1. Introduction
Antimicrobial resistance (AMR) is a pressing global concern, driven in part by the inappropriate and unregulated use of antibiotics in both human and companion animal veterinary medicine. This study focuses exclusively on companion animals, specifically dogs and cats, which represent over 95% of the caseload in small-animal veterinary practice in Brazil. Ensuring the rational use of these substances depends not only on professional education but also on robust and transparent national regulatory systems [
1,
2].
The prescription and regulation of veterinary antibiotics differ markedly across geopolitical and economic contexts. In high-income countries such as the United Kingdom, regulations have been in place for a longer period and are well established. In contrast, in the United States, stricter controls were implemented more recently. For instance, the U.S. Food and Drug Administration (FDA) only mandated veterinary prescriptions for medically important antibiotics for all animal species, including livestock, starting in 2017 with the Veterinary Feed Directive (VFD), which was further reinforced in 2023 [
3], whereas in the UK, despite the growing use of antimicrobials in companion animals, prescription tracking and oversight remain limited [
4]. These examples illustrate the variability in regulatory maturity and the enforcement of prudent practices in high-income settings.
Conversely, regulatory challenges are even more pronounced in low- and middle-income countries (LMICs), where enforcement capacity is frequently limited. For instance, studies from Kenya report that 100% of veterinary pharmacies sell antibiotics without requiring prescriptions [
5]. Similarly, in Vietnam, antimicrobials intended for veterinary use can be legally purchased without a prescription in approximately 12,000 veterinary drug shops [
6]. This pattern reflects a widespread problem across LMICs, where weak regulatory enforcement, fragmented oversight systems, and high demand for antimicrobials in animal health contribute significantly to inappropriate use.
Brazil exemplifies these regulatory gaps commonly seen in LMICs. In the Brazilian context, medications intended for veterinary use fall under the jurisdiction of the Ministry of Agriculture and Livestock (MAPA), whereas antibiotics intended for human use are regulated by the National Health Surveillance Agency (ANVISA) of the Ministry of Health. Although ANVISA permits the dispensing of antibiotics approved for human use under veterinary prescription [
7], MAPA’s regulations, specifically Normative Instruction No. 26 [
8], do not universally mandate prescriptions for veterinary drugs. Prescription requirements are generally restricted to certain antimicrobials used as zootechnical additives in livestock production. This regulatory gap enables the widespread, and often uncontrolled, sale of antimicrobials for veterinary purposes, particularly in the care of companion animals.
Often, there is limited enforcement of prescription requirements, and over-the-counter sales of antibiotics—intended for either human or veterinary use—are commonplace. In some settings, veterinary drugs are under the jurisdiction of agricultural ministries, while human-use drugs fall under health authorities, creating fragmented oversight systems. This division may lead to inconsistencies in prescription policies and facilitate the misuse of antimicrobials in animals, particularly in the care of companion animals.
These regulatory gaps are deeply rooted in a combination of factors. Politically, the fragmented responsibilities between health (ANVISA) and agricultural (MAPA) authorities hinder the development of unified surveillance systems. Economically, limited investment in veterinary public health infrastructure reduces the capacity for enforcement and monitoring [
9]. Culturally, informal practices such as over-the-counter antibiotic sales and self-medication in animal care are widespread, reflecting a lack of awareness and educational outreach regarding antimicrobial stewardship [
2]
This lack of regulation, combined with the increasing demand for antibiotics to treat various conditions in pets, contributes to their indiscriminate use. Despite recommendations from veterinary associations promoting the prudent use of antimicrobials, adherence to these guidelines is often inconsistent [
4]. The over-the-counter availability of these drugs, frequently without veterinary oversight, exacerbates the risk of AMR emergence in both animal and human populations.
Within the One Health framework, AMR is a critical and urgent issue that requires coordinated action across sectors—including public health, veterinary practice, pharmaceutical manufacturing, and environmental surveillance [
10,
11]. Thus, this study aimed to contribute to this integrated perspective by critically evaluating the use of antibiotics approved for human use in companion animals in Brazil—representing LMICs—through a retrospective, population-level analysis. Specifically, we examined veterinary prescriptions dispensed in human pharmacies, developed a national consumption profile by antibiotic class and route of administration, and analyzed regional disparities in prescription patterns. Additionally, we normalized prescription volume by the number of veterinarians practicing small-animal medicine to better understand potential misuse or overprescription.
2. Materials and Methods
2.1. Study Design
This study is a retrospective time-series analysis covering the period from 2017 to 2021. It investigates trends in the number of veterinary prescriptions for antibiotics approved for human use, the most commonly used antibiotic classes, and the regional distribution of prescriptions in Brazil. This study focuses specifically on antibiotics for oral and injectable use, as recorded in human pharmacies.
