Optimizing Low Crude Protein Diets with Coated Cysteamine Hydrochloride and Exogenous Alkaline Protease Supplementation in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experiment Design and Bird Husbandry
2.3. Sampling and Measurements
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Carcass Characteristics
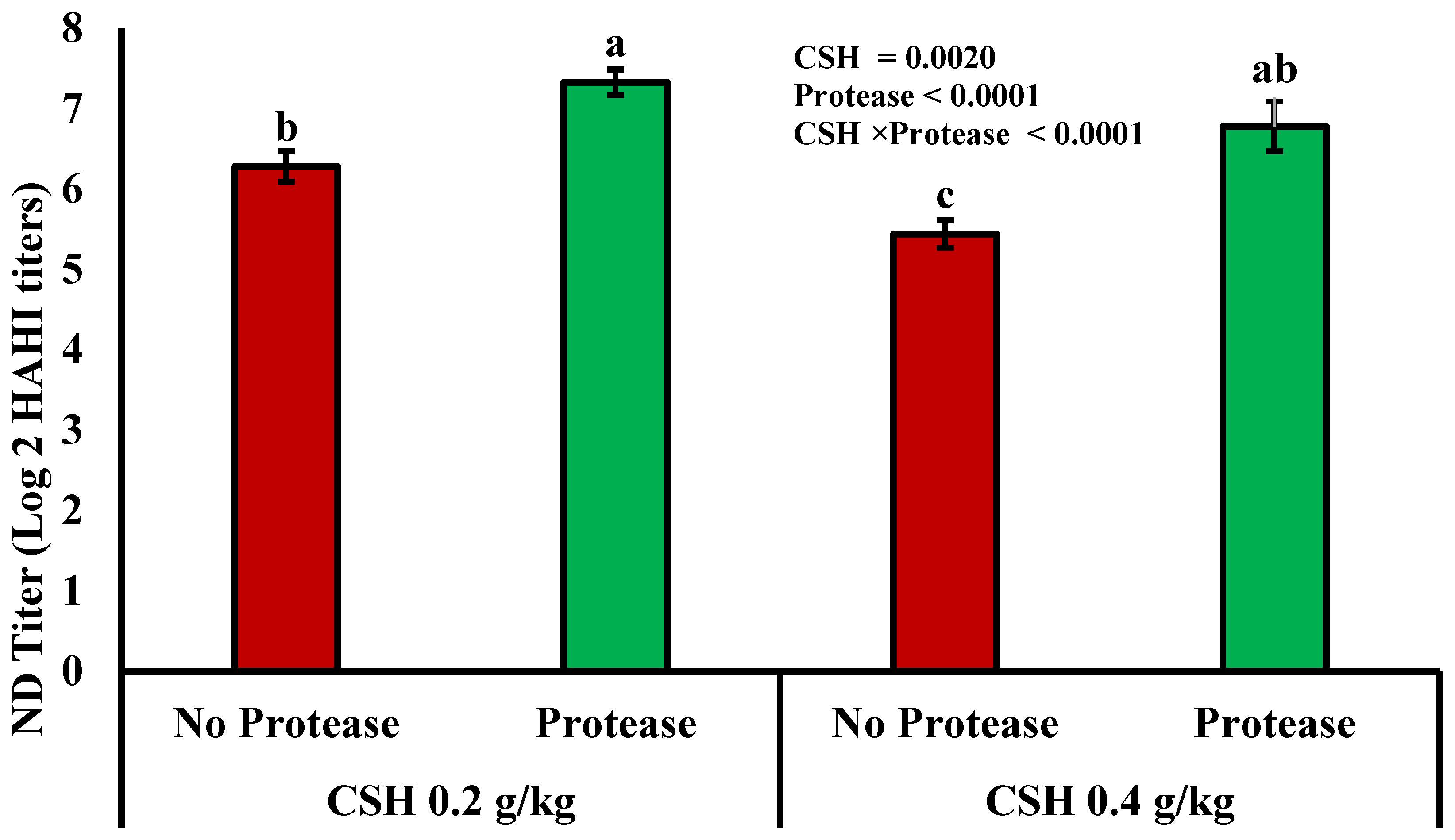
3.3. Immune Status
3.4. Gut Morphology
3.5. Nutrient Digestibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Moosavi, M.; Eslami, M.; Chaji, M.; Boujarpour, M. Economic value of diets with different levels of energy and protein with constant ratio on broiler chickens. J. Anim. Vet. Adv. 2011, 10, 709–711. [Google Scholar] [CrossRef]
- Esonu, B.; Ogbonna, U.; Anyanwu, G.; Emenalom, O.; Uchegbu, M.; Etuk, E.; Udedibie, A. Evaluation of performance, organ characteristics and economic analysis of broiler finisher fed dried rumen digesta. Int. J. Poult. Sci. 2006, 5, 1116–1118. [Google Scholar] [CrossRef]
- Shi, S.; Lu, J.; Tong, H.; Zou, J.; Wang, K. Effects of graded replacement of soybean meal by sunflower seed meal in laying hen diets on hen performance, egg quality, egg fatty acid composition, and cholesterol content. J. Appl. Poult. Res. 2012, 21, 367–374. [Google Scholar] [CrossRef]
- Pettersson, D.; Pontoppidan, K. Soybean meal and the potential for upgrading its feeding value by enzyme supplementation. Soybean-Bio-Act. Compd. 2013, 13, 288–307. [Google Scholar] [CrossRef]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Passantino, A.; Khan, R.; Tufarelli, V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Han, Q.; Guo, Y.; Zhang, B.; D’inca, R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Br. J. Nutr. 2016, 116, 1878–1888. [Google Scholar] [CrossRef]
- Parsons, C.; Castanon, F.; Han, Y. Protein and amino acid quality of meat and bone meal. Poult. Sci. 1997, 76, 361–368. [Google Scholar] [CrossRef]
- Nir, I.; Nitsan, Z.; Mahagna, M. Comparative growth and development of the digestive organs and of some enzymes in broiler and egg type chicks after hatching. Br. Poult. Sci. 1993, 34, 523–532. [Google Scholar] [CrossRef]
- Le Huerou-Luron, I.; Lhoste, E.; Wicker-Planquart, C.; Dakka, N.; Toullec, R.; Corring, T.; Guilloteau, P.; Puigserver, A. Molecular aspects of enzyme synthesis in the exocrine pancreas with emphasis on development and nutritional regulation. Proc. Nutr. Soc. 1993, 52, 301–313. [Google Scholar] [CrossRef]
- Alam, S.; Masood, S.; Zaneb, H.; Rabbani, I.; Khan, R.U.; Shah, M.; Ashraf, S.; Alhidary, I.A. Effect of Bacillus cereus and phytase on the expression of musculoskeletal strength and gut health in Japanese quail (Coturnix japonica). J. Poult. Sci. 2020, 57, 200–204. [Google Scholar] [CrossRef]
