Nationwide Seroprevalence of Coxiella burnetii Infection in Saudi Farm Animals: Implications for Public Health
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
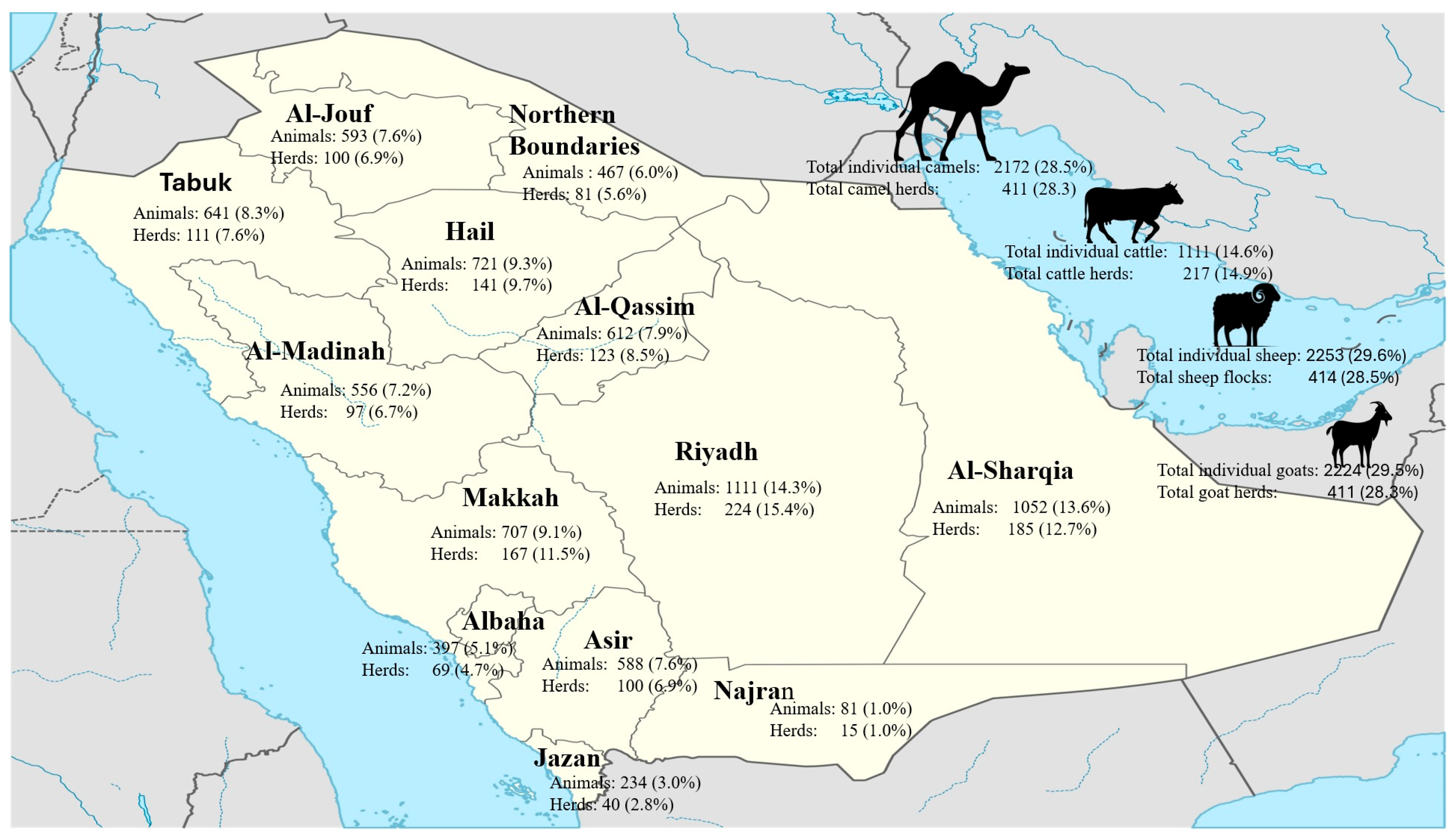
2.1. Study Design
2.2. Serological Assay
2.3. Data Management and Statistical Analysis
3. Results
3.1. Prevalence of Seropositivity
3.2. Q Fever Seroprevalence in Sheep
3.3. Q Fever Seroprevalence in Goats
3.4. Q Fever Seroprevalence in Cattle
3.5. Q Fever Seroprevalence in Camels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chmielewski, T.; Tylewska-Wierzbanowska, S. Q fever at the turn of the century. Pol. J. Microbiol. 2012, 61, 81. [Google Scholar] [CrossRef]
- Derrick, E. “Q” Fever, a New Fever Entity: Clinical Features, Diagnosis and Laboratory Investigation. Med. J. Aust. 1937, 2, 281–299. [Google Scholar] [CrossRef]
- Norlander, L. Q fever epidemiology and pathogenesis. Microbes Infect. 2000, 2, 417–424. [Google Scholar] [CrossRef]
- Parker, N.R.; Barralet, J.H.; Bell, A.M. Q fever. Lancet 2006, 367, 679–688. [Google Scholar] [CrossRef]
- WOAH. Q Fever. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/tahm/3.01.16_Q_FEVER.pdf (accessed on 15 January 2025).
