Appraisal of Allostatic Load in Wild Boars Under a Controlled Environment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Rearing and Observation Area
2.3. Animals and Sampling
2.4. Laboratory Analysis
2.5. Statistical Analysis
2.6. Ethical Note
- (1)
- freedom from hunger, thirst and poor nutrition (water ad libitum and daily distribution of a complete diet);
- (2)
- freedom from environmental disturbances (the animals were housed in a fenced wooded area);
- (3)
- freedom from disease and injury (monthly veterinary check-up);
- (4)
- freedom to freely express species-specific behavioral characteristics (adequate spaces in which to develop the correct intraspecific competitions necessary for the formation of the family group);
- (5)
- freedom from fear and stress (less anthropogenic disturbance during the entire observation period; evaluation of HPA axis activity through cortisol concentrations).
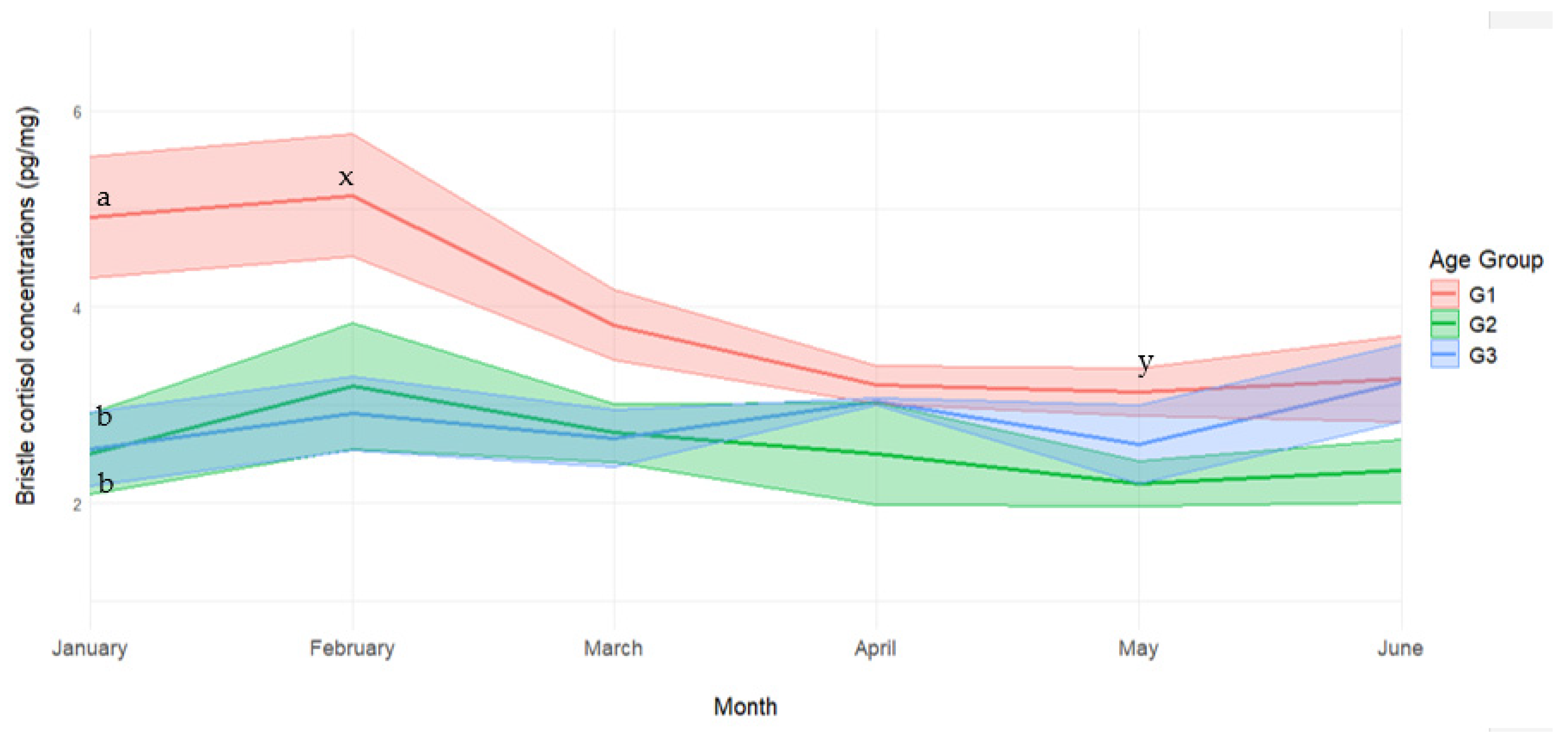
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Jankord, R.; Herman, J.P. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N. Y. Acad. Sci. 2008, 1148, 64–73. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef]
- Hing, S.; Currie, A.; Broomfield, S.; Keatley, S.; Jones, K.; Thompson, R.C.; Narayan, E.; Godfrey, S.S. Host stress physiology and Trypanosoma haemoparasite infection influence innate immunity in the woylie (Bettongia penicillata). Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Karaer, M.C.; Cebulj-Kadunc, N.; Snoj, T. Stress in wildlife: Comparison of the stress response among domestic, captive, and free-ranging animals. Front. Vet. Sci. 2023, 10, 1167016. [Google Scholar] [CrossRef] [PubMed]
- Brearley, G.; Rhodes, J.; Bradley, A.; Baxter, G.; Seabrook, L.; Lunney, D.; Liu, Y.; McAlpine, C. Wildlife disease prevalence in human-modified landscapes. Biol. Rev. Camb. Philos. Soc. 2013, 88, 427–442. [Google Scholar] [CrossRef]
- Matteri, R.L.; Dyer, C.J.; Touchette, K.J.; Carroll, J.A.; Allee, G.L. Effects of weaning on somatotrophic gene expression and circulating levels of insulin-like growth factor-1 (IGF-1) and IGF-2 in pigs. Domest. Anim. Endocrinol. 2000, 19, 247–259. [Google Scholar] [CrossRef]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol determination in hair and faeces from domestic cats and dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Hayssen, V. Measuring cortisol in hair and saliva from dogs: Coat color and pigment differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Auletta, L.; Ciani, F.; Pelagalli, A.; Pasolini, M.P. Hair cortisol levels in captive brown hare (Lepus europaeus): Potential effect of sex, age, and breeding technology. Eur. J. Wildl. Res. 2017, 63, 62. [Google Scholar] [CrossRef]
- Esposito, L.; Tafuri, S.; Cocchia, N.; Fasanelli, R.; Piscopo, N.; Lamagna, B.; Eguren, V.; Amici, A.; Iorio, E.L.; Ciani, F. Assessment of living conditions in wild boars by analysis of oxidative stress markers. J. Appl. Anim. Welf. Sci. 2021, 24, 64–71. [Google Scholar] [CrossRef]