- Applegate, T.J.; Angel, R. Nutrient requirements of poultry publication: History and need for an update. J. Appl. Poult. Res. 2014, 23, 567–575. [Google Scholar] [CrossRef]
- Angel, C.R.; Saylor, W.; Vieira, S.L.; Ward, N. Effects of a monocomponent protease on performance and protein utilization in 7-to 22-day-old broiler chickens. Poult. Sci. 2011, 90, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Vieira, S.; Angel, C.; Favero, A.; Maiorka, A. Performance and nutrient utilization of broilers fed diets supplemented with a novel mono component protease. J. Appl. Poult. Res. 2011, 20, 322–334. [Google Scholar] [CrossRef]
- Hafeez, A.; Shah, S.A.A.; Khan, R.U.; Ullah, Q.; Naz, S. Effect of diet supplemented with phytogenics and protease enzyme on performance, serum biochemistry and muscle histomorphology in broilers. J. Appl. Anim. Res. 2020, 48, 326–330. [Google Scholar] [CrossRef]
- Kiarie, E.; Walsh, M.; Nyachoti, C. Performance, digestive function, and mucosal responses to selected feed additives for pigs. J. Anim. Sci. 2016, 94, 169–180. [Google Scholar] [CrossRef]
- Ghazi, S.; Rooke, J.; Galbraith, H.; Bedford, M. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br. Poult. Sci. 2002, 43, 70–77. [Google Scholar] [CrossRef]
- Law, F.L.; Zulkifli, I.; Soleimani, A.F.; Liang, J.B.; Awad, E.A. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian-Australas. J. Anim. Sci. 2018, 31, 1291. [Google Scholar] [CrossRef]
- Mahmood, T.; Mirza, M.; Nawaz, H.; Shahid, M. Effect of different exogenous proteases on growth performance, nutrient digestibility, and carcass response in broiler chickens fed poultry by-product meal-based diets. Livest. Sci. 2017, 200, 71–75. [Google Scholar] [CrossRef]
- Maqsood, M.A.; Khan, E.U.; Qaisrani, S.N.; Rashid, M.A.; Shaheen, M.S.; Nazir, A.; Talib, H.; Ahmad, S. Interactive effect of amino acids balanced at ideal lysine ratio and exogenous protease supplemented to low CP diet on growth performance, carcass traits, gut morphology, and serum metabolites in broiler chicken. Trop. Anim. Health Prod. 2022, 54, 186. [Google Scholar] [CrossRef]
- Ajayi, H. Effect of protease supplementation on performance and carcass weights of broiler chickens fed low protein diets. Niger. J. Agric. Food Environ. 2015, 11, 29–32. [Google Scholar]
- Varia, A.D.; Shukla, V.Y.; Tipre, D.R. Alkaline protease-a versatile enzyme. Int. J. Res. Anal. Rev. 2019, 6, 208–217. [Google Scholar]
- Evnin, L.B.; Vásquez, J.R.; Craik, C.S. Substrate specificity of trypsin investigated by using a genetic selection. Proc. Natl. Acad. Sci. USA 1990, 87, 6659–6663. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, A.; Hong, Q.; Liu, J.; Liu, J. Effects of cysteamine on growth performance, digestive enzyme activities, and metabolic hormones in broilers. Poult. Sci. 2006, 85, 1912–1916. [Google Scholar] [CrossRef]