- Berri, M.; Arricau-Bouvery, N.; Rodolakis, A. PCR-based detection of Coxiella burnetii from clinical samples. PCR Detect. Microb. Pathog. 2003, 216, 153–161. [Google Scholar]
- Sheek-Hussein, M.; Zewude, A.; Abdullahi, A.S.; Abdelgaleel, N.H.; Ishag, H.Z.A.; Yusof, M.F.; MS, A.L.; Shah, A.M.A.; AlNeyadi, J.; Osman, B.; et al. One health approach based descriptive study on Coxiella burnetii infections in camels and abattoir workers in the United Arab Emirates. Sci. Rep. 2025, 15, 12308. [Google Scholar] [CrossRef]
- Angulo, F.J.; LeJeune, J.T.; Rajala-Schultz, P.J. Unpasteurized milk: A continued public health threat. Clin. Infect. Dis. 2009, 48, 93–100. [Google Scholar]
- Karagiannis, I.; Schimmer, B.; Van Lier, A.; Timen, A.; Schneeberger, P.; Van Rotterdam, B.; De Bruin, A.; Wijkmans, C.; Rietveld, A.; Van Duynhoven, Y. Investigation of a Q fever outbreak in a rural area of The Netherlands. Epidemiol. Infect. 2009, 137, 1283–1294. [Google Scholar] [CrossRef]
- van der Hoek, W.; Hunink, J.; Vellema, P.; Droogers, P. Q fever in The Netherlands: The role of local environmental conditions. Int. J. Environ. Health Res. 2011, 21, 441–451. [Google Scholar] [CrossRef]
- Fenollar, F.; Fournier, P.-E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 2001, 33, 312–316. [Google Scholar] [CrossRef]
- Berri, M.; Laroucau, K.; Rodolakis, A. The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Vet. Microbiol. 2000, 72, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Nicollet, P.; Valognes, A. Current review of Q fever diagnosis in animals. Bull. L’académie Vétérinaire Fr. 2007, 160, 289–295. [Google Scholar] [CrossRef]
- Gelpi, A.P. Q fever in Saudi Arabia. Am. J. Trop. Med. Hyg. 1966, 15, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.F.; Alshaikh, M.; El-Rab, M.G.; Aljumaah, R.; El-Nabi, A.G.; Bagi, A.A. Serological prevalence of Q fever and chlamydiosis in camels in Saudi Arabia. J. Anim. Vet. Adv. 2008, 7, 685–688. [Google Scholar]
- Hussein, M.F.; Al-Khalifa, I.M.; Aljumaah, R.S.; Elnabi, A.G.; Mohammed, O.B.; Omer, S.A.; Macasero, W.V. Serological prevalence of Coxiella burnetii in captive wild ruminants in Saudi Arabia. Comp. Clin. Pathol. 2012, 21, 33–38. [Google Scholar] [CrossRef]
- Mohammed, O.B.; Jarelnabi, A.A.; Aljumaah, R.S.; Alshaikh, M.A.; Bakhiet, A.O.; Omer, S.A.; Alagaili, A.N.; Hussein, M.F. Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: Molecular detection from camel and other domestic livestock. Asian Pac. J. Trop. Med. 2014, 7, 715–719. [Google Scholar] [CrossRef]
- Almogren, A.; Shakoor, Z.; Hasanato, R.; Adam, M.H. Q fever: A neglected zoonosis in Saudi Arabia. Ann. Saudi Med. 2013, 33, 464–468. [Google Scholar] [CrossRef]