Data were sourced from the National System for the Management of Controlled Products (Sistema Nacional de Gerenciamento de Produtos Controlados, SNGPC), maintained by the Brazilian National Health Surveillance Agency (ANVISA). The decision to use ANVISA data was based on the agency’s rigorous standards for tracking antimicrobial sales. According to Collegiate Board Resolution (RDC) No. 22 of 29 April 2014 [
12], pharmacies are required to retain copies of prescriptions and report sales electronically to the SNGPC. This system includes prescriptions issued by veterinarians registered with the Regional Veterinary Medicine Councils. While the MAPA regulates veterinary drugs, it lacks a centralized and digital prescription-tracking system comparable to the SNGPC. Therefore, using ANVISA data offers a more detailed and standardized source for evaluating antimicrobial use in companion animals.
It is important to note that the SNGPC database captures antimicrobial sales dispensed through human pharmacies, including prescriptions issued by veterinarians. However, it does not include sales from veterinary clinics, agro-veterinary establishments regulated by the Ministry of Agriculture (MAPA), pet shops, or online platforms authorized to sell veterinary medicines. Thus, this dataset only represents a portion of the total antimicrobial use in companion animals in Brazil, potentially underestimating the actual consumption. Due to the nature of the SNGPC database, information related to animal age, breed, or clinical condition is not available; therefore, our analysis is restricted to prescription patterns regardless of these variables.
It is important to note that in November 2021, the Brazilian Ministry of Health suspended Articles 3 and 4 of RDC 22/2014, interrupting data reporting to the SNGPC [
13]. As a result, data from November 2021 onward became incomplete, with no data for December 2021 and minimal records in 2022 (less than 10% of the previous year’s average). Data for 2023 were not available at the time of submission. Consequently, our study period was defined as January 2017 to October 2021.
2.2. Data Extraction and Preprocessing
Monthly datasets were downloaded from the ANVISA/SNGPC portal in CSV format, corresponding to industrialized medicine sales. A total of 59 monthly prescription datasets were collected (12 months per year for 5 years, excluding December 2021).
Due to the volume of records exceeding Excel’s native row limits (1,048,576 rows), data wrangling was performed using Power Query® within Microsoft Excel®. Files were processed and separated by year to accommodate size limitations. Records were filtered to retain only those issued by veterinary professionals and those containing antibiotics as active ingredients. Additional cleaning steps excluded records for antibiotics administered via non-oral and non-injectable routes.
To address inconsistencies and known anomalies in the ANVISA database, three outlier cases were removed:
A single sales record of 798,365 bottles of amoxicillin–clavulanate suspension (Jardim Silva, RJ).
A sale of 117,187 boxes of amoxicillin, clarithromycin, and lansoprazole (Rio de Janeiro, December 2018).
A sale of 17,802 boxes of amoxicillin (Belém, Pará, July 2020).
2.3. Inclusion and Exclusion Criteria
This study included all medication records of molecules pharmacologically classified as antibiotics with systemic activity, dispensed through human pharmacies. When uncertainties arose regarding specific drug names or formulations, pharmacology textbooks were consulted to ensure accurate classification. Only antibiotics intended for oral (per os, PO) use or injectable use when available in human pharmacies were retained, considering their systemic action and relevance to discussions of antimicrobial resistance (AMR), particularly due to their excretion pathways. It is important to note that antibiotics commonly administered via subcutaneous (SC), intramuscular (IM), or intravenous (IV) routes within veterinary clinical settings are generally not dispensed to clients but applied directly by veterinarians or purchased through veterinary channels. Therefore, these routes are not fully represented in this dataset, which predominantly reflects oral antibiotics and the limited number of injectable formulations suitable for at-home use.
Medications administered through non-systemic routes were excluded to improve the specificity of the analysis. These included formulations intended for topical, ophthalmic, otologic, buccal, vaginal, and nasal use. The lack of standardization in how these routes were abbreviated in the prescription and sales records posed a significant data-cleaning challenge. To address this, a multi-step filtering and normalization process was implemented.
For topical use, the following abbreviations were identified and excluded: “derm,” “crem derm,” “pom derm,” “sun top,” “top powder,” “gel ct,” and “pom ct.”
Additionally, all formulations intended for dermatological, otic, ophthalmic, buccal, vaginal, nasal, and perianal use were systematically excluded from the analysis. Our study focused exclusively on antibiotics intended for systemic administration, specifically oral and injectable formulations, given their greater relevance to antimicrobial resistance dynamics. Ophthalmic medications were labeled as “oft,” “sol ocu,” and “sol oft.” Otologic routes were recorded as “sol oto,” “sus otol,” and “sus oto.” Buccal use appeared as “buccal” and “sol buc.” Vaginal formulations included labels such as “gel vag,” “gele vag,” “ovl vag,” “gynec,” and “pom vag.” Finally, nasal preparations were listed as “sol nas” and “nasal.” Abbreviations such as “sun,” “top,” “gel,” and “oto” were particularly ambiguous, as they could apply to more than one route, thus requiring broader exclusion rules.
To mitigate these inconsistencies, we applied a manual and programmatic standardization protocol using Power Query®. This ensured that all included data referred exclusively to antibiotics with systemic use via oral or injectable routes, thereby enhancing the accuracy and reliability of this study’s conclusions regarding antibiotic usage patterns in veterinary practice.