- Hayssen, V.; Harper, J.M.; DeFina, R. Fecal corticosteroids in agouti and non-agouti deer mice (Peromyscus maniculatus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 439–446. [Google Scholar] [CrossRef]
- Stelwagen, K.; van Espen, D.C.; Verkerk, G.A.; McFadden, H.A.; Farr, V.C. Elevated plasma cortisol reduces permeability of mammary tight junctions in the lactating bovine mammary epithelium. J. Endocrinol. 1998, 159, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Vining, R.F.; McGinley, R.A.; Maksvytis, J.J.; Ho, K.Y. Salivary cortisol: A better measure of adrenal cortical function than serum cortisol. Ann. Clin. Biochem. 1983, 20, 329–335. [Google Scholar] [CrossRef]
- Peric, T.; Mazzoni, C.; Quai, F.; Cotticelli, A.; Pividori, I.; Corazzin, M.; Comin, A.; Bresciani, C.; Prandi, A. Sow’s pre- and post-delivery in different confinement systems evaluated by hair hormones concentrations. Livest. Sci. 2023, 272, 105235. [Google Scholar] [CrossRef]
- Cordeschi, G.; Peric, T.; Prandi, A.; Zoratto, F.; Mori, E. Environmental variability and allostatic load in the Eurasian red squirrel (Sciurus vulgaris). Rend. Lincei. Sci. Fis. E Nat. 2021, 32, 437–448. [Google Scholar] [CrossRef]
- Franchini, M.; Peric, T.; Frangini, L.; Prandi, A.; Comin, A.; Rota, M.; Filacorda, S. You’re stressing me out! Effect of interspecific competition from red deer on roe deer physiological stress response. J. Zool. 2023, 320, 63–74. [Google Scholar] [CrossRef]
- Montillo, M.; Caslini, C.; Peric, T.; Prandi, A.; Netto, P.; Tubaro, F.; Pedrotti, L.; Bianchi, A.; Mattiello, S. Analysis of 19 Minerals and Cortisol in Red Deer Hair in Two Different Areas of the Stelvio National Park: A Preliminary Study. Animals 2019, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Prandi, A.; Peric, T.; Corazzin, M.; Comin, A.; Colitti, M. A first survey on hair cortisol of an Alpine ibex (Capra ibex ibex) population. Anim. Sci. Pap. Rep. 2018, 36, 57–74. [Google Scholar]
- Bryan, H.M.; Smits, J.E.G.; Koren, L.; Paquet, P.C.; Wynne-Edwards, K.E.; Musiani, M. Heavily hunted wolves have higher stress and reproductive steroids than wolves with lower hunting pressure. Funct. Ecol. 2015, 29, 347–356. [Google Scholar] [CrossRef]
- Doss, E.M.; Jouffroy, M.; Rey, B.; Cohas, A.; von Hardenberg, A.; Smith, T.E. Technical validation and a comparison of two methods to quantify individual levels of glucocorticoids in Alpine marmot hair. MethodsX 2023, 11, 102418. [Google Scholar] [CrossRef] [PubMed]
- Terwissen, C.V.; Mastromonaco, G.F.; Murray, D.L. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Goldsmith, A.R. Noninvasive endocrine data for behavioural studies: The importance of validation. Anim. Bahaviour 2004, 67, 183–185. [Google Scholar] [CrossRef]
