Dual-Energy Computed Tomography for the Detection of Bone Edema-Like Lesions in the Equine Foot: Standing Horses and Cadaveric Specimens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnostic Imaging
2.2.1. Magnetic Resonance Imaging (MRI)
2.2.2. Computed Tomography (CT)
2.2.3. Image Reconstruction
2.2.4. Image Analysis
2.3. Statistical Analysis
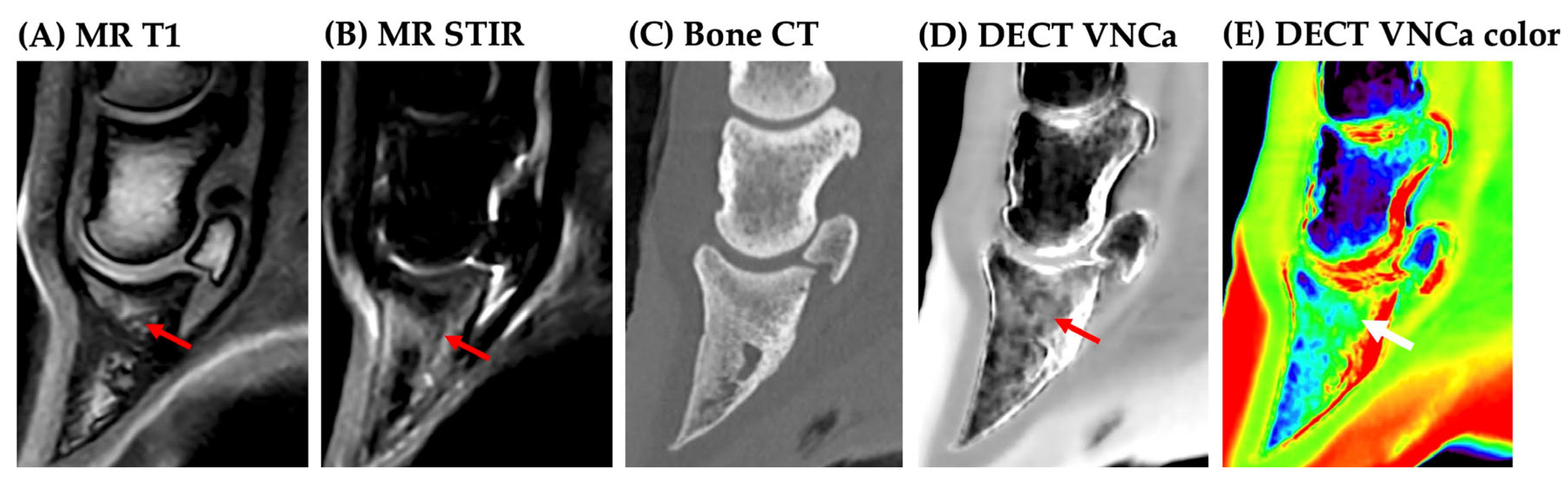
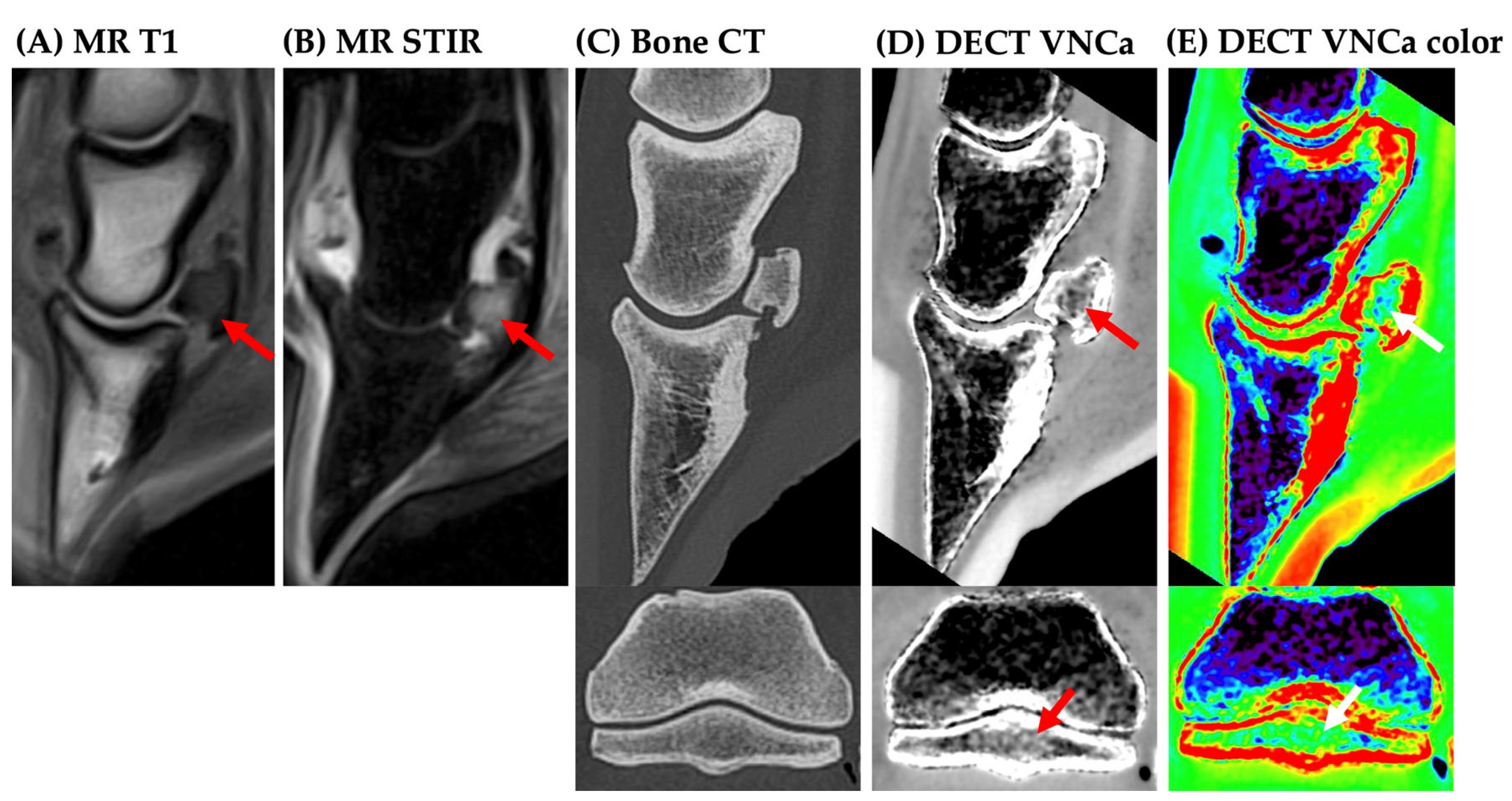
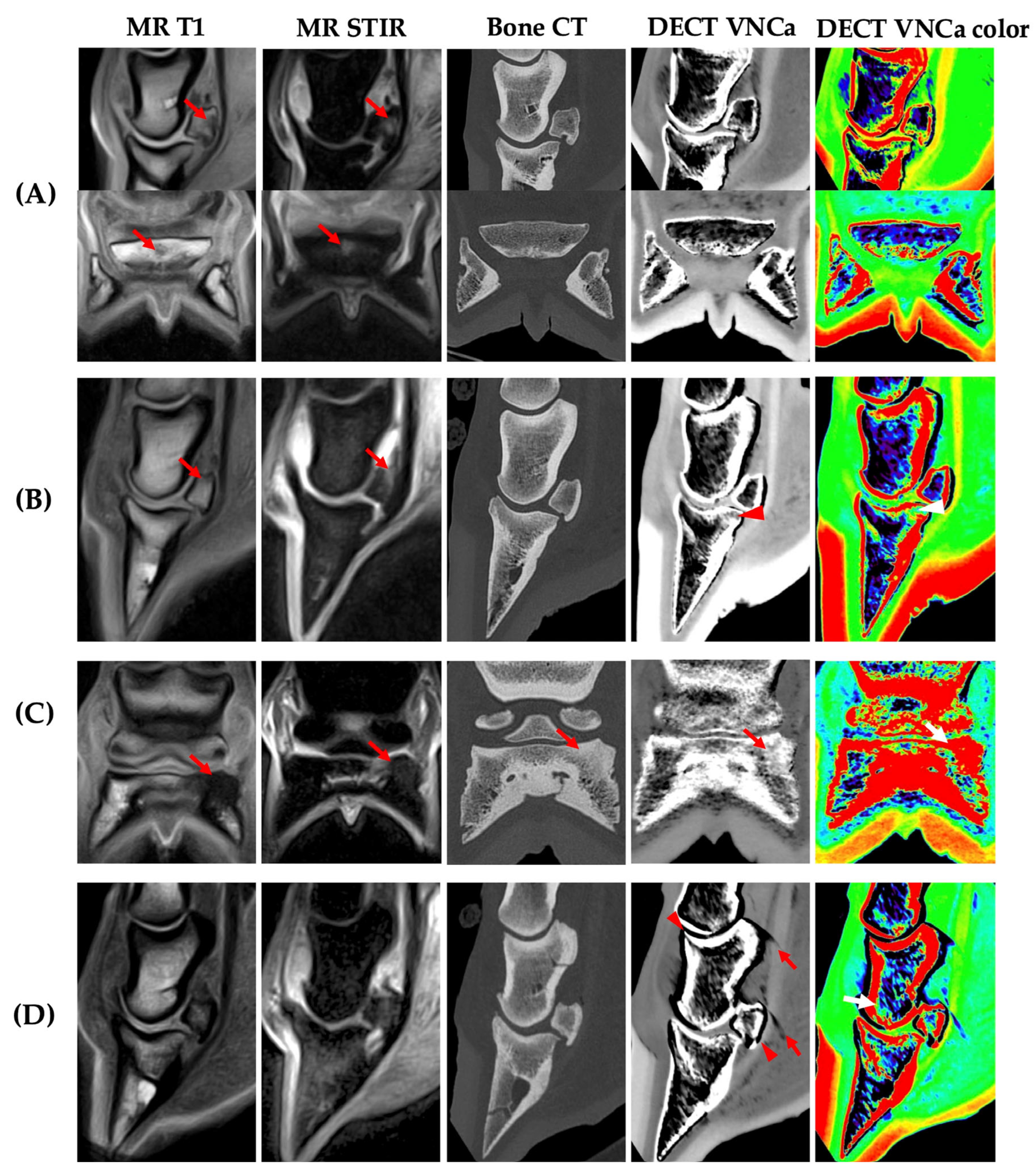
3. Results
3.1. Study Cohort Characteristics
3.2. Image Analysis
3.3. Standing Versus Recumbent Group
3.4. Effect of BME Type
3.5. Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDR | Adaptive Iterative Dose Reduction |
| BME | Bone edema-like lesion |
| CT | Computed tomography |
| DECT | Dual-energy computed tomography |
| ET | Echo time |
| FC | Filter convolution |
| GE | Gradient echo |
| IT | Inversion time |
| kVp | Kilovoltage peak |
| MRI | Magnetic resonance imaging |
| PACS | Picture archiving and communication system |
| PM | Post-mortem |
| RT | Repetition time |
| STIR | Short-tau inversion recovery |
| VNCa | Virtual-non-calcium |
References
- Dixon, J.; Müksch, G.; Witte, T.; Perkins, J.; Weller, R. Standing equine computed tomography: Technique and clinical use. In Yearbook of European Association of Veterinary Diagnostic Imaging Forum; European Association of Veterinary Diagnostic Imaging: Cambridge, UK, 2016; pp. 31–50. [Google Scholar]
- Mageed, M. Standing computed tomography of the equine limb using a multi-slice helical scanner: Technique and feasibility study. Equine Vet. Educ. 2020, 34, 77–83. [Google Scholar] [CrossRef]
- Moliner, L.; Bolas, N.; Pallas, E.; Rodríguez-Álvarez, M.J.; Benlloch, J.M. Development of a standing equine Leg CT (slCT). J. Instrum. 2022, 17, C03003. [Google Scholar] [CrossRef]
- Porter, E.G.; Werpy, N.M. New concepts in standing advanced diagnostic equine imaging. Vet. Clin. N. Am. Equine Pract. 2014, 30, 239–268. [Google Scholar] [CrossRef]