It is important to note that the SNGPC database only provides the number of prescriptions and units sold but does not capture the total quantity of active pharmaceutical ingredients dispensed (e.g., milligrams or kilograms). Therefore, this study does not allow the calculation of antibiotic exposure per animal based on tonnage or mg/kg metrics, representing a restriction in fully assessing the antibiotic burden on the animal population. Future studies integrating data on dosage and treatment duration are essential for a more comprehensive understanding of antimicrobial exposure in companion animals.
2.4. Data Organization
In the data wrangling phase (data wrangling refers to the process of cleaning raw data by identifying and resolving errors, addressing inconsistencies, and organizing variables into a usable format for analysis. No missing or incomplete values were supplemented in this study; the analysis was conducted solely based on the available dataset extracted from the SNGPC. This process is essential to ensure the integrity and usability of complex datasets (Harvard Business School Online, 2021):
https://online.hbs.edu/blog/post/data-wrangling (accessed on 2 October 2023)). The dataset was systematically structured to enable consistent and accurate analysis. Antibiotic-containing products were initially classified by pharmacological class: aminoglycosides, amphenicols, carbapenems, cephalosporins, lincosamides, macrolides, nitroimidazoles, penicillins, quinolones, sulfonamides, and tetracyclines.
Subsequently, each annual file was aggregated by key analytical attributes, including the following: the total number of prescriptions issued by veterinarians; the total number of units sold (noting that some prescriptions comprised multiple packages); and the number of prescriptions assigned to each antibiotic class. These data were also organized by month, year, and federative unit (state) of origin. This step ensured the uniform structure of the database and supported subsequent exploratory analyses and the graphical representation of antibiotic prescription patterns across time and geography in Brazil.
2.5. Exploratory Data Analysis and Data Visualization
Following data wrangling and organization, Exploratory Data Analysis (EDA) was conducted to obtain an initial understanding of patterns, trends, and anomalies within the dataset. Although traditionally considered a preliminary step, in this study, EDA was employed iteratively and cyclically in close association with data cleaning procedures, allowing the identification of inconsistencies and the refinement of filters during processing.
The primary objective of EDA was to generate descriptive insights into veterinary prescription practices involving antibiotics approved for human use over time and across regions. To achieve this, the analysis employed frequency distributions and summary statistics to quantify prescription volume, antibiotic classes used, and monthly trends. These descriptive measures supported hypothesis generation and guided a deeper contextual interpretation of the findings.
In addition to numerical summaries, data visualization techniques were applied to illustrate temporal fluctuations and potential seasonal patterns in antibiotic use. Monthly and yearly plots of total prescriptions and antibiotic sales volume allowed for the identification of deviations, peaks, or reductions possibly linked to public health events (e.g., the COVID-19 pandemic) or regulatory changes (e.g., suspension of SNGPC data reporting in late 2021).
The combination of tabular analysis and visual representation provided a more intuitive and comprehensive understanding of the dataset. This approach facilitated the identification of regional differences and helped highlight critical points of concern in veterinary antimicrobial use within Brazil, insights that may have broader relevance for other low- and middle-income countries facing similar regulatory challenges.
2.6. Statistical Analysis
Data were expressed as monthly or annual means. Comparisons among years were performed using one-way ANOVA, followed by Tukey’s Honest Significant Difference (HSD) test for multiple comparisons. Statistical significance was set at p < 0.05. All analyses were conducted using Statistica® version 8 (StatSoft Inc., Tulsa, OK, USA).
3. Results
The initial findings were derived from the analysis of veterinary prescriptions for antibiotics approved for human use dispensed in private pharmacies across Brazil between 2017 and 2021. In addition to antibiotics, prescriptions issued by veterinarians also included other drug classes controlled by ANVISA, such as antidepressants, anxiolytics, opioids, dopamine antagonists, anticonvulsants, antidiabetics, diuretics, and amphetamines. When all prescriptions issued by veterinarians and filled in pharmacies were aggregated (
Table 1), the data revealed a consistent upward trend over the five-year period. Prescription volumes in 2019, 2020, and 2021 were significantly higher compared to 2017 (
p = 0.0497,
p = 0.00013, and
p = 0.00013, respectively), indicating that the growth in prescriptions became statistically evident starting in 2019. The most substantial increase occurred between 2019 and 2020 (
p = 0.00926).
When isolating antibiotic prescriptions from the broader dataset, similar trends were observed (
Table 2). Antibiotics accounted for approximately 70% of all prescriptions issued by veterinarians, highlighting a remarkably high proportion. Prescription volumes showed a progressive increase over the years, with 2020 and 2021 being significantly higher compared to 2017 (
p = 0.000161 and
p = 0.000544) and 2018 (
p = 0.001354 and
p = 0.013044).
The most substantial annual growth occurred between 2019 and 2020, with a 12% increase, although this difference was marginally non-significant (p = 0.0667). Monthly peaks were particularly notable between July 2019 and July 2020 (17%) and March 2020 and March 2021 (18%), reflecting the impact of the COVID-19 pandemic on veterinary prescribing patterns.