- Zhou, P.; Luo, Y.; Zhang, L.; Li, J.; Zhang, B.; Xing, S.; Zhu, Y.; Gao, F.; Zhou, G. Effects of cysteamine supplementation on the intestinal expression of amino acid and peptide transporters and intestinal health in finishing pigs. Anim. Sci. J. 2017, 88, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P. Somatostatin receptor expression in clinical immunology. Metabolism 1996, 45, 86–87. [Google Scholar] [CrossRef]
- Yang, Q.; Lian, G.; Gong, X. Enhancement of mucosal immune responses in chickens by oral administration of cysteamine. Poult. Sci. 2007, 86, 1323–1328. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, B.; Feng, Y.; Zhang, H.; Zhang, Q.; Hou, J.; Wang, Y.; Sa, R.; Zhao, F.; Xie, J. In vitro release and in vivo growth-promoting effects of coated cysteamine in broilers. Poult. Sci. 2023, 102, 102475. [Google Scholar] [CrossRef]
- Wang, S.; Bai, M.; Xu, K.; Shao, Y.; Yang, Z.; Xiong, X.; Huang, R.; Li, Y.; Liu, H. Effects of Coated Cysteamine on Oxidative Stress and Inflammation in Weaned Pigs. Animals 2021, 11, 2217. [Google Scholar] [CrossRef]
- Santana-Sánchez, P.; Vaquero-García, R.; Legorreta-Haquet, M.V.; Chávez-Sánchez, L.; Chávez-Rueda, A.K. Hormones and B-cell development in health and autoimmunity. Front. Immunol. 2024, 15, 1385501. [Google Scholar] [CrossRef]
- Yaqoob, M.U.; Hou, J.; Zhe, L.; Qi, Y.; Wu, P.; Zhu, X.; Cao, X.; Li, Z. Coated cysteamine, a potential feed additive for ruminants—An updated review. Anim. Biosci. 2024, 37, 161–172. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Fazli, A.; Morammazi, S.; Saadati, N.; Ahmad, H.I.; Hassan, F. Cysteamine administration in lambs grazing on mountain pastures: Effects on the body weight, antioxidant capacity, thyroid hormones and growth hormone secretion. Vet. Med. Sci. 2022, 8, 328–335. [Google Scholar] [CrossRef]
- Aviagen. Ross-308 Broiler Management Handbook; Aviagen: Huntsville, AL, USA, 2018. [Google Scholar]
- Aviagen. Ross-308 Nutrition Specifications; Aviagen: Huntsville, AL, USA, 2022. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 22nd ed.; AOAC: Washington, DC, USA, 2023. [Google Scholar]
- Muneeb, M.; Khan, E.U.; Ali, M.; Suleman, M.; Shaheen, M.S.; Zafar, M.S.; Ahmad, S. Effects of replacing antibiotics with probiotics and antimicrobial peptides on performance, gut health, carcass traits, meat quality, and welfare in broilers infected with Eimeria and Clostridium perfringens. Trop. Anim. Health Prod. 2025, 57, 184. