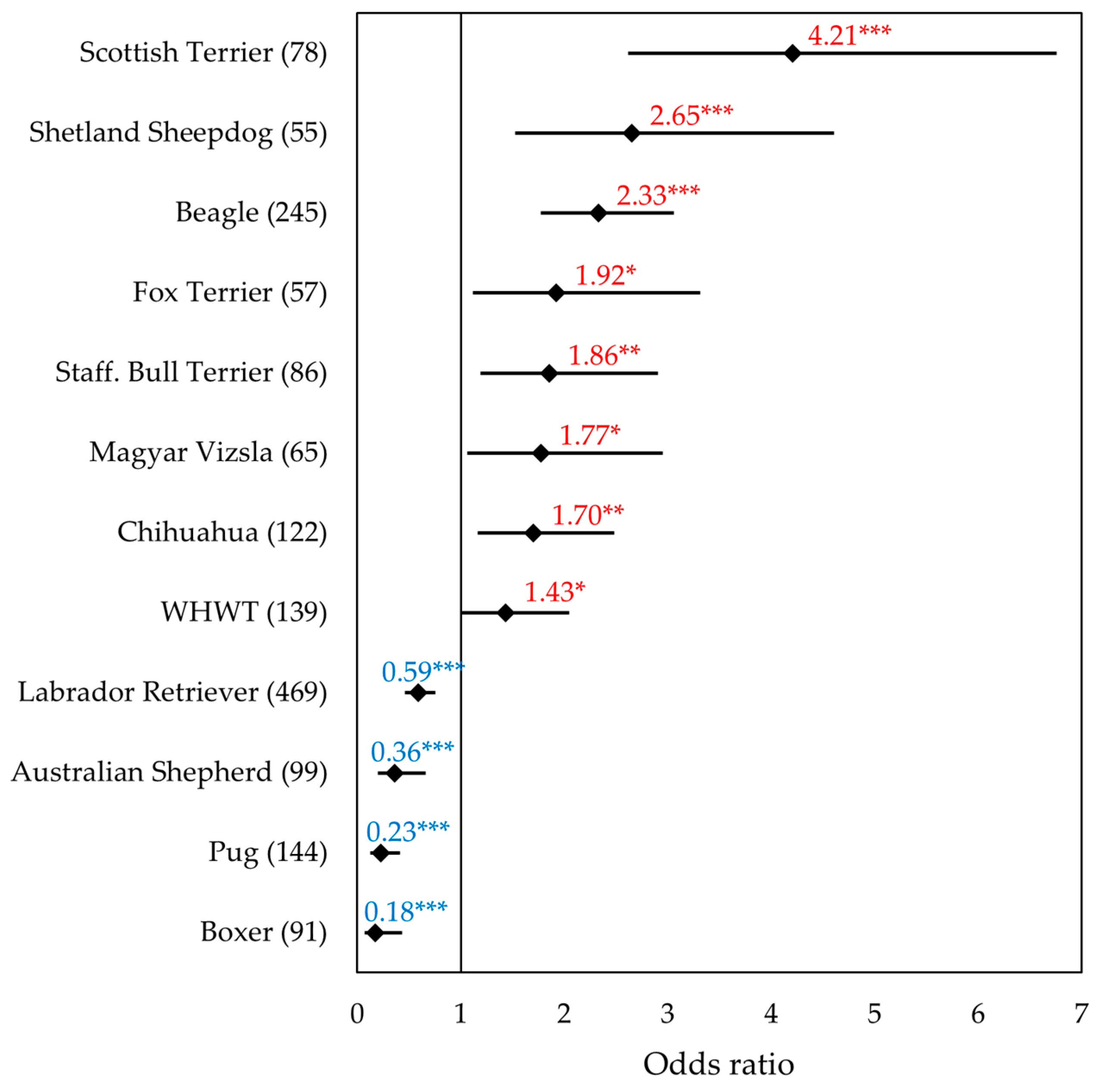

| Breed (n; BRAF+ in %) | M | MN | F | FN | Significance p |
|---|---|---|---|---|---|
| n (BRAF+/Total) BRAF+ in % | |||||
| Shetland Sheepdog (55; 54.5%) | 4/17 | 8/10 | 8/12 | 10/16 | a < 0.01 *, b = 0.03 * |
| 23.5 a,b | 80.0 a | 66.7 b | 62.5 | ||
| Beagle (245; 53.1%) | 21/55 | 29/57 | 34/61 | 46/72 | n.s. |
| 38.2 | 50.9 | 55.7 | 63.9 | ||
| Magyar Vizsla (65; 44.6%) | 6/17 | 12/19 | 5/10 | 6/19 | n.s. |
| 35.3 | 63.2 | 50.0 | 31.6 | ||
| Chihuahua (122; 44.3%) | 9/35 | 14/30 | 11/23 | 20/34 | n.s. |
| 25.7 | 46.7 | 47.8 | 58.8 | ||
| German Spitz (49; 42.9%) | 2/9 | 7/13 | 2/12 | 10/15 | a < 0.01 * |
| 22.2 | 53.8 | 16.7 a | 66.7 a | ||
| Labrador Retriever (469; 20.0%) | 20/151 | 34/130 | 16/85 | 24/103 | a < 0.01 * |
| 13.2 a | 26.1 a | 18.8 | 23.3 | ||
| Australian Shepherd (99; 13.1%) | 1/37 | 5/17 | 4/18 | 3/27 | a < 0.01 *, b = 0.04 * |
| 2.7 a,b | 29.4 a | 22.2 b | 11.1 | ||
| Pug (144; 8.3%) | 4/32 | 2/13 | 2/41 | 4/58 | n.s. |
| 12.5 | 15.4 | 4.9 | 6.9 | ||
| Boxer (91; 5.5%) | 2/41 | 0/19 | 2/17 | 1/14 | n.s. |
| 4.9 | 0 | 11.8 | 7.1 | ||
| Breed (n) | Age (Total) | Age (BRAF+) | Age (BRAF−) |
|---|---|---|---|
| Range (Median) in Years | |||
| Shetland Sheepdog (55) | 6–16 (11) | 7–16 (11) | 6–16 (11) |
| Beagle (245) | 2–17 (11) | 6–17 (11) | 2–17 (11) |
| Magyar Vizsla (65) | 4–15 (11) | 9–15 (11) | 4–15 (11) |
| Chihuahua (122) | 1–16 (11) | 7–16 (11) | 1–16 (11) |
| German Spitz (49) | 5–18 (11) | 9–17 (12) | 5–18 (11) |
| Labrador Retriever (469) | 1–18 (10) | 6–16 (11) | 1–18 (10) |
| Australian Shepherd (99) | 1–15 (10) | 7–14 (11) | 1–15 (10) |
| Pug (144) | 3–16 (9) | 8–14 (10) | 3–16 (9) |
| Boxer (91) | 1–14 (9) | 8–10 (10) | 1–14 (9) |
| Breed (n; BRAF+ in %) | M | MN | F | FN | Significance p |
|---|---|---|---|---|---|
| n (BRAF+/Total) BRAF+ in % | |||||
| Scottish Terrier (78; 57.7%) | 14/32 | 3/6 | 15/22 | 13/18 | n.s. |
| 43.8 | 50.0 | 68.2 | 72.2 | ||
| Fox Terrier (57; 47.4%) | 5/21 | 10/20 | 4/6 | 8/10 | n.s. |