Although the absolute number of prescriptions in 2021 was slightly lower than in 2020 mainly due to missing data for December, the monthly average remained 2% higher. No significant difference was observed between 2020 and 2021 (p = 0.959), indicating that the elevated prescription levels achieved during the pandemic were sustained.
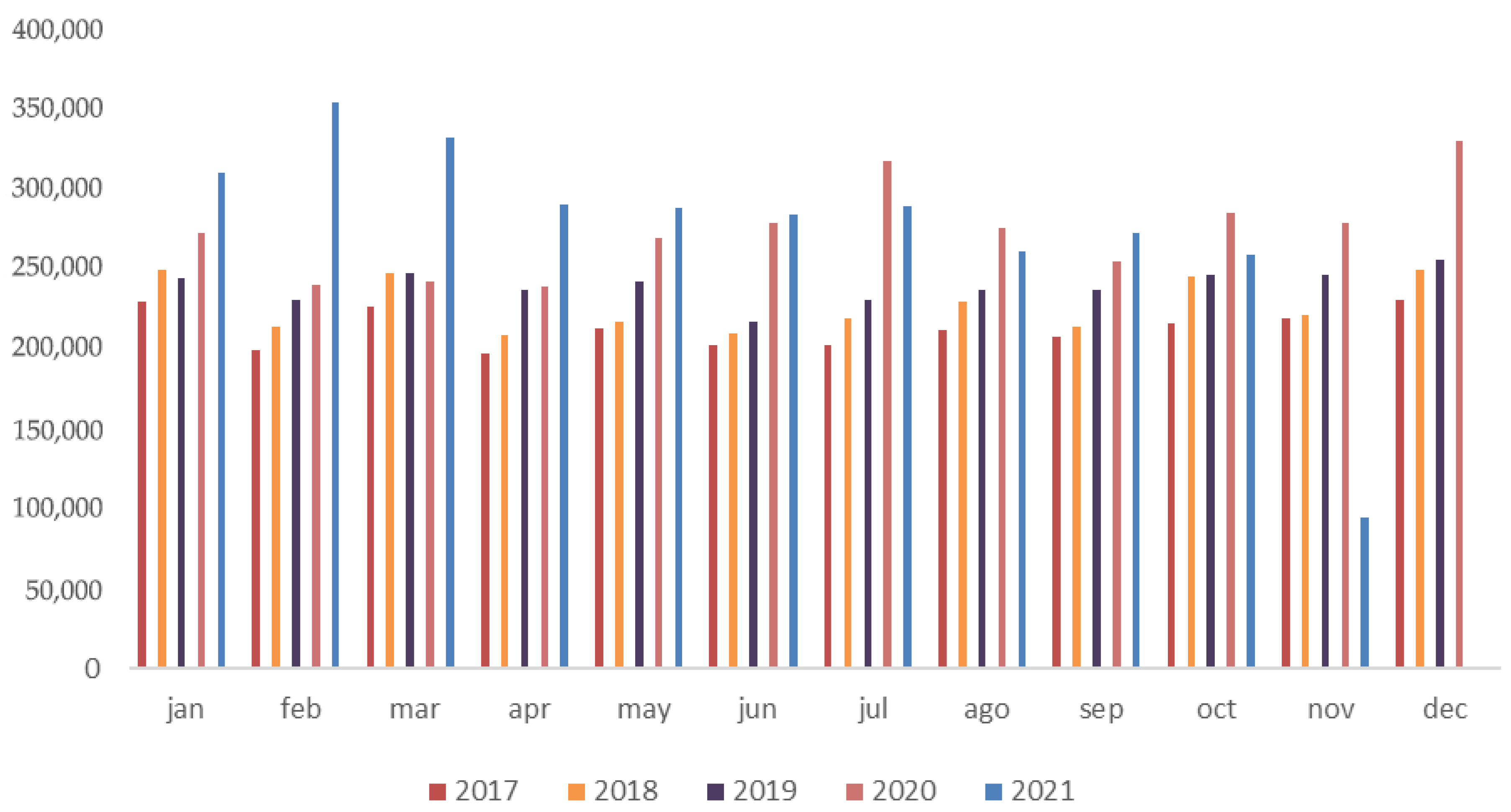
Figure 1 displays the total number of antibiotic units (boxes, vials, or packages) prescribed by veterinarians and purchased in human pharmacies in Brazil from 2017 to 2021, on a monthly basis. The descriptive analysis shows progressive increases of 4.46%, 5.29%, and 9.25% in monthly totals between 2017 and 2018, 2018 and 2019, and 2019 and 2020, respectively, followed by a reduction of 11.76% from 2020 to 2021, which corroborates the observed decline in the second half of 2021.
The most pronounced increases were observed in June, July, and December 2020, likely associated with the impact of the COVID-19 pandemic on veterinary clinical routines. Over the five-year period, there was a cumulative mean monthly increase of 24.64% when comparing 2017 to 2021. Annual totals were 2,554,757 units in 2017; 2,723,921 in 2018; 2,870,350 in 2019; 3,280,243 in 2020; and 3,032,058 in 2021, representing increases of 6.2%, 5.1%, and 12.5%, followed by a decrease of 8.2% in 2021.
