In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Isolation of 6PP
2.2. Cell Culture and Virus
2.3. Viral Titration
2.4. Cytotoxicity Assay
2.5. TBARS Assay
2.6. Viricidal Activity Assay
2.7. Antiviral Activity Assays
2.7.1. Protocol A: Cell Protection After Viral Infection
- (1)
- The IC20 concentration of the 6PP was added to the cell monolayers, and plates were incubated for 72 h at 37 °C.
- (2)
- The IC20 concentration of the 6PP was added to the cell monolayers, and plates were incubated for 3 h at 37 °C. Following incubation, the compound was removed, the wells were washed with DMEM, and then, replaced with 1 mL of DMEM, and the plates were further incubated for 72 h at 37 °C.
2.7.2. Protocol B: Cell Protection Before Viral Infection
2.7.3. Protocol C: Viral Internalization Inhibition Assay
2.8. Fluorescence Quantification
2.9. RT-qPCR for BCoV
2.10. Data Analysis
3. Results
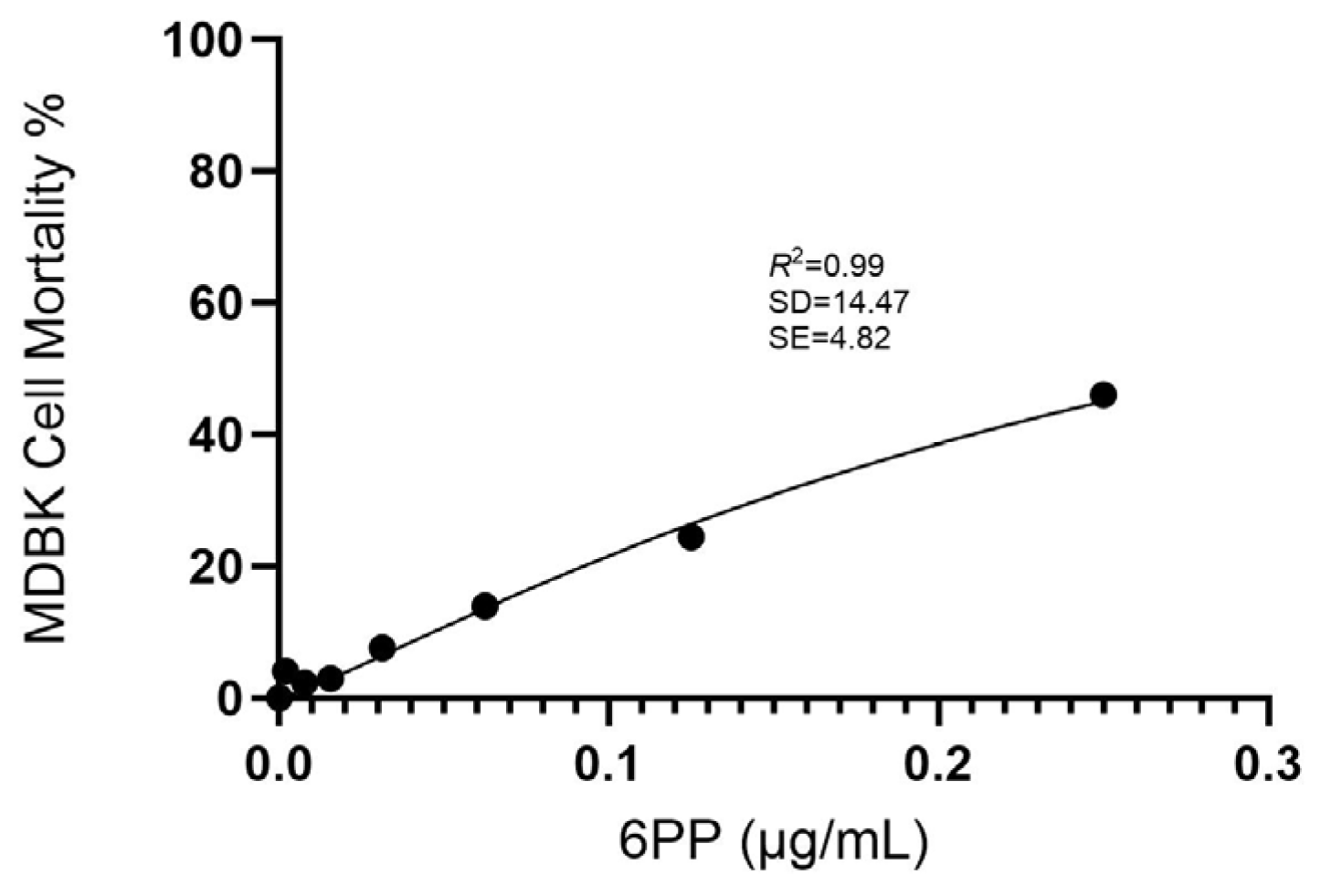
3.1. Cytotoxicity Assay
3.2. TBARS
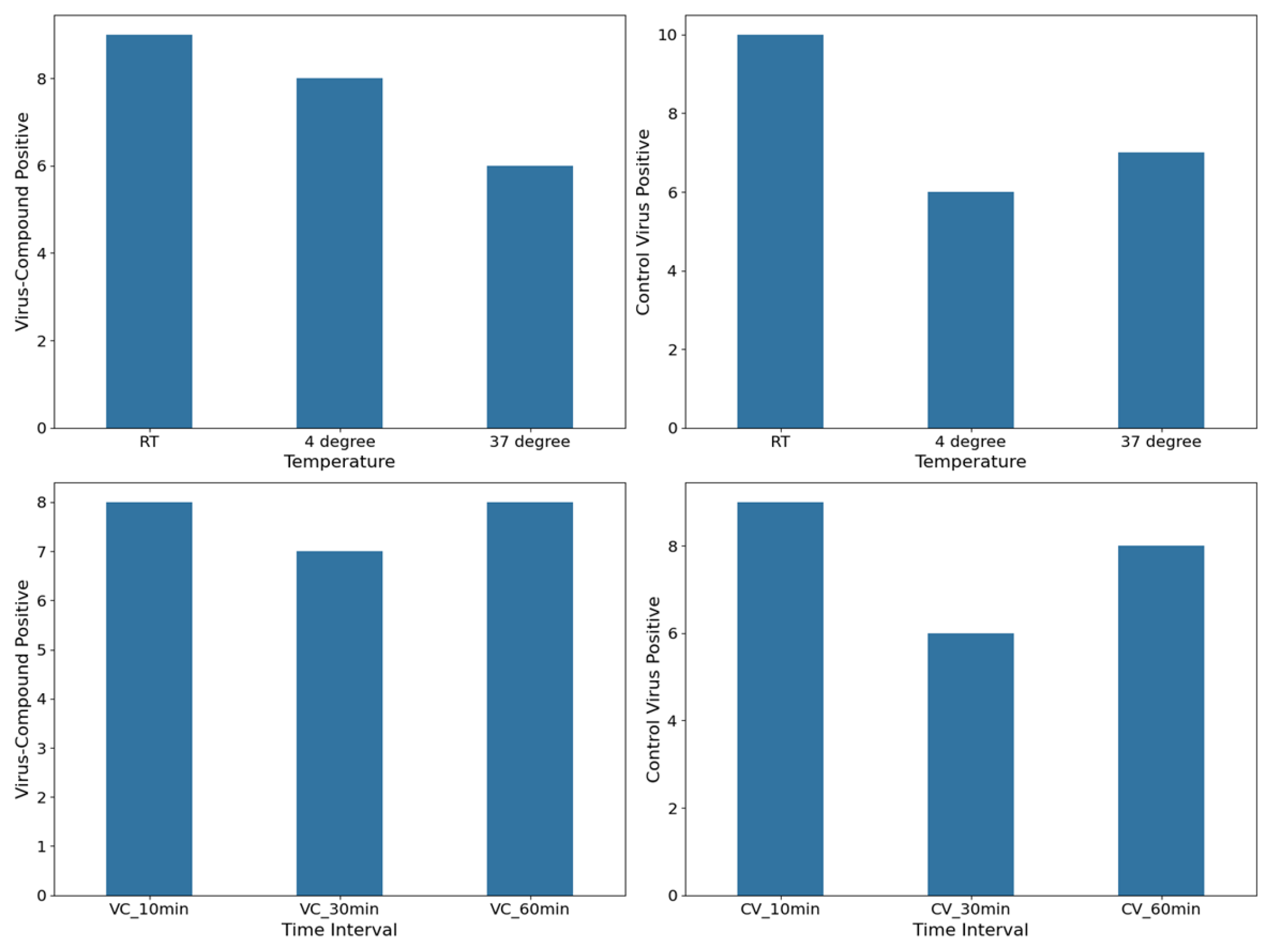
3.3. Virucidal Activity
3.4. Antiviral Activity
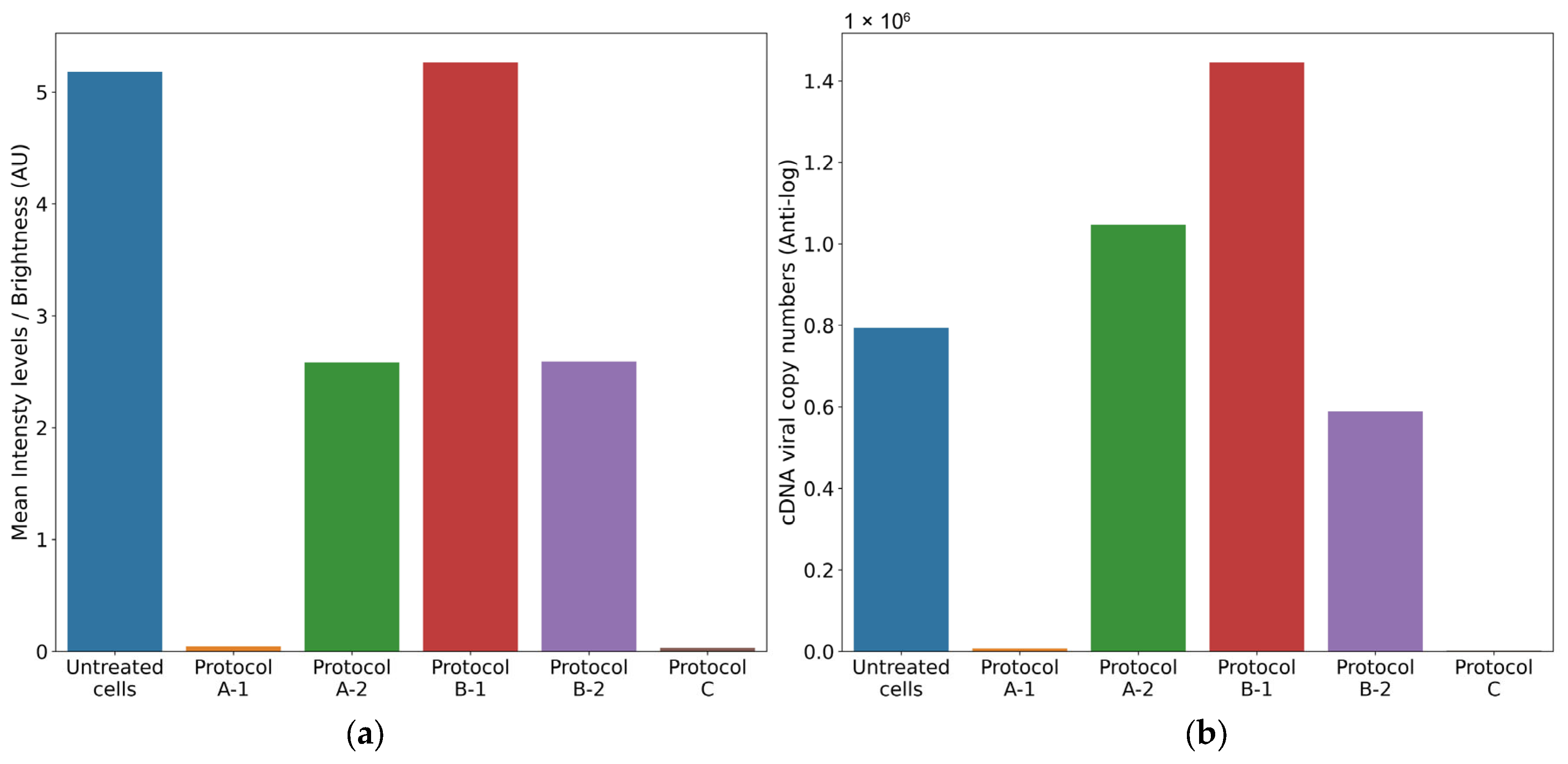
3.4.1. Protocol A: Cell Protection After Viral Infection
- (1)
- Treatment of cell monolayer with 6PP for 72 h: comparing the log10 viral cDNA copies/mL of 6PP-treated infected cells (mean = 3.85 log10 cDNA viral copy numbers) with untreated infected cells (mean = 5.9 log10 cDNA viral copy numbers), a significant decrease of 2.02 log10 (p = 0.0175) was detected in treated cells. Similarly, a statistically significant difference in mean fluorescence was observed between infected cells treated with 6PP for 72 h (mean = 0.04 brightness intensity levels) and untreated infected cells (mean = 5.18 brightness intensity levels) (p = 0.0169).
- (2)