- Mizobe, F.; Nomura, M.; Ueno, T.; Yamada, K. Bone marrow oedema-type signal in the proximal phalanx of Thoroughbred racehorses. J. Vet. Med. Sci. 2019, 81, 593–597. [Google Scholar] [CrossRef]
- Olive, J.; Mair, T.; Charles, B. Use of standing low-field magnetic resonance imaging to diagnose middle phalanx bone marrow lesions in horses. Equine Vet. Educ. 2009, 21, 116–123. [Google Scholar] [CrossRef]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in X-ray computerized tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Avrin, D.E.; Macovski, A.; Zatz, L.E. Clinical application of Compton and photo-electric reconstruction in computed tomography: Preliminary results. Investig. Radiol. 1978, 13, 217–222. [Google Scholar] [CrossRef]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Omoumi, P.; Becce, F.; Racine, D.; Ott, J.G.; Andreisek, G.; Verdun, F.R. Dual-energy CT: Basic principles, technical approaches, and applications in musculoskeletal imaging (Part 1). Semin. Musculoskelet. Radiol. 2015, 19, 431–437. [Google Scholar] [CrossRef]
- Booz, C.; Nöske, J.; Albrecht, M.H.; Lenga, L.; Martin, S.S.; Bucher, A.M.; Huizinga, N.A.; Wichmann, J.L.; Vogl, T.J.; Yel, I. Diagnostic accuracy of color-coded virtual noncalcium dual-energy CT for the assessment of bone marrow edema in sacral insufficiency fracture in comparison to MRI. Eur. J. Radiol. 2020, 129, 109046. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Herregods, N.; Jaremko, J.L.; Carron, P.; Elewaut, D.; Van den Bosch, F.; Jans, L. Bone marrow edema in sacroiliitis: Detection with dual-energy CT. Eur. Radiol. 2020, 30, 3393–3400. [Google Scholar] [CrossRef]
- Diekhoff, T.; Ziegeler, K.; Feist, E.; Kiefer, T.; Mews, J.; Hamm, B.; Hermann, K.G. First experience with single-source dual-energy computed tomography in six patients with acute arthralgia: A feasibility experiment using joint aspiration as a reference. Skelet. Radiol. 2015, 44, 1573–1577. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-energy CT for the musculoskeletal system. