Starch Digestion Characteristics of Different Starch Sources and Their Effects on Goslings’ Apparent Nutrient Utilization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Extraction and Purification of Starch
2.1.2. In Vitro Starch Digestion
2.2. In Vivo Study
2.2.1. Experimental Design
2.2.2. Continuous Blood Glucose Monitoring
2.2.3. Nutrient Utilization Rate
2.2.4. Determination Method
2.3. Statistical Analysis
3. Results
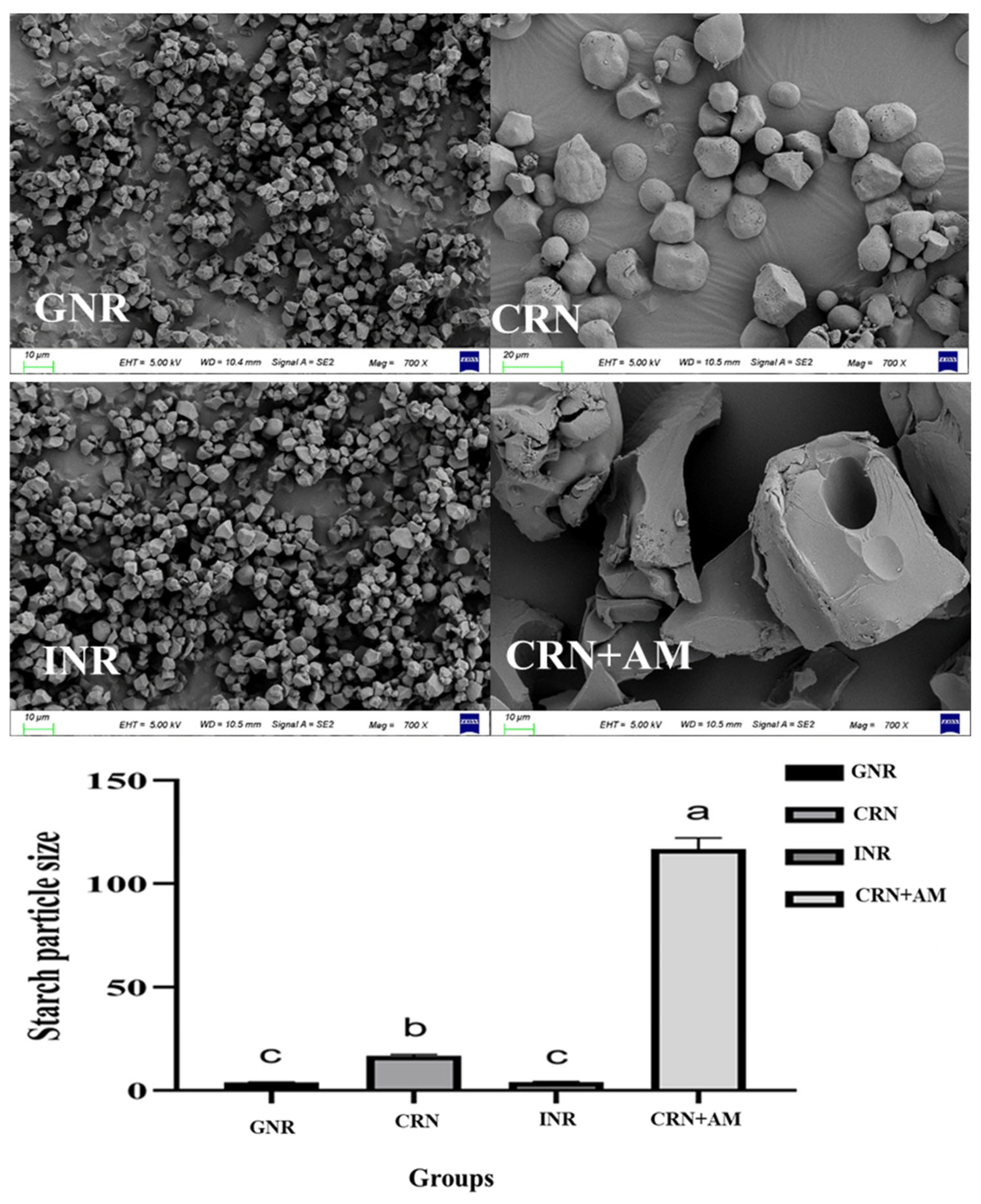
3.1. Starch Particle Size and Morphology
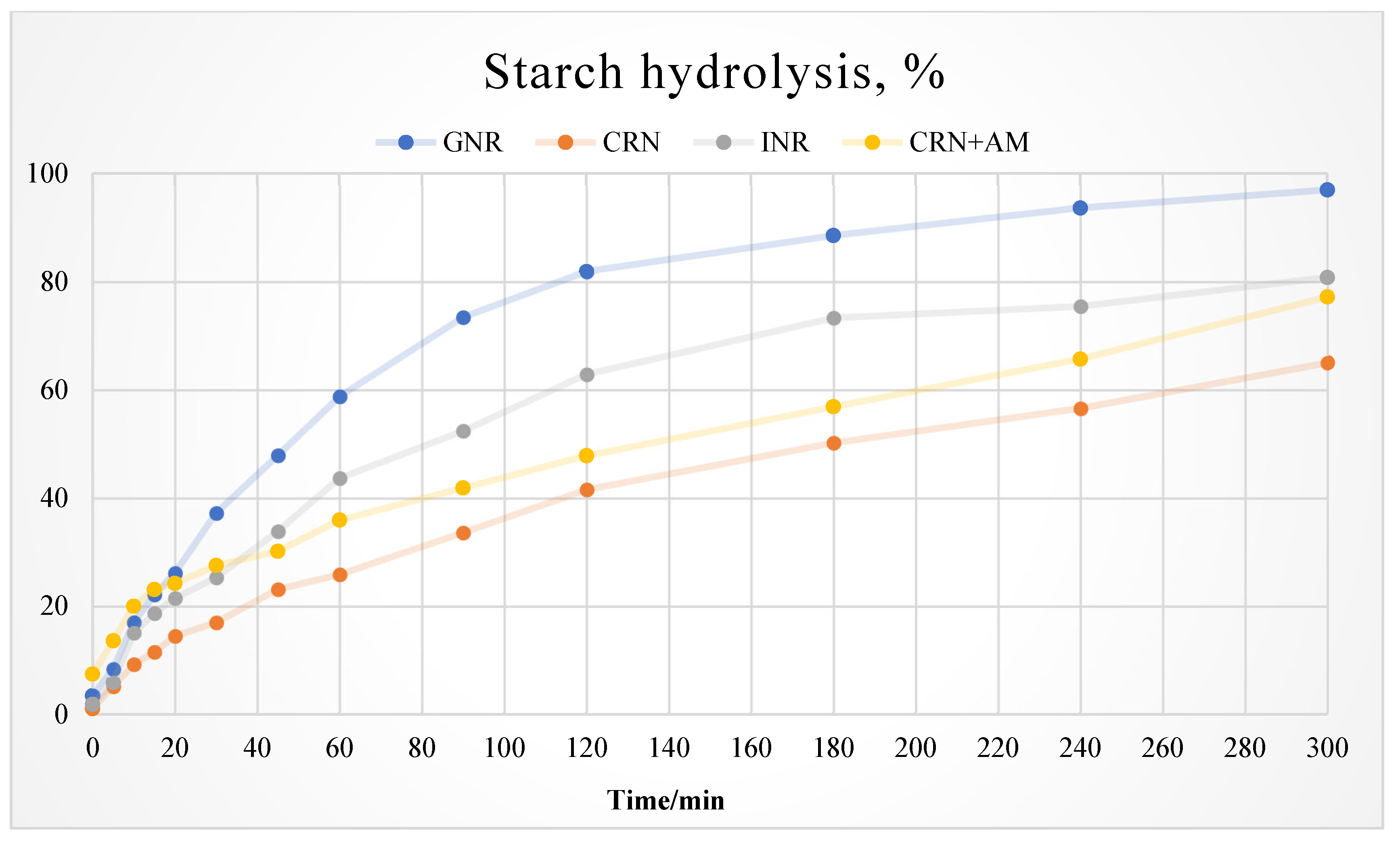
3.2. In Vitro Starch Digestion Rate
3.3. In Vivo Blood Glucose Monitoring
3.4. Growth Performance
3.5. Nutrient Digestibility
3.6. Amino Acid Availability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Punia, S. Barley Starch: Structure, Properties and in Vitro Digestibility—A Review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Hallfrisch, J. Plasma Glucose and Insulin Reduction after Consumption of Breads Varying in Amylose Content. Eur. J. Clin. Nutr. 2002, 56, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Nadia, J.; Olenskyj, A.G.; Subramanian, P.; Hodgkinson, S.; Stroebinger, N.; Estevez, T.G.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Influence of Food Macrostructure on the Kinetics of Acidification in the Pig Stomach after the Consumption of Rice- and Wheat-Based Foods: Implications for Starch Hydrolysis and Starch Emptying Rate. Food Chem. 2022, 394, 133410. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Wu, Y.; Ouyang, J. Factors Influencing the Starch Digestibility of Starchy Foods: A Review. Food Chem. 2023, 406, 135009. [Google Scholar] [CrossRef]
- Nadia, J.; Bronlund, J.; Singh, R.P.; Singh, H.; Bornhorst, G.M. Structural Breakdown of Starch-Based Foods during Gastric Digestion and Its Link to Glycemic Response: In Vivo and in Vitro Considerations. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2660–2698. [Google Scholar] [CrossRef]