- Treatment of cell monolayer with 6PP for 3 h: comparing the log10 viral cDNA copies/mL of 6PP-treated infected cells (mean = 6.02 log10 cDNA viral copy numbers) with untreated infected cells (mean = 5.9 log10 cDNA viral copy numbers), a difference of 0.12 log10 was observed without any statistical significance (p = 0.4465). Similarly, the mean fluorescence of the cell monolayer treated with 6PP for 3 h (mean = 2.58 brightness intensity levels) and untreated infected cells (mean = 5.18 brightness intensity levels) was not significantly different (p = 0.127).
3.4.2. Protocol B: Cell Protection Before Viral Infection
- (1)
- Treatment with 6PP at 4 °C: the log10 viral cDNA copies/mL of 6PP-treated infected cells (mean = 6.16 log10 cDNA viral copy numbers) compared with the untreated infected cells (mean = 5.9 log10 cDNA viral copy numbers) revealed a 0.26 log10 difference, although without any statistical significance (p = 0.0924). This is consistent with the fluoroscopy quantification method, which did not reveal any statistical significance (p = 0.964) between the infected cells treated with 6PP at 4 °C versus untreated infected cells, with computed mean pixels of 5.26 and 5.18 brightness intensity levels, respectively.
- (2)
- Treatment with 6PP at 37 °C: when comparing the log10 viral cDNA copies/mL of 6PP-treated infected cells (mean = 5.77 log10 cDNA viral copy numbers) with untreated infected cells (mean = 5.9 log10 cDNA viral copy numbers), no statistically significant difference was observed (p = 0.4223). Consistent with these results, the fluorescence quantification technique did not detect any significant difference (p = 0.147) in the mean fluorescence between infected cells treated with 6PP at 37 °C (mean = 2.59 brightness intensity levels) and untreated infected cells (mean = 5.18 brightness intensity levels).