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Yadav, H.; Khanduri, S.; Yadav, P.; Pandey, S.; Yadav, V.K.; Khan, S. Diagnostic accuracy of dual energy CT in the assessment of traumatic bone marrow edema of lower limb and its correlation with MRI. Indian J. Radiol. Imaging 2020, 30, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, G.; Chang, X. Diagnostic accuracy of dual-energy computed tomography in bone marrow edema with vertebral compression fractures: A meta-analysis. Eur. J. Radiol. 2018, 99, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.E.; Broeckx, B.J.G.; Martens, A.; Raes, E.; Haardt, H.; Vanderperren, K. Single energy metal artifact reduction performs better than virtual monoenergetic dual-energy reconstruction in CT of the equine proximal phalanx. Vet. Radiol. Ultrasound 2023, 64, 677–685. [Google Scholar] [CrossRef]
- Germonpré, J.; Vandekerckhove, L.M.J.; Raes, E.; Chiers, K.; Jans, L.; Vanderperren, K. Post-mortem feasibility of dual-energy computed tomography in the detection of bone edema-like lesions in the equine foot: A proof of concept. Front. Vet. Sci. 2024, 10, 1201017. [Google Scholar] [CrossRef]
- Steiner, J.; Richter, H.; Kaufmann, R.; Ohlerth, S. Characterization of Normal Bone in the Equine Distal Limb with Effective Atomic Number and Electron Density Determined with Single-Source Dual Energy and Detector-Based Spectral Computed Tomography. Animals 2024, 14, 1064. [Google Scholar] [CrossRef]
- Deppe, D.; Ziegeler, K.; Hermann, K.G.A.; Proft, F.; Poddubnyy, D.; Radny, F.; Makowski, M.R.; Muhle, M.; Diekhoff, T. Dual-Energy-CT for Osteitis and Fat Lesions in Axial Spondyloarthritis: How Feasible is Low-Dose Scanning? Diagnostics 2023, 13, 776. [Google Scholar] [CrossRef]
- Foti, G.; Serra, G.; Iacono, V.; Zorzi, C. Identification of Traumatic Bone Marrow Oedema: The Pearls and Pitfalls of Dual-Energy CT (DECT). Tomography 2021, 7, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Faccioli, N.; Silva, R.; Oliboni, E.; Zorzi, C.; Carbognin, G. Bone marrow edema around the hip in non-traumatic pain: Dual-energy CT vs. MRI. Eur. Radiol. 2020, 30, 4098–4106. [Google Scholar] [CrossRef] [PubMed]