- Adamu, S.G.; Kabir, J.; Umoh, J.U.; Raji, M.A. Seroprevalence of brucellosis and Q fever (Coxiellosis) in cattle herds in Maigana and Birnin Gwari agro-ecological zone of Kaduna State, Nigeria. Trop. Anim. Health Prod. 2018, 50, 1583–1589. [Google Scholar] [CrossRef]
- Alvarez, J.; Perez, A.; Mardones, F.O.; Pérez-Sancho, M.; García-Seco, T.; Pagés, E.; Mirat, F.; Díaz, R.; Carpintero, J.; Domínguez, L. Epidemiological factors associated with the exposure of cattle to Coxiella burnetii in the Madrid region of Spain. Vet. J. 2012, 194, 102–107. [Google Scholar] [CrossRef]
- Capuano, F.; Landolfi, M.; Monetti, D. Influence of three types of farm management on the seroprevalence of Q fever as assessed by an indirect immunofluorescence assay. Vet. Rec. 2001, 149, 669–671. [Google Scholar] [CrossRef]
- Njeru, J.; Henning, K.; Pletz, M.W.; Heller, R.; Neubauer, H. Q fever is an old and neglected zoonotic disease in Kenya: A systematic review. BMC Public Health 2016, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- El-Mahallawy, H.S.; Lu, G.; Kelly, P.; Xu, D.; Li, Y.; Fan, W.; Wang, C. Q fever in China: A systematic review, 1989–2013. Epidemiol. Infect. 2015, 143, 673–681. [Google Scholar] [CrossRef]
- Jarelnabi, A.A.; Alshaikh, M.A.; Bakhiet, A.O.; Omer, S.A.; Aljumaah, R.S.; Harkiss, G.D.; Mohammed, O.B.; Hussein, M.F. Seroprevalence of Q fever in farm animals in Saudi Arabia. Biomed. Res. 2018, 29, 895–900. [Google Scholar] [CrossRef]
- Van den Brom, R.; Vellema, P. Q fever outbreaks in small ruminants and people in the Netherlands. Small Rumin. Res. 2009, 86, 74–79. [Google Scholar] [CrossRef]
- Van den Brom, R.; van Engelen, E.; Roest, H.I.; van der Hoek, W.; Vellema, P. Coxiella burnetii infections in sheep or goats: An opinionated review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii infection in Africa: A OneHealth systematic review. PLoS Neglected Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Beaudeau, F.; Berri, M.; Joly, A.; Rodolakis, A.; Seegers, H. Shedding routes of Coxiella burnetii in dairy cows: Implications for detection and control. Vet. Res. 2006, 37, 827–833. [Google Scholar] [CrossRef]
- Wambua, L.; Bett, B.; Abkallo, H.M.; Muturi, M.; Nthiwa, D.; Nyamota, R.; Kiprono, E.; Kirwa, L.; Gakuya, F.; Bartlow, A.W.; et al. National serosurvey and risk mapping reveal widespread distribution of Coxiella burnetii in Kenya. Sci. Rep. 2025, 15, 9706. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; van Schaik, G.; Vellema, P. Demography of Q fever seroprevalence in sheep and goats in The Netherlands in 2008. Prev. Vet. Med. 2013, 109, 76–82. [Google Scholar] [CrossRef]
- Miller, H.K.; Priestley, R.A.; Kersh, G.J. Q Fever: A troubling disease and a challenging diagnosis. Clin. Microbiolgy Newsl. 2021, 43, 109–118. [Google Scholar] [CrossRef]