These monthly variations are presented as descriptive trends, with statistical significance testing applied exclusively to annual mean values, as detailed in the
Section 3.
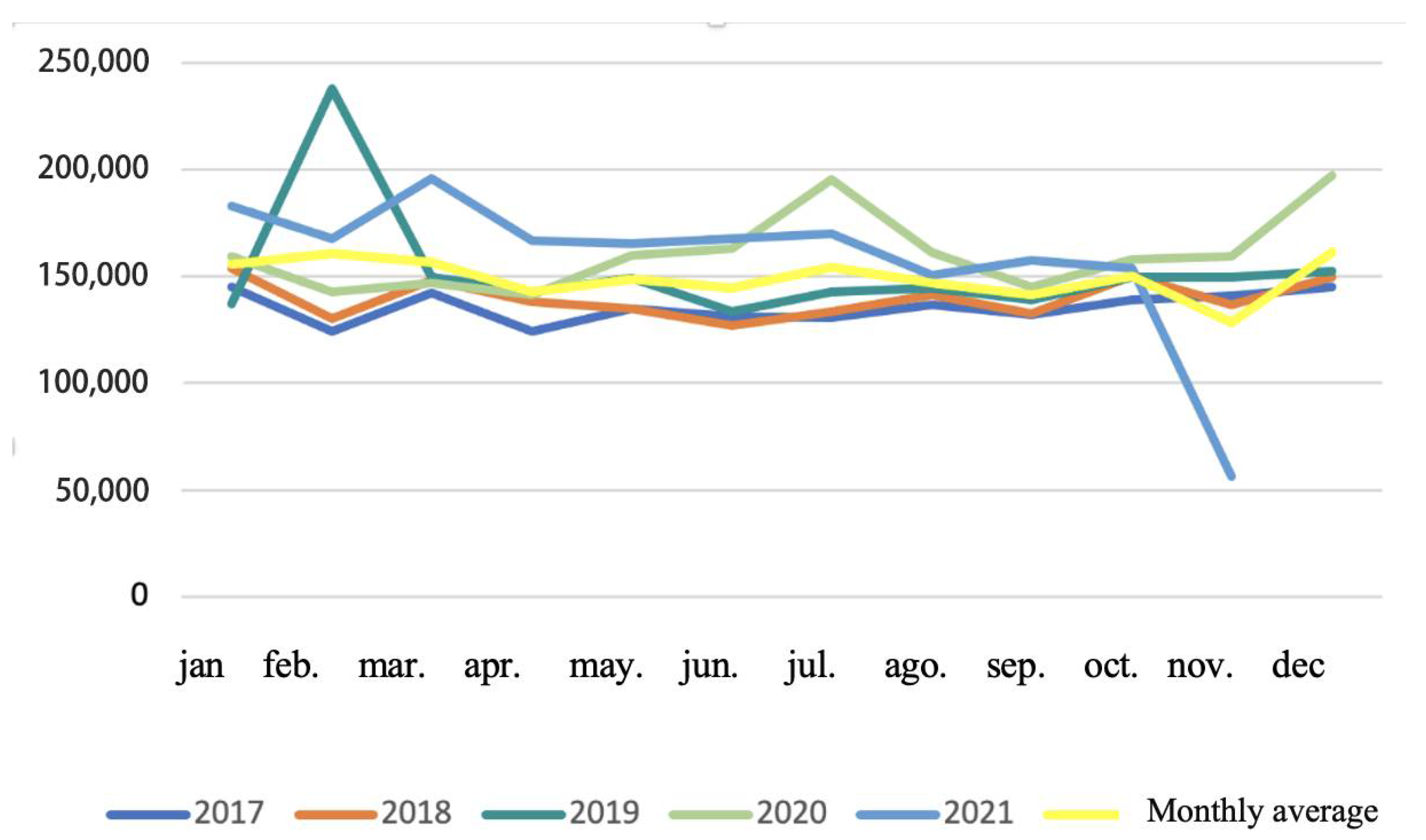
After applying the inclusion criteria to exclude non-oral and non-injectable formulations, the dataset was reduced by 35%, with oral and injectable antibiotics representing approximately 65% of total prescriptions. Although the number of prescriptions ranged from 40,000 to 62,000 monthly, individual records could include single or multiple units, with some reflecting bulk purchases for clinical stock.
Monthly trends in oral and injectable antibiotic sales showed moderate variability over time (
Figure 2). Notably, February consistently displayed higher variability across years, and significant peaks were observed in July and December 2020, likely associated with the impacts of the COVID-19 pandemic. Despite the database interruption in December 2021 and the unusually low figure for November 2021, the overall trend indicates that prescription activity remained stable over time. These patterns are presented as descriptive trends, with statistical significance testing applied only to annual mean values, as described in the
Section 3.