- Diekhoff, T.; Hermann, K.G.; Pumberger, M.; Hamm, B.; Putzier, M.; Fuchs, M. Dual-energy CT virtual non-calcium technique for detection of bone marrow edema in patients with vertebral fractures: A prospective feasibility study on a single-source volume CT scanner. Eur. J. Radiol. 2017, 87, 59–65. [Google Scholar] [CrossRef]
- Eustace, S.; Keogh, C.; Blake, M.; Ward, R.; Oder, P.; Dimasi, M. MR imaging of bone oedema: Mechanisms and interpretation. Clin. Radiol. 2001, 56, 4–12. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Breitenseher, M.J.; Kramer, J.; Aigner, N.; Norden, C.; Hofmann, S. STIR vs. T1-weighted fat-suppressed gadolinium-enhanced MRI of bone marrow edema of the knee: Quantitative comparison. J. Magn. Reson. Imaging 2005, 22, 788–793. [Google Scholar] [CrossRef]
- Cao, J.X.; Wang, Y.M.; Kong, X.Q.; Yang, C.; Wang, P. Good interrater reliability of a new grading system in detecting traumatic bone marrow lesions in the knee by dual energy CT virtual noncalcium images. Eur. J. Radiol. 2015, 84, 1109–1115. [Google Scholar] [CrossRef]
- Diekhoff, T.; Hermann, K.G.A.; Lambert, R.G. Future of low-dose computed tomography and dual-energy computed tomography in axial spondyloarthritis. Curr. Rheumatol. Rep. 2022, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, R.; Gnannt, R.; Hodler, J.; Krauss, B.; Wanner, G.A.; Csuka, E.; Payne, B.; Frauenfelder, T.; Andreisek, G.; Alkadhi, H. Diagnostic performance of dual-energy CT for the detection of traumatic bone marrow lesions in the ankle: Comparison with MR imaging. Radiology 2012, 264, 164–173. [Google Scholar] [CrossRef]
- Vandersmissen, M.; Evrard, L.; Busoni, V. Prevalence and distribution of distal phalanx STIR hypersignal on MR images of 129 equine feet [Poster presentation]. In Proceedings of the European Veterinary Diagnostic Imaging (EVDI) Meeting, Basel, Switzerland, 21–24 August 2019. [Google Scholar]