- Lurier, T.; Rousset, E.; Gasqui, P.; Sala, C.; Claustre, C.; Abrial, D.; Dufour, P.; de Crémoux, R.; Gache, K.; Delignette-Muller, M.L.; et al. Evaluation using latent class models of the diagnostic performances of three ELISA tests commercialized for the serological diagnosis of Coxiella burnetii infection in domestic ruminants. Vet. Res. 2021, 52, 56. [Google Scholar] [CrossRef] [PubMed]
- Joulié, A.; Sidi-Boumedine, K.; Bailly, X.; Gasqui, P.; Barry, S.; Jaffrelo, L.; Poncet, C.; Abrial, D.; Yang, E.; Leblond, A.; et al. Molecular epidemiology of Coxiella burnetii in French livestock reveals the existence of three main genotype clusters and suggests species-specific associations as well as regional stability. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2017, 48, 142–149. [Google Scholar] [CrossRef]
- Astobiza, I.; Tilburg, J.J.; Piñero, A.; Hurtado, A.; García-Pérez, A.L.; Nabuurs-Franssen, M.H.; Klaassen, C.H. Genotyping of Coxiella burnetii from domestic ruminants in northern Spain. BMC Vet. Res. 2012, 8, 241. [Google Scholar] [CrossRef]
- Tomaiuolo, S.; Boarbi, S.; Fancello, T.; Michel, P.; Desqueper, D.; Grégoire, F.; Callens, J.; Fretin, D.; Devriendt, B.; Cox, E.; et al. Phylogeography of Human and Animal Coxiella burnetii Strains: Genetic Fingerprinting of Q Fever in Belgium. Front. Cell. Infect. Microbiol. 2020, 10, 625576. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Alam, M.M.; Islam, M.A.; Bhuiyan, A.K.; Rahman, A.K. Serological and molecular evidence of q fever in domestic ruminants in Bangladesh. Vet. Med. Int. 2016, 2016, 9098416. [Google Scholar] [CrossRef] [PubMed]
| Region | Sheep | Goats | Camels | Cattle | Cumulative | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | ||||||
| Individuals | Herds | Individuals | Herds | Individuals | Herds | Individuals | Herds | Individuals | Herds | |
| Hail | 104/243 (42.8%) | 33/45 (73.3%) | 160/238 (67.2%) | 41/45 (91%) | 164/214 (76.6%) | 42/45 (93.3%) | 0/26 (0%) | 0/6 (0%) | 428/721 (59.3%) | 116/141 (82.2%) |
| Riyadh | 89/300 (29.7%) | 48/59 (81.4%) | 139/295 (47.1%) | 48/59 (81.4%) | 87/298 (29.2%) | 57/60 (95%) | 39/218 (17.9%) | 20/46 (43.5%) | 354/1111 (31.8%) | 173/224 (77.2%) |
| Al-Sharqia | 107/313 (34.2%) | 48/55 (87.4%) | 134/312 (42.9%) | 52/55 (94.5%) | 136/308 (44.2%) | 47/55 (85.6%) | 3/119 (2.5%) | 3/20 (15%) | 380/1052 (36.1%) | 150/185 (81%) |
| Al-Qassim | 71/180 (39.4%) | 29/36 (87.3%) | 73/160 (45.6%) | 29/32 (90.6%) | 125/180 (69.4%) | 35/36 (97.2%) | 3/92 (3.3%) | 2/19 (10.5%) | 272/612 (44.4%) | 95/123 (77.2%) |
| N. Boundaries | 29/145 (20%) | 14/25 (87.3%) | 34/142 (23.9%) | 21/25 (84%) | 71/144 (49.3%) | 23/25 (92%) | 1/36 (2.8%) | 1/6 (16.7%) | 135/467 (28.9%) | 59/81 (72.8%) |
| Al-Gouf | 51/149 (34.2%) | 24/25 (96%) | 80/139 (57.6%) | 23/25 (92%) | 55/157 (35%) | 24/25 (96%) | 6/148 (4%) | 3/25 (12%) | 192/593 (32.3%) | 74/100 (74%) |