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.E. Growth performance and nutrient digestibility of broilers fed low-energy corn-soybean meal-based diets supplemented with an exogenous enzyme cocktail as a combined activity. Rev. Bras. De Zootec. 2024, 53, e20230100. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Al Sulaiman, A.R.; Alharthi, A.S.; Abudabos, A.M. Effect of Exogenous Enzymes Cocktail on Performance, Carcass Traits, Biochemical Metabolites, Intestinal Morphology, and Nutrient Digestibility of Broilers Fed Normal and Low-Energy Corn-Soybean Diets. Animals 2022, 12, 1094. [Google Scholar] [CrossRef]
- Muneeb, M.; Khan, E.U.; Ali, M.; Haque, M.N.U.; Khan, M.U.Z.; Ahmad, S. Comparative Effects of Antibiotic and Antimicrobial Peptide on Growth Performance, Gut Morphology, Intestinal Lesion Score, Ileal Microbial Counts, and Immune Status in Broilers Challenged with Necrotic Enteritis. Probiotics Antimicrob. Proteins 2025, 17, 1–15. [Google Scholar] [CrossRef]
- Ahmadi-Sefat, A.A.; Taherpour, K.; Ghasemi, H.A.; Gharaei, M.A.; Shirzadi, H.; Rostami, F. Effects of an emulsifier blend supplementation on growth performance, nutrient digestibility, intestinal morphology, and muscle fatty acid profile of broiler chickens fed with different levels of energy and protein. Poult. Sci. 2022, 101, 102145. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Zaefarian, F.; Gu, Y.; Xiao, W.; Jia, J.; Ravindran, V. Influence of soybean bioactive peptides on growth performance, nutrient utilisation, digestive tract development and intestinal histology in broilers. J. Appl. Anim. Nutr. 2017, 5, e7. [Google Scholar] [CrossRef]
- Zavy, M.; Lindsey, T. Effect of cysteamine administration on growth and efficiency of food utilisation in chicks. Br. Poult. Sci. 1988, 29, 409–417. [Google Scholar] [CrossRef]
- Nunes, J.O.; Bertechini, A.G.; Brito, J.Á.G.d.; Makiyama, L.; Mesquita, F.R.; Nishio, C.M. Evaluation of cysteamine associated with different energy patterns in diets for broiler chickens. Rev. Bras. Zootec. 2012, 41, 1956–1960. [Google Scholar] [CrossRef][Green Version]
- Ai, X.; Han, Z. Effect of cysteamine on the pancreatic secretion and lipase activity of geese. Chin. J. Vet. Sci. 2002, 18, 297–300. [Google Scholar][Green Version]
- Ndazigaruye, G.; Kim, D.-H.; Kang, C.-W.; Kang, K.-R.; Joo, Y.-J.; Lee, S.-R.; Lee, K.-W. Effects of low-protein diets and exogenous protease on growth performance, carcass traits, intestinal morphology, cecal volatile fatty acids and serum parameters in broilers. Animals 2019, 9, 226. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.L.; Pan, L.; Ma, X.K.; Tian, Q.Y.; Xu, Y.T.; Long, S.F.; Zhang, Z.H.; Piao, X.S. Effects of coated proteases on the performance, nutrient retention, gut morphology and carcass traits of broilers fed corn or sorghum based diets supplemented with soybean meal. Anim. Feed. Sci. Technol. 2017, 223, 119–127. [Google Scholar] [CrossRef]