- Cavallaro, M.; D’Angelo, T.; Albrecht, M.H.; Yel, I.; Martin, S.S.; Wichmann, J.L.; Lenga, L.; Mazziotti, S.; Blandino, A.; Ascenti, G.; et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur. Radiol. 2022, 32, 561–571. [Google Scholar] [CrossRef]
- Heales, C.J.; Summers, I.R.; Fulford, J.; Knapp, K.M.; Winlove, C.P. Investigation of changes in bone density and chemical composition associated with bone marrow oedema-type appearances in magnetic resonance images of the equine forelimb. BMC Musculoskelet. Disord. 2019, 20, 330. [Google Scholar] [CrossRef]
- Diekhoff, T.; Engelhard, N.; Fuchs, M.; Pumberger, M.; Putzier, M.; Mews, J.; Makowski, M.; Hamm, B.; Hermann, K.-G.A. Single-source dual-energy computed tomography for the assessment of bone marrow oedema in vertebral compression fractures: A prospective diagnostic accuracy study. Eur. Radiol. 2019, 29, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-K.; Tsai, J.-M.; Chuang, M.-T.; Wang, M.-T.; Huang, K.-Y.; Lin, R.-M. Bone marrow edema in vertebral compression fractures: Detection with dual-energy CT. Radiology 2013, 269, 525–533. [Google Scholar] [CrossRef]
- Mens, M.A.; de Geus, A.; Wellenberg, R.H.H.; Streekstra, G.J.; Weil, N.L.; Bus, S.A.; Busch-Westbroek, T.E.; Nieuwdorp, M.; Maas, M. Preliminary evaluation of dual-energy CT to quantitatively assess bone marrow edema in patients with diabetic foot ulcers and suspected osteomyelitis. Eur. Radiol. 2023, 33, 5645–5652. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Catania, M.; Caia, S.; Romano, L.; Beltramello, A.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the ankle: Diagnostic accuracy of dual-energy CT in comparison with MRI. Radiol. Medica 2019, 124, 1028–1036. [Google Scholar] [CrossRef]