- Qadir, N.; Wani, I.A. In-Vitro Digestibility of Rice Starch and Factors Regulating Its Digestion Process: A Review. Carbohydr. Polym. 2022, 291, 119600. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Xue, Y.; Shen, Q. In Vitro Starch Digestibility and Estimated Glycemic Index of Mung Bean (Vigna radiata L.) as Affected by Endogenous Proteins and Lipids, and Exogenous Heat-Processing Methods. Plant Foods Hum. Nutr. 2020, 75, 547–552. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Tu, Q.; Yang, H. Glucose and Amino Acid in Enterocyte: Absorption, Metabolism and Maturation. Front. Biosci.- Landmark 2018, 23, 1721–1739. [Google Scholar] [CrossRef]
- Pennisi, M.; Arfuso, F.; Giudice, E.; Giannetto, C.; Bruschetta, G.; Piccione, G.; Fiore, E. Saccharomyces Cerevisiae Diet Supplementation Influences Haematological Parameters in Healthy Steers. Large Anim. Rev. 2023, 29, 113–117. [Google Scholar]
- Van Den Borne, J.J.G.C.; Schrama, J.W.; Heetkamp, M.J.W.; Verstegen, M.W.A.; Gerrits, W.J.J. Synchronising the Availability of Amino Acids and Glucose Increases Protein Retention in Pigs. Animal 2007, 1, 666–674. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and Measurement of Nutritionally Important Starch Fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Yang, Z.; Xu, C.; Wang, W.; Xu, X.; Yang, H.M.; Wang, Z.Y.; Rose, S.P.; Pirgozliev, V. Dietary Amylose and Amylopectin Ratio Changes Starch Digestion and Intestinal Microbiota Diversity in Goslings. Br. Poult. Sci. 2022, 63, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, H.; Yang, H.M.; Wang, Z.Y. Effects of Dietary Paddy Rice on Growth Performance, Carcass Traits, Bare Skin Color, and Nutrient Digestibility in Geese. Poult. Sci. 2022, 101, 101865. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, Z.; Yang, Z.F.; He, X.X.; Zhang, C.Y.; Yang, H.M.; Rose, S.P.; Wang, Z.Y. Effects of Different Dietary Starch Sources on Growth and Glucose Metabolism of Geese. Poult. Sci. 2023, 102, 102362. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. In Vitro Digestibility of Starches with Different Crystalline Polymorphs at Low α-Amylase Activity to Substrate Ratio. Food Chem. 2021, 349, 129170. [Google Scholar] [CrossRef]
- Oh, J.H.; Chung, J.O.; Lee, C.Y.; Yun, Y.; Park, M.Y.; Hong, Y.D.; Kim, W.G.; Cha, H.Y.; Shin, K.S.; Hong, G.P.; et al. Characterized Polysaccharides from Green Tea Inhibited Starch Hydrolysis and Glucose Intestinal Uptake by Inducing Microstructural Changes of Wheat Starch. J. Agric. Food Chem. 2021, 69, 14075–14085. [Google Scholar] [CrossRef]