- Jabbar, A.; Tahir, M.; Khan, R.U.; Ahmad, N. Interactive effect of exogenous protease enzyme and dietary crude protein levels on growth and digestibility indices in broiler chickens during the starter phase. Trop. Anim. Health Prod. 2021, 53, 23. [Google Scholar] [CrossRef] [PubMed]
- McLeod, K.R.; Harmon, D.L.; Schillo, K.K.; Hileman, S.M.; Mitchell, G.E. Effects of cysteamine on pulsatile growth hormone release and plasma insulin concentrations in sheep. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 523–533. [Google Scholar] [CrossRef]
- Hanauska-Brown, L.A.; Dufty, A.M., Jr.; Roloff, G.J. Blood chemistry, cytology, and body condition in adult northern goshawks (Accipiter gentilis). J. Raptor Res. 2003, 37, 5. [Google Scholar]
- Ruan, T.; Li, L.; Peng, X.; Wu, B. Effects of methionine on the immune function in animals. Health 2017, 9, 857–869. [Google Scholar] [CrossRef]
- Barnett, M.; Hegarty, R. Cysteamine: A human health dietary additive with potential to improve livestock growth rate and efficiency. Anim. Prod. Sci. 2016, 56, 1330–1338. [Google Scholar] [CrossRef]
- Peek, H.; Van der Klis, J.; Vermeulen, B.; Landman, W. Dietary protease can alleviate negative effects of a coccidiosis infection on production performance in broiler chickens. Anim. Feed. Sci. Technol. 2009, 150, 151–159. [Google Scholar] [CrossRef]
- Geyra, A.; Uni, Z.; Sklan, D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult Sci. 2001, 80, 776–782. [Google Scholar] [CrossRef]
- Uni, Z.; Geyra, A.; Ben-Hur, H.; Sklan, D. Small intestinal development in the young chick: Crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 2000, 41, 544–551. [Google Scholar] [CrossRef]
- Tzora, A.; Giannenas, I.; Karamoutsios, A.; Papaioannou, N.; Papanastasiou, D.; Bonos, E.; Skoufos, S.; Bartzanas, T.; Skoufos, I. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. J. Poult. Sci. 2017, 54, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Prosser, Z.; Chee, E.Y.; Hansen, C.F.; Dunshea, F.R.; Mullan, B.P.; Pluske, J.R. Dietary stimulation of the endogenous somatotropic axis in weaner and grower-finisher pigs using medium chain triglycerides and cysteamine hydrochloride. J. Anim. Sci. Biotechnol. 2016, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.M.; Li, D.D.; Li, Z.R.; Wang, J.P.; Zeng, Q.F.; Bai, S.P.; Su, Z.W.; Zhang, K.Y. Effects of dietary crude protein levels and exogenous protease on performance, nutrient digestibility, trypsin activity and intestinal morphology in broilers. Livest. Sci. 2016, 193, 26–31. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Roos, F.F. Toward optimal value creation through the application of exogenous mono-component protease in the diets of non-ruminants. Anim. Feed. Sci. Technol. 2016, 221, 331–340. [Google Scholar] [CrossRef]