- Gow, R.; Thomson, S.; Rieder, M.; Van Uum, S.; Koren, G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int. 2010, 196, 32–37. [Google Scholar] [CrossRef]
- Keckeis, K.; Lepschy, M.; Schopper, H.; Moser, L.; Troxler, J.; Palme, R. Hair cortisol: A parameter of chronic stress? Insights from a radiometabolism study in guinea pigs. J. Comp. Physiol. B 2012, 182, 985–996. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Van Uum, S.H.; Sauve, B.; Fraser, L.A.; Morley-Forster, P.; Paul, T.L.; Koren, G. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress 2008, 11, 483–488. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gacic, D.; Sprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozolins, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest. Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, N.; Costanzo, M.; Gelzo, M.; Sacchettino, L.; Vitiello, C.; Balestrieri, A.; Napolitano, F.; Esposito, L. Effect of the sarcoptic mange upon metabolome profiling in wild boars. Res. Vet. Sci. 2025, 183, 105505. [Google Scholar] [CrossRef] [PubMed]
- Herrero, J.; García-Serrano, A.; Couto, S.; Ortuño, V.M.; García-González, R. Diet of wild boar Sus scrofa L. and crop damage in an intensive agroecosystem. Eur. J. Wildl. Res. 2006, 52, 245–250. [Google Scholar] [CrossRef]
- Geisser, H.; REYER, H.U. Efficacy of hunting, feeding, and fencing to reduce Crop damage by wild boars. J. Wildl. Manag. 2004, 68, 939–946. [Google Scholar] [CrossRef]
- Ficetola, G.; Bonardi, A.; Mairota, P.; Leronni, V.; Padoa-Schioppa, E. Predicting wild boar damages to croplands in a mosaic of agricultural and natural areas. Curr. Zool. 2014, 2, 170–179. [Google Scholar] [CrossRef]
- Kruuse, M.; Enno, S.E.; Oja, T. Temporal patterns of wild boar-vehicle collisions in Estonia, at the northern limit of its range. Eur. J. Wildl. Res. 2016, 62, 787–791. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Mentaberre, G.; Aguilar, X.F.; Conejero, C.; Colom-Cadena, A.; Ráez-Bravo, A.; González-Crespo, C.; Espunyes, J.; Lavín, S.; López-Olvera, J.R. Wild boar in the city: Phenotypic responses to urbanisation. Sci. Total Environ. 2021, 773, 145593. [Google Scholar] [CrossRef]
- Zani, L.; Dietze, K.; Dimova, Z.; Forth, J.H.; Denev, D.; Depner, K.; Alexandrov, T. African Swine Fever in a Bulgarian Backyard Farm-A Case Report. Vet. Sci. 2019, 6, 94. [Google Scholar] [CrossRef]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and Sexual Steroid Concentrations in Fattening Pigs’ Hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef]
- Falco, A.; Girardi, D.; Elfering, A.; Peric, T.; Pividori, I.; Dal Corso, L. Is Smart Working Beneficial for Workers’ Wellbeing? A Longitudinal Investigation of Smart Working, Workload, and Hair Cortisol/Dehydroepiandrosterone Sulfate during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 6220. [Google Scholar] [CrossRef]