- Lee, Y.E.; Yoo, S.H.; Chung, J.O.; Park, M.Y.; Hong, Y.D.; Park, S.H.; Park, T.S.; Shim, S.M. Hypoglycemic Effect of Soluble Polysaccharide and Catechins from Green Tea on Inhibiting Intestinal Transport of Glucose. J. Sci. Food Agric. 2020, 100, 3979–3986. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, J.; Hu, J.; Li, X.; Du, X. Susceptibility of Glutinous Rice Starch to Digestive Enzymes. Carbohydr. Polym. 2015, 128, 154–162. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, S.; Chen, D.; Zhu, X.; Yuan, H. Structure Characterization and Hypoglycemic Effects of Dual Modified Resistant Starch from Indica Rice Starch. Carbohydr. Polym. 2014, 103, 81–86. [Google Scholar] [CrossRef]
- Tatsumi, H.; Katano, H.; Ikeda, T. Kinetic Analysis of Glucoamylase-Catalyzed Hydrolysis of Starch Granules from Various Botanical Sources. Biosci. Biotechnol. Biochem. 2007, 71, 946–950. [Google Scholar] [CrossRef]
- Hasjim, J.; Lavau, G.C.; Gidley, M.J.; Gilbert, R.G. In Vivo and in Vitro Starch Digestion: Are Current in Vitro Techniques Adequate? Biomacromolecules 2010, 11, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Eskandari, R.; Jones, K.; Reddy, K.R.; Quezada-Calvillo, R.; Nichols, B.L.; Rose, D.R.; Hamaker, B.R.; Pinto, B.M. Modulation of Starch Digestion for Slow Glucose Release through “Toggling” of Activities of Mucosal α-Glucosidases. J. Biol. Chem. 2012, 287, 31929–31938. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, G. Oxidation of Amino Acids, Glucose, and Fatty Acids as Metabolic Fuels in Enterocytes of Developing Pigs. Amino Acids 2022, 54, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Furukawa, K.; Bailey, C.A.; Wu, G. Oxidation of Amino Acids, Glucose, and Fatty Acids as Metabolic Fuels in Enterocytes of Post-Hatching Developing Chickens. J. Anim. Sci. 2022, 100, skac053. [Google Scholar] [CrossRef]
- Tu, J.; Chen, Q.; Zhou, J.; Fan, Y.; Li, Y.; Ma, Y.; Zeng, X.; Qiao, S.; Cai, S. Characteristics of Amino Acid and Glucose Digestion and Metabolism in Energy and Protein Feedstuffs for Pigs. Animals 2025, 15, 1510. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318. [Google Scholar] [CrossRef]