- Lu, P.; Choi, J.; Yang, C.; Mogire, M.; Liu, S.; Lahaye, L.; Adewole, D.; Rodas-Gonzalez, A.; Yang, C. Effects of antibiotic growth promoter and dietary protease on growth performance, apparent ileal digestibility, intestinal morphology, meat quality, and intestinal gene expression in broiler chickens: A comparison. J. Anim. Sci. 2020, 98, skaa254. [Google Scholar] [CrossRef]
| A 2 × 2 Factorial Arrangement Under Completely Randomized Design | Coated Cysteamine Hydrochloride (CSH) | ||
|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | ||
| Exogenous Alkaline Protease (EAP) | No Protease added (−) | Male broilers 150 birds, 6 replicates 25 birds/replicate | Male broilers 150 birds, 6 replicates 25 birds/replicate |
| Protease at 0.2 g/kg (+) | Male broilers 150 birds, 6 replicates 25 birds/replicate | Male broilers 150 birds, 6 replicates 25 birds/replicate | |
| Feed Ingredients (%) | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–35 d) |
|---|---|---|---|
| Corn Grain | 54.65 | 58.54 | 63.10 |
| Wheat Bran | 2.00 | 1.00 | 1.27 |
| Rice Polishing | 2.80 | 4.20 | 5.10 |
| Rice Tips | 2.50 | 3.00 | 2.00 |
| Molasses | 3.00 | 3.00 | 3.00 |
| Oil Canola | 1.25 | 1.45 | 1.70 |
| Soybean Meal | 6.50 | 6.50 | 6.50 |
| Canola Meal | 9.00 | 8.00 | 3.20 |
| Sunflower Meal | 8.10 | 5.00 | 3.80 |
| Fish Meal (52%) | 4.00 | 4.00 | 4.00 |
| Poultry BP Meal | 4.00 | 4.00 | 4.00 |
| DL-Methionine | 0.13 | 0.11 | 0.11 |
| L-Lysine HCl | 0.17 | 0.10 | 0.12 |
| Limestone | 1.80 | 1.00 | 1.00 |
| Celite/Marker | - | - | 1.00 |
| Micro Mineral Premix * | 0.05 | 0.05 | 0.05 |
| Vitamin Premix * | 0.05 | 0.05 | 0.05 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient Contents | |||
| CP (%) | 20.70 | 19.35 | 17.55 |
| ME (Calculated, Kcal/kg) | 2975 | 3050 | 3100 |
| Ca (%) | 0.95 | 0.75 | 0.65 |
| P (Av) (%) | 0.50 | 0.42 | 0.36 |
| Lysine (dig) (%) | 1.32 | 1.18 | 1.08 |
| Methionine (dig) (%) | 0.55 | 0.51 | 0.48 |
| Arginine (dig) (%) | 1.40 | 1.27 | 1.17 |
| Tryptophan (dig) (%) | 0.21 | 0.19 | 0.17 |
| Analyzed Nutrients | |||
| CP (%) | 20.65 | 19.28 | 17.47 |
| Crude fiber (%) | 3.72 | 3.50 | 3.29 |
| Crude fat (%) | 3.50 | 4.13 | 4.81 |
| Ca (%) | 0.92 | 0.71 | 0.63 |
| Ph (Av) (%) | 0.51 | 0.40 | 0.35 |
| Lysine (dig) (%) | 1.30 | 1.15 | 1.05 |
| Methionine (dig) (%) | 0.52 | 0.50 | 0.46 |
| Arginine (dig) (%) | 1.39 | 1.25 | 1.16 |
| Tryptophan (dig) (%) | 0.20 | 0.17 | 0.16 |
| Threonine (dig) (%) | 0.90 | 0.80 | 0.82 |
| Isoleucine (dig) (%) | 0.91 | 0.81 | 0.73 |