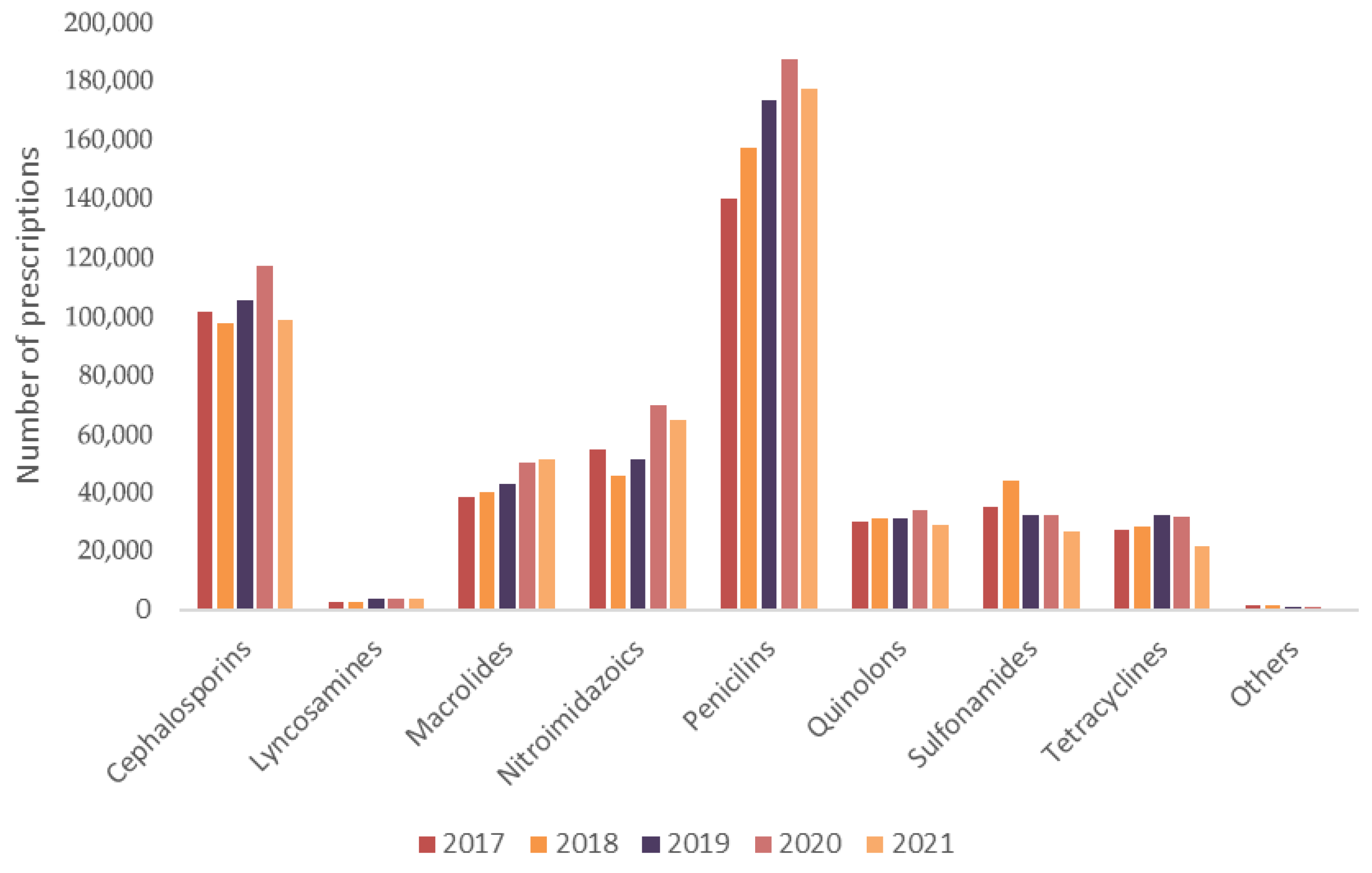
An analysis by antibiotic class revealed that penicillins (35.67%) and cephalosporins (22.08%) dominated prescription volumes, followed by nitroimidazoles (12.28%) and macrolides (9.54%). Quinolones (6.41%), sulfonamides (7.20%), and tetracyclines (5.86%) were also frequently prescribed. Less common classes included lincosamides (0.73%), amphenicols and penicillin combinations (0.10%), aminoglycosides (0.05%), and carbapenems (
n = 10), all grouped under the “Other” category in
Figure 3.
Trends over time showed a consistent increase in the use of penicillins and a notable rise in macrolide prescriptions—particularly azithromycin—during 2020 and 2021. Amoxicillin accounted for nearly 95% of all penicillin prescriptions, with more than 60% including clavulanic acid. Among cephalosporins, cephalexin represented 90% of the total. Other frequently used antibiotics included metronidazole, sulfamethoxazole + trimethoprim, ciprofloxacin, and doxycycline.