3.4.3. Protocol C: Viral Internalization Inhibition Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saif, L.J.; Jung, K.; McAdam, A.J. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J. Clin. Microbiol. 2020, 58, e01355-20. [Google Scholar] [CrossRef] [PubMed]
- Faustino, R.; Faria, M.; Teixeira, M.; Palavra, F.; Sargento, P.; Do Céu Costa, M. Systematic review and meta-analysis of the prevalence of coronavirus: One health approach for a global strategy. One Health 2022, 14, 100383. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Sun, D. Advances in Bovine Coronavirus Epidemiology. Viruses 2022, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, J.J.; Ježek, J.; Starič, J. Coronaviruses in cattle. Trop. Anim. Health Prod. 2020, 52, 2809–2816. [Google Scholar] [CrossRef]
- Padalino, B.; Cirone, F.; Zappaterra, M.; Tullio, D.; Ficco, G.; Giustino, A.; Ndiana, L.A.; Pratelli, A. Factors Affecting the Development of Bovine Respiratory Disease: A Cross-Sectional Study in Beef Steers Shipped From France to Italy. Front. Vet. Sci. 2021, 8, 627894. [Google Scholar] [CrossRef]
- Pratelli, A.; Lucente, M.S.; Cordisco, M.; Ciccarelli, S.; Di Fonte, R.; Sposato, A.; Mari, V.; Capozza, P.; Pellegrini, F.; Carelli, G.; et al. Natural Bovine Coronavirus Infection in a Calf Persistently Infected with Bovine Viral Diarrhea Virus: Viral Shedding, Immunological Features and S Gene Variations. Animals 2021, 11, 3350. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Saif, L.J. Bovine Coronavirus and the Associated Diseases. Front. Vet. Sci. 2021, 8, 643220. [Google Scholar] [CrossRef]
- Boileau, M.J.; Kapil, S. Bovine Coronavirus Associated Syndromes. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 123–146. [Google Scholar] [CrossRef]
- Clark, M.A. Bovine coronavirus. Br. Vet. J. 1993, 149, 51–70. [Google Scholar] [CrossRef]
- Saif, L.J. Bovine Respiratory Coronavirus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef]
- Reynolds, D.J.; Debney, T.G.; Hall, G.A.; Thomas, L.H.; Parsons, K.R. Studies on the relationship between coronaviruses from the intestinal and respiratory tracts of calves. Arch. Virol. 1985, 85, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Martínez-Salas, E.; Sobrino, F.; de la Torre, J.C.; Portela, A.; Ortín, J.; López-Galindez, C.; Pérez-Breña, P.; Villanueva, N.; Nájera, R.; et al. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: Biological relevance—A review. Gene 1985, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, B.; Soggiu, A.; Musella, V.; Britti, D.; Sanguinetti, M.; Urbani, A.; Roncada, P. Molecular basis of COVID-19 relationships in different species: A one health perspective. Microbes Infect. 2020, 22, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, B.; Soggiu, A.; Sanguinetti, M.; Musella, V.; Britti, D.; Bonizzi, L.; Urbani, A.; Roncada, P. Comparative computational analysis of SARS-CoV-2 nucleocapsid protein epitopes in taxonomically related coronaviruses. Microbes Infect. 2020, 22, 188–194. [Google Scholar] [CrossRef]
- Domańska-Blicharz, K.; Woźniakowski, G.; Konopka, B.; Niemczuk, K.; Welz, M.; Rola, J.; Socha, W.; Orłowska, A.; Antas, M.; Śmietanka, K.; et al. Animal coronaviruses in the light of COVID-19. J. Vet. Res. 2020, 64, 333–345. [Google Scholar] [CrossRef]
- Van De Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef]
- Tian, W.J.; Wang, X.J. Broad-Spectrum Antivirals Derived from Natural Products. Viruses 2023, 15, 1100. [Google Scholar] [CrossRef]
- Aggarwal, V.; Bala, E.; Kumar, P.; Raizada, P.; Singh, P.; Verma, P.K. Natural Products as Potential Therapeutic Agents for SARS-CoV-2: AMedicinal Chemistry Perspective. Curr. Top. Med. Chem. 2023, 23, 1664–1698. [Google Scholar] [CrossRef]