| Items | Treatments | Pooled SEM | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | |||||||
| − | + | − | + | CSH | Protease | Interaction | ||
| Starter (1–10 d) | ||||||||
| FI (g) | 548 | 547 | 547 | 547 | 0.81 | 0.9860 | 0.7270 | 0.9785 |
| BWG (g) | 293 | 295 | 290 | 291 | 0.88 | 0.3392 | 0.3386 | 0.2092 |
| FCR | 1.05 | 1.05 | 1.07 | 1.07 | 0.00 | 0.0672 | 0.4825 | 0.1874 |
| Grower (11–24 d) | ||||||||
| FI (g) | 1457 b | 1477 a | 1426 c | 1458 b | 3.90 | <0.0001 | <0.0001 | <0.0001 |
| BWG (g) | 979 b | 1067 a | 920 d | 966 c | 11.12 | <0.0001 | <0.0001 | <0.0001 |
| FCR | 1.34 b | 1.23 c | 1.41 a | 1.35 b | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| Finisher (25–53 d) | ||||||||
| FI (g) | 1103 b | 1126 a | 1025 b | 1103 c | 7.96 | <0.0001 | <0.0001 | <0.0001 |
| BWG (g) | 910 b | 783 a | 783 d | 871 c | 30.03 | <0.0001 | <0.0001 | <0.0001 |
| FCR | 1.79 c | 1.42 d | 1.97 a | 1.87 b | 0.04 | <0.0001 | <0.0001 | <0.0001 |
| Overall (1–35 d) | ||||||||
| FI (g) | 3108 b | 3150 a | 2989 c | 3108 b | 11.86 | <0.0001 | <0.0001 | <0.0001 |
| BWG (g) | 2182 b | 2531 a | 1992 d | 2128 c | 41.47 | <0.0001 | <0.0001 | <0.0001 |
| FCR | 1.49 c | 1.30 d | 1.58 a | 1.53 b | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| Liv % | 98.97 | 99.00 | 98.31 | 98.56 | 0.12 | 0.0034 | 0.2288 | 0.0945 |
| EPEF | 442 b | 586 a | 380 d | 419 c | 16.30 | <0.0001 | <0.0001 | <0.0001 |
| Items | Treatments | Pooled SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | |||||||
| − | + | − | + | CSH | Protease | Interaction | ||
| Body weight (g) | 2228 b | 2567 a | 2030 d | 2165 c | 24.83 | <0.0001 | <0.0001 | <0.0001 |
| Carcass weight (g) | 1420 b | 1697 a | 1240 d | 1379 c | 20.28 | <0.0001 | <0.0001 | <0.0001 |
| Dressing (%) | 63.78 b | 66.09 a | 61.08 c | 63.70 b | 0.22 | <0.0001 | <0.0001 | <0.0001 |
| Leg (%) | 11.72 b | 12.64 c | 10.95 d | 11.79 b | 0.07 | <0.0001 | <0.0001 | <0.0001 |
| Breast (%) | 28.35 b | 32.16 a | 26.42 d | 28.08 c | 0.25 | <0.0001 | <0.0001 | <0.0001 |
| Heart (%) | 0.71 a | 0.73 a | 0.67 b | 0.71 a | 0.01 | 0.0290 | 0.0178 | 0.0123 |
| Liver (%) | 2.64 c | 2.75 b | 2.90 a | 2.62 c | 0.02 | <0.0001 | <0.0001 | <0.0001 |
| Gizzard (%) | 1.49 | 1.57 | 1.52 | 1.50 | 0.02 | 0.5907 | 0.3124 | 0.2268 |
| Fat (%) | 2.39 d | 2.52 b | 2.50 c | 2.60 a | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| Items | Treatments | Pooled SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | |||||||
| − | + | − | + | CSH | Protease | Interaction | ||
| Bursa (%) | 0.37 a | 0.39 a | 0.30 b | 0.36 a | 0.01 | <0.0001 | 0.0004 | <0.0001 |
| Spleen (%) | 0.27 | 0.30 | 0.28 | 0.28 | 0.01 | 0.3662 | 0.3119 | 0.3596 |