| Tabuk | 39/200 (19.5%) | 28/35 (80%) | 90/207 (43.5%) | 34/35 (97.1%) | 124/201 (61.7) | 34/35 (97.1%) | 4/33 (12.1%) | 3/6 (50%) | 257/641 (40%) | 99/111 (89.1%) |
| Gazan | 21/60 (35%) | 9/10 (90%) | 28/57 (49.1%) | 10/10 (100%) | 18/57 (31.6%) | 8/10 (80%) | 2/60 (3.3%) | 1/10 (10%) | 69/234 (29.4%) | 28/40 (70%) |
| Najran | 5/29 (17.2%) | 4/5 (80%) | 10/26 (38.5%) | 3/5 (60%) | 15/26 (57.7%) | 5/5 (100%) | - | - | 30/81 (37%) | 12/15 (80%) |
| Asir | 32/150 (21.3%) | 17/25 (68%) | 70/148 (47.3%) | 24/25 (96%) | 67/145 (46.2%) | 23/25 (92%) | 4/145 (2.8%) | 3/25 (12%) | 173/588 (29.4%) | 67/100 (67%) |
| Al-Baha | 35/108 (32.4%) | 16/19 (84.2%) | 62/117 (53%) | 18/20 (90%) | 35/86 (40.7%) | 14/15 (93.3%) | 8/86 (9.3%) | 6/15 (40%) | 140/397 (35.2%) | 54/69 (78.2%) |
| Madinah | 47/173 (27.2%) | 25/30 (83.3%) | 96/177 (54.2%) | 30/30 (100%) | 82/170 (48.2%) | 26/30 (86.7%) | 5/36 (15.2%) | 4/7 (57.1%) | 230/556 (41.3%) | 85/97 (87.6%) |
| Makkah | 51/203 (25.1%) | 36/45 (80%) | 91/206 (44.2%) | 45/45 (100%) | 35/186 (18.8%) | 44/45 (97.8%) | 16/112 (14.2%) | 14/32 (43.8%) | 193/707 (27.2%) | 139/167 (83.2%) |
| Total | 681/2253 (30.2%) | 331/414 (80%) | 1067/2224 (48%) | 378/411 (92%) | 1014/2172 (46.7%) | 382/411 (92.9%) | 91/1111 (8.2%) | 60/217 (27.6%) | 2853/7760 (36.7%) | 1151/1453 (79.2%) |
| p-Value | 0.000 ** | 0.07 * | 0.000 ** | 0.005 ** | 0.000 ** | 0.31 NS | 0.000 ** | 0.0014 ** | 0.001 ** | 0.003 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasem, S.; Alsubki, R.A.; Saad, A.; Zidan, K.H.; Qasim, I.; Hashim, O.; Alkarar, A.; Abu-Obeida, A.; Damra, E.; Al-Jabri, Z.; et al. Nationwide Seroprevalence of Coxiella burnetii Infection in Saudi Farm Animals: Implications for Public Health. Vet. Sci. 2025, 12, 629. https://doi.org/10.3390/vetsci12070629
Kasem S, Alsubki RA, Saad A, Zidan KH, Qasim I, Hashim O, Alkarar A, Abu-Obeida A, Damra E, Al-Jabri Z, et al. Nationwide Seroprevalence of Coxiella burnetii Infection in Saudi Farm Animals: Implications for Public Health. Veterinary Sciences. 2025; 12(7):629. https://doi.org/10.3390/vetsci12070629
Chicago/Turabian StyleKasem, Samy, Roua A. Alsubki, Ahmed Saad, Kamal H. Zidan, Ibrahim Qasim, Osman Hashim, Ali Alkarar, Ali Abu-Obeida, Eman Damra, Zaaima Al-Jabri, and et al. 2025. "Nationwide Seroprevalence of Coxiella burnetii Infection in Saudi Farm Animals: Implications for Public Health" Veterinary Sciences 12, no. 7: 629. https://doi.org/10.3390/vetsci12070629
APA StyleKasem, S., Alsubki, R. A., Saad, A., Zidan, K. H., Qasim, I., Hashim, O., Alkarar, A., Abu-Obeida, A., Damra, E., Al-Jabri, Z., Abdel-Moneim, A. S., & Al-Salem, W. (2025). Nationwide Seroprevalence of Coxiella burnetii Infection in Saudi Farm Animals: Implications for Public Health. Veterinary Sciences, 12(7), 629. https://doi.org/10.3390/vetsci12070629