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52. [Google Scholar] [CrossRef]
- Salvatore, M.M.; DellaGreca, M.; Andolfi, A.; Nicoletti, R. New Insights into Chemical and Biological Properties of Funicone-like Compounds. Toxins 2022, 14, 466. [Google Scholar] [CrossRef]
- Raihan, T.; Rabbee, M.F.; Roy, P.; Choudhury, S.; Baek, K.H.; Azad, A.K. Microbial Metabolites: The Emerging Hotspot of Antiviral Compounds as Potential Candidates to Avert Viral Pandemic Alike COVID-19. Front. Mol. Biosci. 2021, 8, 732256. [Google Scholar] [CrossRef] [PubMed]
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of Bioactive Fungal Secondary Metabolites: Cytotoxic and Antimicrobial Compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Agrawal, S.; Gupta, M.K.; Patidar, R.K.; Ranjan, N. Recent Advances in the Discovery of Antiviral Metabolites from Fungi. Curr. Pharm. Biotechnol. 2022, 23, 495–537. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Barbosa, B.V.R.; Lima, M.T.N.S.; Cardoso, P.G.; Contigli, C.; Pimenta, L.P.S. Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorg Med. Chem. 2021, 46, 116366. [Google Scholar] [CrossRef]
- Cerracchio, C.; Del Sorbo, L.; Serra, F.; Staropoli, A.; Amoroso, M.G.; Vinale, F.; Fiorito, F. Fungal metabolite 6-pentyl-α-pyrone reduces canine coronavirus infection. Heliyon 2024, 10, e28351. [Google Scholar] [CrossRef]
- Nakajima, S.; Watashi, K.; Kamisuki, S.; Tsukuda, S.; Takemoto, K.; Matsuda, M.; Suzuki, R.; Aizaki, H.; Sugawara, F.; Wakita, T. Specific inhibition of hepatitis C virus entry into host hepatocytes by fungi-derived sulochrin and its derivatives. Biochem. Biophys. Res. Commun. 2013, 440, 515–520. [Google Scholar] [CrossRef]
- Nzimande, B.; Makhwitine, J.P.; Mkhwanazi, N.P.; Ndlovu, S.I. Developments in Exploring Fungal Secondary Metabolites as Antiviral Compounds and Advances in HIV-1 Inhibitor Screening Assays. Viruses 2023, 15, 1039. [Google Scholar] [CrossRef]
- Guo, Y.-W.; Liu, X.-J.; Yuan, J.; Li, H.-J.; Mahmud, T.; Hong, M.-J.; Yu, J.-C.; Lan, W.-J. l-Tryptophan Induces a Marine-Derived Fusarium sp. to Produce Indole Alkaloids with Activity against the Zika Virus. J. Nat. Prod. 2020, 83, 3372–3380. [Google Scholar] [CrossRef]
- Thaisrivongs, S.; Romero, D.L.; Tommasi, R.A.; Janakiraman, M.N.; Strohbach, J.W.; Turner, S.R.; Biles, C.; Morge, R.R.; Johnson, P.D.; Aristoff, P.A.; et al. Structure-Based Design of HIV Protease Inhibitors: 5,6-Dihydro-4-hydroxy-2-pyrones as Effective, Nonpeptidic Inhibitors. J. Med. Chem. 1996, 39, 4630–4642. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Sujarit, K.; Pattananandecha, T.; Saenjum, C.; Lumyong, S. Natural Bioactive Compounds from Fungi as Potential Candidates for Protease Inhibitors and Immunomodulators to Apply for Coronaviruses. Molecules 2020, 25, 1800. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Marra, R.; Barbetti, M.; Li, H.; Woo, S.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Staropoli, A.; Iacomino, G.; De Cicco, P.; Woo, S.L.; Di Costanzo, L.; Vinale, F. Induced secondary metabolites of the beneficial fungus Trichoderma harzianum M10 through OSMAC approach. Chem. Biol. Technol. Agric. 2023, 10, 28. [Google Scholar] [CrossRef]
- Fiorito, F.; Marfè, G.; De Blasio, E.; Granato, G.E.; Tafani, M.; De Martino, L.; Montagnaro, S.; Florio, S.; Pagnini, U. 2,3,7,8-Tetrachlorodibenzo-p-dioxin regulates Bovine Herpesvirus type 1 induced apoptosis by modulating Bcl-2 family members. Apoptosis 2008, 13, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; pp. 302–310. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0076687978520326 (accessed on 15 May 2025).