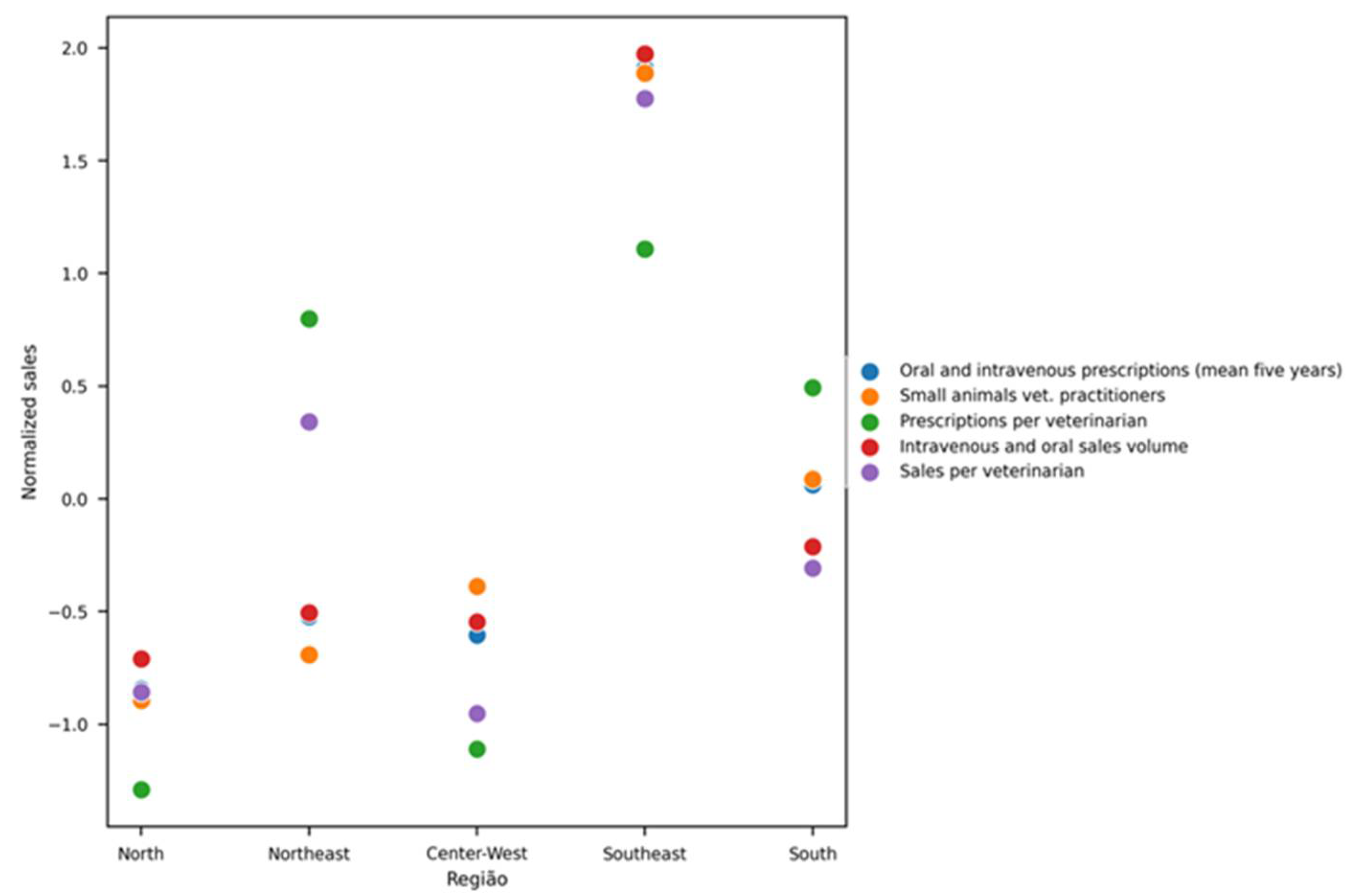
When analyzed by geographic region, the Southeast presented the highest rate of antibiotic prescriptions normalized by the number of veterinarians in small-animal practice (9.27 prescriptions per veterinarian), followed by the Northeast (8.6), South (7.94), Central-West (4.48), and North (4.09). In terms of total antibiotic units sold per veterinarian, the Southeast again led (38.27 units), followed by the Northeast (25.70), South (20.00), North (15.16), and Central-West (14.33) (
Figure 4).
These normalized results align with regional demographic data of veterinary professionals [
14], showing a higher concentration of veterinarians in the Southeast. However, an unexpected finding was the high number of prescriptions and antibiotic units sold in the Northeast, despite its relatively lower number of professionals in small-animal practice. This discrepancy suggests regional differences in access, prescription behavior, or drug sourcing practices that merit further investigation.
4. Discussion
Antimicrobial resistance (AMR) is a global health challenge, exacerbated by inappropriate antibiotic use in both human and veterinary medicine. While high-income countries have established regulations and surveillance systems, many low- and middle-income countries (LMICs) still face regulatory gaps and insufficient professional training, leading to the widespread and poorly controlled use of critical antimicrobials [
1,
2].
Brazil represents a clear example of this scenario. Although human-use antibiotics are monitored through ANVISA’s SNGPC system, veterinary antimicrobials lack a unified prescription-monitoring policy. MAPA does not require veterinary prescriptions for all antimicrobial classes, and no classes are explicitly prohibited for veterinary use. In contrast, the European Union has adopted WHO categorizations to restrict the veterinary use of antibiotics critical to human health [
15], highlighting a regulatory disparity that may directly influence resistance patterns.