- Cunningham, K.; Hinton, T.G.; Luxton, J.J.; Bordman, A.; Okuda, K.; Taylor, L.E.; Hayes, J.; Gerke, H.C.; Chinn, S.M.; Anderson, D.; et al. Evaluation of DNA damage and stress in wildlife chronically exposed to low-dose, low-dose rate radiation from the Fukushima Dai-ichi Nuclear Power Plant accident. Environ. Int. 2021, 155, 106675. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Malkinson, D.; Schonblum, A.; Koren, L.; Shanas, U. Do boars compensate for hunting with higher reproductive hormones? Conserv. Physiol. 2021, 9, coab068. [Google Scholar] [CrossRef] [PubMed]
- Tajchman, K.; Janiszewski, P.; Staniszewska, P.; Hanzal, V.; Kasperek, K.; Strachecka, A. The impact of stalking hunt season on long-term stress in big game. BMC Vet. Res. 2024, 20, 549. [Google Scholar] [CrossRef]
- Heimburge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth—Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 2020, 288, 113359. [Google Scholar] [CrossRef]
- Scollo, A.; Cotticelli, A.; Peric, T.; Perrucci, A.; Prandi, A.; Ferrari, P. Hair Dehydroepiandrosterone Sulfate (DHEA(S)) and Cortisol/DHEA(S) Ratio as Long-Lasting Biomarkers of Clinical Syndromes Exhibited by Piglets Early in Life. Animals 2025, 15, 1032. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P.; Wright-Lichter, J.; Romero, L.M. Chronic stress and the introduction to captivity: How wild house sparrows (Passer domesticus) adjust to laboratory conditions. Gen. Comp. Endocrinol. 2018, 259, 85–92. [Google Scholar] [CrossRef]
- Heimburge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
| Age | Mean | SE |
|---|---|---|
| G1 | 3.90 B | 0.21 |
| G2 | 2.57 A | 0.25 |
| G3 | 2.83 A | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscopo, N.; Balestrieri, A.; D’Alessio, N.; Silvestre, P.; Bifulco, G.; Cotticelli, A.; Peric, T.; Prandi, A.; d’Angelo, D.; Napolitano, F.; et al. Appraisal of Allostatic Load in Wild Boars Under a Controlled Environment. Vet. Sci. 2025, 12, 667. https://doi.org/10.3390/vetsci12070667
Piscopo N, Balestrieri A, D’Alessio N, Silvestre P, Bifulco G, Cotticelli A, Peric T, Prandi A, d’Angelo D, Napolitano F, et al. Appraisal of Allostatic Load in Wild Boars Under a Controlled Environment. Veterinary Sciences. 2025; 12(7):667. https://doi.org/10.3390/vetsci12070667
Chicago/Turabian StylePiscopo, Nadia, Anna Balestrieri, Nicola D’Alessio, Pasqualino Silvestre, Giovanna Bifulco, Alessio Cotticelli, Tanja Peric, Alberto Prandi, Danila d’Angelo, Francesco Napolitano, and et al. 2025. "Appraisal of Allostatic Load in Wild Boars Under a Controlled Environment" Veterinary Sciences 12, no. 7: 667. https://doi.org/10.3390/vetsci12070667
APA StylePiscopo, N., Balestrieri, A., D’Alessio, N., Silvestre, P., Bifulco, G., Cotticelli, A., Peric, T., Prandi, A., d’Angelo, D., Napolitano, F., & Esposito, L. (2025). Appraisal of Allostatic Load in Wild Boars Under a Controlled Environment. Veterinary Sciences, 12(7), 667. https://doi.org/10.3390/vetsci12070667