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Jones, E.; Oliphant, T.; Peterson, P. SciPy: Open Source Scientific Tools for Python. ScienceOpen. 2001. Available online: https://www.researchgate.net/publication/213877848_SciPy_Open_Source_Scientific_Tools_for_Python (accessed on 21 May 2025).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach Learn Res. 2012, 12, 2825–2830. [Google Scholar]
- Rossum, G. Python Reference Manual; Centrum voor Wiskunde en Informatica: Amsterdam, NY, USA, 1995. [Google Scholar]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference (SciPy 2010), Austin, TX, USA, 3–28 July 2010; pp. 92–96. Available online: https://doi.curvenote.com/10.25080/Majora-92bf1922-011 (accessed on 28 April 2025).
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Hao, J.; Wuyun, D.; Xi, X.; Dong, B.; Wang, D.; Quan, W.; Zhang, Z.; Zhou, H. Application of 6-Pentyl-α-Pyrone in the Nutrient Solution Used in Tomato Soilless Cultivation to Inhibit Fusarium oxysporum HF-26 Growth and Development. Agronomy 2023, 13, 1210. [Google Scholar] [CrossRef]
- Lim, J.S.; Hong, J.-H.; Lee, D.Y.; Li, X.; Lee, D.E.; Choi, J.U.; Lee, K.Y.; Kim, K.H.; Cho, Y.-C. 6-Pentyl-α-Pyrone from Trichoderma gamsii Exert Antioxidant and Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Mouse Macrophages. Antioxidants 2023, 12, 2028. [Google Scholar] [CrossRef]
- Minesso, S.; Odigie, A.E.; Franceschi, V.; Cotti, C.; Cavirani, S.; Tempesta, M.; Donofrio, G. A Simple and Versatile Method for Ex Vivo Monitoring of Goat Vaginal Mucosa Transduction by Viral Vector Vaccines. Vaccines 2024, 12, 851. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; León-Juárez, M.; Lira-Hernández, F.I.; Rivas-Santiago, B.; Velázquez-Cervantes, M.A.; Méndez-Delgado, I.M.; Macías-Guerrero, D.I.; Hernández-Castillo, J.; Hernández-Rodríguez, X.; Calderón-Sandate, D.N.; et al. Advances and Challenges in Antiviral Development for Respiratory Viruses. Pathogens 2024, 14, 20. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L.; et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell 2020, 182, 1284–1294.e9. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasinioti, V.I.; Odigie, A.E.; Lucente, M.S.; Del Sorbo, L.; Catella, C.; Casalino, E.; Salvatore, M.M.; Staropoli, A.; Vinale, F.; Tempesta, M.; et al. In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2. Vet. Sci. 2025, 12, 634. https://doi.org/10.3390/vetsci12070634
Vasinioti VI, Odigie AE, Lucente MS, Del Sorbo L, Catella C, Casalino E, Salvatore MM, Staropoli A, Vinale F, Tempesta M, et al. In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2. Veterinary Sciences. 2025; 12(7):634. https://doi.org/10.3390/vetsci12070634
Chicago/Turabian StyleVasinioti, Violetta Iris, Amienwanlen Eugene Odigie, Maria Stella Lucente, Luca Del Sorbo, Cristiana Catella, Elisabetta Casalino, Maria Michela Salvatore, Alessia Staropoli, Francesco Vinale, Maria Tempesta, and et al. 2025. "In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2" Veterinary Sciences 12, no. 7: 634. https://doi.org/10.3390/vetsci12070634
APA StyleVasinioti, V. I., Odigie, A. E., Lucente, M. S., Del Sorbo, L., Catella, C., Casalino, E., Salvatore, M. M., Staropoli, A., Vinale, F., Tempesta, M., Fiorito, F., Andolfi, A., Buonavoglia, A., Pratelli, A., & Capozza, P. (2025). In Vitro Antiviral Activity of the Fungal Metabolite 6-Pentyl-α-Pyrone Against Bovine Coronavirus: A Translational Study to SARS-CoV-2. Veterinary Sciences, 12(7), 634. https://doi.org/10.3390/vetsci12070634