- Foti, G.; Mantovani, W.; Faccioli, N.; Crivellari, G.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the knee: Diagnostic accuracy of dual-energy CT in comparison with MRI. Radiol. Medica 2021, 126, 405–413. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Liang, Z.; Liu, Y. Diagnostic efficacy of dual-energy CT virtual non-calcium technique in the diagnosis of bone marrow edema of sacroiliac joints in ankylosing spondylitis. J. Orthop. Surg. Res. 2025, 20, 28. [Google Scholar] [CrossRef]
- Hagen, F.; Fritz, J.; Mair, A.; Horger, M.; Bongers, M.N. Dual-energy computed tomography-based quantitative bone marrow imaging in non-hematooncological subjects: Associations with age, gender and other variables. J. Clin. Med. 2022, 11, 4094. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Y.; Gill, K.G.; Rebsamen, S.L.; Nguyen, J.C. MR imaging of pediatric bone marrow. Radiographics 2016, 36, 1911–1930. [Google Scholar] [CrossRef]
- Laor, T.; Jaramillo, D. MR imaging insights into skeletal maturation: What is normal? Radiology 2009, 250, 28–38. [Google Scholar] [CrossRef]
- Małkiewicz, A.; Dziedzic, M. Bone marrow reconversion—Imaging of physiological changes in bone marrow. Pol. J. Radiol. 2012, 77, 45–50. [Google Scholar] [CrossRef]
- D’Angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: Clinical applications. Eur. Radiol. Exp. 2021, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Yun, S.J.; Jin, W.; Lee, S.H.; Park, S.Y.; Ryu, C.W. Dual-energy CT for the diagnosis of vertebral bone marrow edema: A systematic review and meta-analysis. Eur. Radiol. 2023, 33, 1201–1210. [Google Scholar] [CrossRef]


| Esaote Vet-MR Grande | Hallmarq Standing MRI | |||||||
|---|---|---|---|---|---|---|---|---|
| Sequence | TR | TE | TI | Slice | TR | TE | TI | Slice |
| T1 | 22 | 9 | - | 0.43 | 24 | 7 | - | 3 |
| STIR | 4480–5580 | 30 | 70–75 | 3.5 | 1500 | 8 | 90 | 5 |
| T2 | 4260 | 100 | - | 4 | 1848 | 81 | - | 5 |
| GET2 | 1925 | 22 | - | 3.5 | 34 | 13 | - | 3 |
| Category | Modality | Scores | |
|---|---|---|---|
| Bone sclerosis | DE Bone CT | (0) none, (1) mild, (2) moderate, (3) severe | |
| Beam-hardening artifact | DE Bone CT | (0) none, (1) mild, (2) moderate, (3) severe | |