- Weurding, R.E.; Enting, H.; Verstegen, M.W.A. The Effect of Site of Starch Digestion on Performance of Broiler Chickens. Anim. Feed Sci. Technol. 2003, 110, 175–184. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, C.; Ran, T.; Jiao, J.; Liu, Y.; Tan, Z.; Tang, S.; Kang, J.; Xie, J.; Chen, L.; et al. Dietary Amylose:Amylopectin Ratio Influences the Expression of Amino Acid Transporters and Enzyme Activities for Amino Acid Metabolism in the Gastrointestinal Tract of Goats. Br. J. Nutr. 2022, 127, 1121–1131. [Google Scholar] [CrossRef]
- Zhu, J.; Schwörer, S.; Berisa, M.; Kyung, Y.J.; Ryu, K.W.; Yi, J.; Jiang, X.; Cross, J.R.; Thompson, C.B. Mitochondrial NADP(H) Generation Is Essential for Proline Biosynthesis. Science 2021, 372, 968–972. [Google Scholar] [CrossRef]
- Phang, J.M.; Liu, W.; Hancock, C.; Christian, K.J. The Proline Regulatory Axis and Cancer. Front. Oncol. 2012, 2, 60. [Google Scholar] [CrossRef]
- Silao, F.G.S.; Ward, M.; Ryman, K.; Wallström, A.; Brindefalk, B.; Udekwu, K.; Ljungdahl, P.O. Mitochondrial Proline Catabolism Activates Ras1/CAMP/PKA-Induced Filamentation in Candida Albicans. PLoS Genet. 2019, 15, e1007976. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ichikawa, H.; Fujita, T.; Takemura, S.; Takagi, T.; Osada-Oka, M.; Minamiyama, Y. Histidine and Arginine Modulate Intestinal Cell Restitution via Transforming Growth Factor-Β1. Eur. J. Pharmacol. 2019, 850, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, R.; Chen, X.; He, M.; Zhang, Y.; Chen, S. Micelles Based on Lysine, Histidine, or Arginine: Designing Structures for Enhanced Drug Delivery. Front. Bioeng. Biotechnol. 2021, 9, 744657. [Google Scholar] [CrossRef] [PubMed]

| Items 1 | Groups | |||
|---|---|---|---|---|
| GNR | CRN | INR | CRN + AM | |
| Ingredients, % | ||||
| Corn | - | 57.60 | - | 49.40 |
| Indica rice | - | - | 53.75 | - |
| Glutinous rice | 51.00 | - | - | - |
| Amylose | - | - | - | 6.00 |
| Soybean meal | 23.20 | 24.20 | 23.45 | 25.00 |
| Rice husk | 9.60 | 9.50 | 10.10 | 9.30 |
| Bran | 12.45 | 4.90 | 9.05 | 6.40 |
| Stone powder | 1.10 | 1.10 | 0.90 | 1.20 |