Our findings show a predominant use of penicillins, cephalosporins, and macrolides in companion animals, classes that are commonly used in human medicine and classified by the WHO as important or critically important [
16]. Drugs such as azithromycin, ciprofloxacin, and cefuroxime, categorized as “watch group” antibiotics, were frequently prescribed [
16]. The use of these classes in veterinary medicine, especially without standardized diagnostic confirmation, contributes to selective pressure and the potential transmission of resistant bacteria across species [
17,
18].
This trend is not exclusive to Brazil. In other LMICs, similar patterns have been documented. In Kenya, 100% of veterinary pharmacies surveyed sold antibiotics without prescriptions [
5]. In Vietnam and Bangladesh, more than 45% of antibiotic dispensing sites operated without professional oversight [
19]. In Latin America, a survey of online pharmacies showed that 65% of websites offered antibiotics without prescription, with high availability of fluoroquinolones, macrolides, and third-/fourth-generation cephalosporins [
20].
Even in regulated systems, the overuse of broad-spectrum antibiotics persists. In Australia, 14.5% of dog consultations involved antibiotic prescriptions—38 per 1000 involving WHO high-importance drugs [
21]. In Spain, nearly 40% of antimicrobials used in pets were antimicrobials approved for human use, with amoxicillin–clavulanate as the most prescribed [
22]. Across Europe, penicillins, first-/second-generation cephalosporins, and macrolides remain the most used in companion animals [
23], aligning with the pattern observed in our study.
The high proportion of antibiotic prescriptions in Brazil’s companion animal sector may also reflect data blind spots. Veterinary industry reports [
24] suggest the declining production of veterinary antibiotics, but these figures do not include the sales of antibiotics approved for human use that were prescribed for animals. This discrepancy indicates a possible shift in procurement sources rather than an actual reduction in antimicrobial use.
It is important to note that this study does not include antibiotic sales from agro-veterinary stores, which are not captured by ANVISA’s SNGPC system. These unrecorded sales likely represent a significant portion of antimicrobial use in veterinary practice, especially in regions with limited pharmacy access. This restriction suggests that our findings may underestimate the true volume of antibiotic consumption in companion animals.
The higher prescription rates observed in the Northeast region may reflect several contributing factors. Incomplete access to veterinary-specific antibiotics in certain areas may lead veterinarians to rely more heavily on antibiotic formulations approved for human use [
18]. Additionally, economic considerations, both from the perspective of pet owners seeking more affordable options and veterinarians operating in lower-income regions, may incentivize the use of widely accessible antibiotics approved for human use [
2]. Furthermore, the sociocultural context, including owner expectations and informal dispensing practices, likely plays a role in this pattern.
Empirical prescribing, often performed without laboratory confirmation, remains widespread in veterinary practice. This approach is particularly evident in the frequent use of broad-spectrum antibiotics such as metronidazole and amoxicillin–clavulanate, which are commonly selected as first-line treatments for gastrointestinal and soft tissue infections in dogs and cats. This pattern, reported in Brazilian veterinary hospitals [
25], aligns with our findings, suggesting that treatment decisions are frequently based on clinical judgment rather than diagnostic testing. Similarly, in Chile, only 15% of veterinarians reported requesting laboratory tests before prescribing antibiotics, highlighting a broader regional trend of reliance on empirical therapy [
26]. Similar trends appear in New Zealand, South Africa, and Greece, where empirical treatment and owner influence often guide prescribing decisions [
27,
28].
These behaviors are influenced by gaps in veterinary education. Although Brazil’s National Curricular Guidelines recommend content on pharmacology and disease control [
29], implementation varies widely. In surveys from Poland, Australia, and South Africa, veterinary students consistently expressed concern over their preparedness to prescribe antibiotics responsibly [
28,
30,
31]. Moreover, studies show that veterinarians often feel pressure from pet owners to prescribe antibiotics unnecessarily [
32,
33].
It is also important to acknowledge the significant contribution of animals to biomedical research. Animal models have been essential for the development, safety assessment, and efficacy validation of antimicrobials used in both human and veterinary medicine. This contribution reinforces the One Health framework by underscoring the intrinsic interconnectedness between human, animal, and environmental health in addressing antimicrobial resistance [
34].
Within the One Health framework, our findings highlight the critical need for integrated policies that address the interconnected risks of antimicrobial resistance (AMR) across human, animal, and environmental health. The patterns observed in Brazil, although geographically specific, reflect structural challenges common to many low- and middle-income countries (LMICs), in which fragmented regulatory oversight and limited surveillance compromise AMR control efforts. Addressing this issue requires coordinated actions that go beyond veterinary clinical practice, involving stringent prescription regulations, comprehensive surveillance systems that include both human and animal sectors, continuing professional education for veterinarians, and public engagement to raise awareness about the prudent use of antibiotics. This integrated approach is essential to mitigate the contribution of companion animal antimicrobial use to the broader AMR crisis.