| Items | Treatments | Pooled SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | ||||||||
| − | + | − | + | CSH | Protease | Interaction | |||
| Duodenum (µm) | VH | 1438 b | 1478 a | 1412 c | 1460 ab | 4.81 | 0.0058 | <0.0001 | <0.0001 |
| CD | 214 | 212 | 216 | 212 | 1.67 | 0.6873 | 0.3566 | 0.7814 | |
| VH: CD | 6.73 ab | 7.02 a | 6.57 b | 6.91 a | 0.05 | 0.1730 | 0.0033 | 0.0150 | |
| Ileum (µm) | VH | 753 bc | 774 a | 739 c | 765 ab | 3.27 | 0.0491 | 0.0002 | 0.0005 |
| CD | 154 | 155 | 153 | 154 | 0.76 | 0.5207 | 0.6833 | 0.8993 | |
| VH: CD | 4.88 bc | 4.99 a | 4.82 c | 4.96 ab | 0.02 | 0.1455 | <0.0001 | 0.0004 | |
| Items | Treatments | Pooled SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CSH 0.2 g/kg | CSH 0.4 g/kg | |||||||
| − | + | − | + | CSH | Protease | Interaction | ||
| Total Tract | ||||||||
| DM% | 71.86 b | 72.70 a | 69.11 d | 71.56 c | 0.16 | <0.0001 | <0.0001 | <0.0001 |
| CP% | 63.35 b | 63.80 a | 61.37 d | 62.36 c | 0.12 | <0.0001 | <0.0001 | <0.0001 |
| OM% | 81.39 b | 81.57 a | 79.11 d | 79.92 c | 0.12 | <0.0001 | <0.0001 | <0.0001 |
| Ileum | ||||||||
| CP% | 82.80 b | 84.71 a | 77.88 d | 79.89 c | 0.31 | <0.0001 | <0.0001 | <0.0001 |
| OM% | 78.42 b | 78.92 a | 72.56 d | 75.46 c | 0.30 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, H.A.B.; Khan, E.U.; Muneeb, M.; Naveed, S.; Soumeh, E.A.; Ahmad, S.; Alhotan, R.A.; Alharthi, A.S.; Abudabos, A.E. Optimizing Low Crude Protein Diets with Coated Cysteamine Hydrochloride and Exogenous Alkaline Protease Supplementation in Broiler Chickens. Vet. Sci. 2025, 12, 622. https://doi.org/10.3390/vetsci12070622
Siddique HAB, Khan EU, Muneeb M, Naveed S, Soumeh EA, Ahmad S, Alhotan RA, Alharthi AS, Abudabos AE. Optimizing Low Crude Protein Diets with Coated Cysteamine Hydrochloride and Exogenous Alkaline Protease Supplementation in Broiler Chickens. Veterinary Sciences. 2025; 12(7):622. https://doi.org/10.3390/vetsci12070622
Chicago/Turabian StyleSiddique, Hafiz Abu Bakar, Ehsaan Ullah Khan, Muhammad Muneeb, Saima Naveed, Elham Assadi Soumeh, Sohail Ahmad, Rashed A. Alhotan, Abdulrahman S. Alharthi, and Ala E. Abudabos. 2025. "Optimizing Low Crude Protein Diets with Coated Cysteamine Hydrochloride and Exogenous Alkaline Protease Supplementation in Broiler Chickens" Veterinary Sciences 12, no. 7: 622. https://doi.org/10.3390/vetsci12070622
APA StyleSiddique, H. A. B., Khan, E. U., Muneeb, M., Naveed, S., Soumeh, E. A., Ahmad, S., Alhotan, R. A., Alharthi, A. S., & Abudabos, A. E. (2025). Optimizing Low Crude Protein Diets with Coated Cysteamine Hydrochloride and Exogenous Alkaline Protease Supplementation in Broiler Chickens. Veterinary Sciences, 12(7), 622. https://doi.org/10.3390/vetsci12070622