| Calcium hydrogen | 1.20 | 1.10 | 1.30 | 1.10 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 |
| Lysine | - | 0.10 | - | 0.10 |
| DL-methionine | 0.15 | 0.20 | 0.15 | 0.20 |
| Premix 2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritional level 3 | ||||
| Energy (ME, MJ/kg) | 10.86 | 10.82 | 10.80 | 10.80 |
| Crude protein (%) | 15.93 | 16.18 | 16.01 | 16.14 |
| Crude fiber (%) | 6.86 | 6.87 | 6.83 | 6.80 |
| Lysine (%) | 0.91 | 0.91 | 0.90 | 0.92 |
| Methionine (%) | 0.41 | 0.43 | 0.42 | 0.43 |
| Total phosphorus (%) | 0.61 | 0.59 | 0.60 | 0.61 |
| Calcium (%) | 0.85 | 0.82 | 0.83 | 0.86 |
| Total starch (%) | 37.92 | 38.84 | 39.77 | 38.16 |
| Amylose/Amylopectin ratio | 0.021 | 0.340 | 0.456 | 0.568 |
| Item | Blood Glucose Increase Rate, mmol/L/min |
|---|---|
| Time, min | |
| s~15 2 | 0.123 a |
| 15~30 | 0.052 b |
| 30~60 | −0.009 c |
| 60~120 | −0.023 c |
| 120~180 | −0.023 c |
| 180~240 | −0.007 c |
| 240~300 | −0.011 c |
| SEM | 0.005 |
| Starch source | |
| Glutinous rice diet | 0.020 |
| Corn diet | 0.017 |
| Indica rice diet | 0.015 |
| High-amylose diet | 0.017 |
| SEM | 0.005 |
| p-value | |
| Time | <0.001 |
| Linear | <0.001 |
| Quadratic | <0.001 |
| Starch source | 0.988 |
| Time × Starch source | <0.001 |
| Time, min | Groups, mmol/L/min | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| GNR | CRN | INR | CRN + AM | |||
| s~15 4 | 0.194 a | 0.089 b | 0.112 b | 0.098 b | 0.011 | <0.001 |
| 15~30 | 0.072 a | 0.050 b | 0.044 b | 0.042 b | 0.004 | 0.013 |
| 30~60 | −0.038 c | 0.000 ab | −0.010 b | 0.012 a | 0.005 | <0.001 |
| 60~120 | −0.034 | −0.018 | −0.016 | −0.022 | 0.006 | 0.759 |
| 120~180 | −0.012 | 0.010 | −0.008 | −0.016 | 0.004 | 0.256 |
| 180~240 | −0.006 | −0.014 | −0.010 | 0.002 | 0.005 | 0.714 |
| 240~300 | −0.012 | −0.020 | −0.006 | −0.006 | 0.005 | 0.691 |
| Item, % | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| GNR | CRN | INR | CRN + AM | |||
| Total starch | 92.24 a | 89.94 b | 90.01 b | 89.30 b | 0.324 | 0.002 |
| Crude protein | 87.64 a | 81.32 b | 85.30 ab | 81.44 b | 0.882 | 0.015 |
| Crude fat | 81.76 | 83.43 | 80.73 | 83.23 | 0.874 | 0.776 |
| Item, % | Groups | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| GNR | CRN | INR | CRN + AM | |||