| 23.8 | 50.0 | 66.7 | 80.0 | ||
| Welsh Terrier (26; 46.2%) | 2/6 | 2/8 | 2/4 | 6/8 | n.s. |
| 33.3 | 25.0 | 50.0 | 75.0 | ||
| Staff. Bull Terrier (86; 43.0%) | 9/30 | 9/18 | 5/15 | 14/23 | n.s. |
| 30.0 | 50.0 | 33.3 | 60.9 | ||
| WHWT (139; 42.4%) | 7/35 | 9/18 | 22/42 | 21/44 | a = 0.02 *, b < 0.01 * |
| 20.0 a,b | 50.0 a | 52.4 b | 47.7 | ||
| Breed (n) | Age (Total) | Age (BRAF+) | Age (BRAF−) |
|---|---|---|---|
| Range (Median) in Years | |||
| Scottish Terrier (78) | 4–13 (10) | 5–13 (9) | 4–13 (10) |
| Fox Terrier (57) | 3–16 (12) | 5–15 (12) | 3–16 (11) |
| Welsh Terrier (26) | 1–16 (12) | 9–16 (12) | 1–14 (11) |
| Staff. Bull Terrier (86) | 4–15 (11) | 6–15 (12) | 4–14 (10) |
| WHWT (139) | 1–17 (12) | 5–17 (12) | 1–17 (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appenzeller, M.; Kehl, A.; Törner, K.; Jensen, K.C.; Klopfleisch, R.; Aupperle-Lellbach, H. BRAF Mutation Analysis: A Retrospective Evaluation of 8365 Diagnostic Samples with a Special View on Canine Breeds (2018–2024). Vet. Sci. 2025, 12, 729. https://doi.org/10.3390/vetsci12080729
Appenzeller M, Kehl A, Törner K, Jensen KC, Klopfleisch R, Aupperle-Lellbach H. BRAF Mutation Analysis: A Retrospective Evaluation of 8365 Diagnostic Samples with a Special View on Canine Breeds (2018–2024). Veterinary Sciences. 2025; 12(8):729. https://doi.org/10.3390/vetsci12080729
Chicago/Turabian StyleAppenzeller, Marielle, Alexandra Kehl, Katrin Törner, Katharina Charlotte Jensen, Robert Klopfleisch, and Heike Aupperle-Lellbach. 2025. "BRAF Mutation Analysis: A Retrospective Evaluation of 8365 Diagnostic Samples with a Special View on Canine Breeds (2018–2024)" Veterinary Sciences 12, no. 8: 729. https://doi.org/10.3390/vetsci12080729
APA StyleAppenzeller, M., Kehl, A., Törner, K., Jensen, K. C., Klopfleisch, R., & Aupperle-Lellbach, H. (2025). BRAF Mutation Analysis: A Retrospective Evaluation of 8365 Diagnostic Samples with a Special View on Canine Breeds (2018–2024). Veterinary Sciences, 12(8), 729. https://doi.org/10.3390/vetsci12080729