| Motion artifact | DE Bone CT | (0) none, (1) mild, (2) moderate, (3) severe | |
| Image quality | DECT VNCa | (0) non-diagnostic, (1) poor, (2) good, (3) excellent | |
| BME location | DECT VNCa |
|
|
| MRI | |||
| BME extent | DECT VNCa | (0) none, (1) mild, (2) moderate, (3) severe | |
| MRI | |||
| BME location agreement | DECT VNCa MRI | Yes or no | |
| BME | MRI(+) | MRI(−) |
|---|---|---|
| DECT VNCa(+) | 13 | 1 |
| DECT VNCa(−) | 1 | 4 |
| BME | MRI(+) | MRI(−) |
|---|---|---|
| DECT VNCa(+) | 8 | 0 |
| DECT VNCa(−) | 2 | 9 |
| BME | MRI(+) | MRI(−) |
|---|---|---|
| DECT VNCa(+) | 0 | 1 |
| DECT VNCa(−) | 0 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germonpré, J.; Lorenz, I.; Vandekerckhove, L.M.J.; Duchateau, L.; Diekhoff, T.; Vanderperren, K. Dual-Energy Computed Tomography for the Detection of Bone Edema-Like Lesions in the Equine Foot: Standing Horses and Cadaveric Specimens. Vet. Sci. 2025, 12, 614. https://doi.org/10.3390/vetsci12070614
Germonpré J, Lorenz I, Vandekerckhove LMJ, Duchateau L, Diekhoff T, Vanderperren K. Dual-Energy Computed Tomography for the Detection of Bone Edema-Like Lesions in the Equine Foot: Standing Horses and Cadaveric Specimens. Veterinary Sciences. 2025; 12(7):614. https://doi.org/10.3390/vetsci12070614
Chicago/Turabian StyleGermonpré, Jolien, Ina Lorenz, Louis M. J. Vandekerckhove, Luc Duchateau, Torsten Diekhoff, and Katrien Vanderperren. 2025. "Dual-Energy Computed Tomography for the Detection of Bone Edema-Like Lesions in the Equine Foot: Standing Horses and Cadaveric Specimens" Veterinary Sciences 12, no. 7: 614. https://doi.org/10.3390/vetsci12070614
APA StyleGermonpré, J., Lorenz, I., Vandekerckhove, L. M. J., Duchateau, L., Diekhoff, T., & Vanderperren, K. (2025). Dual-Energy Computed Tomography for the Detection of Bone Edema-Like Lesions in the Equine Foot: Standing Horses and Cadaveric Specimens. Veterinary Sciences, 12(7), 614. https://doi.org/10.3390/vetsci12070614