| Asp | 91.79 a | 87.12 b | 89.66 ab | 87.69 b | 0.565 | 0.004 |
| Thr | 88.78 a | 86.12 ab | 85.77 b | 82.63 c | 0.647 | 0.002 |
| Ser | 91.82 a | 87.54 c | 90.10 ab | 88.12 bc | 0.513 | 0.003 |
| Glu | 93.66 a | 91.40 b | 92.27 ab | 90.99 b | 0.354 | 0.024 |
| Gly | 86.35 a | 80.08 bc | 83.21 ab | 75.88 c | 1.137 | 0.001 |
| Ala | 86.42 a | 84.93 ab | 81.94 b | 81.79 b | 0.649 | 0.011 |
| Cys | 75.19 | 81.38 | 67.99 | 77.44 | 2.117 | 0.146 |
| Val | 88.01 a | 83.73 b | 83.96 b | 81.29 b | 0.742 | 0.004 |
| Met | 95.50 a | 94.75 a | 84.34 b | 84.54 b | 1.263 | <0.001 |
| Ile | 91.61 a | 85.79 b | 87.28 b | 85.01 b | 0.747 | 0.001 |
| Leu | 90.97 a | 89.36 ab | 89.24 ab | 87.11 b | 0.472 | 0.022 |
| Tyr | 91.45 a | 89.34 a | 89.01 a | 80.22 b | 1.077 | <0.001 |
| Phe | 92.30 a | 86.62 c | 90.13 ab | 88.22 bc | 0.616 | 0.001 |
| Lys | 90.76 a | 86.45 b | 89.81 a | 86.91 b | 0.581 | 0.006 |
| His | 96.73 | 95.76 | 94.88 | 93.67 | 0.501 | 0.163 |
| Arg | 95.38 | 92.46 | 93.25 | 95.20 | 0.520 | 0.113 |
| Pro | 86.17 b | 90.53 a | 81.24 c | 86.03 b | 0.970 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Lin, J.; Xu, C.; Xing, X.; Yang, H.; Wang, Z. Starch Digestion Characteristics of Different Starch Sources and Their Effects on Goslings’ Apparent Nutrient Utilization. Vet. Sci. 2025, 12, 630. https://doi.org/10.3390/vetsci12070630
Yang Z, Lin J, Xu C, Xing X, Yang H, Wang Z. Starch Digestion Characteristics of Different Starch Sources and Their Effects on Goslings’ Apparent Nutrient Utilization. Veterinary Sciences. 2025; 12(7):630. https://doi.org/10.3390/vetsci12070630
Chicago/Turabian StyleYang, Zhi, Jun Lin, Chen Xu, Xiyuan Xing, Haiming Yang, and Zhiyue Wang. 2025. "Starch Digestion Characteristics of Different Starch Sources and Their Effects on Goslings’ Apparent Nutrient Utilization" Veterinary Sciences 12, no. 7: 630. https://doi.org/10.3390/vetsci12070630
APA StyleYang, Z., Lin, J., Xu, C., Xing, X., Yang, H., & Wang, Z. (2025). Starch Digestion Characteristics of Different Starch Sources and Their Effects on Goslings’ Apparent Nutrient Utilization. Veterinary Sciences, 12(7), 630. https://doi.org/10.3390/vetsci12070630







