Successful Management of a Pancreatic Abscess in a Dog with Juvenile Diabetes Mellitus Through Ultrasound-Guided Drainage and Medical Therapy
Simple Summary
Abstract
1. Introduction
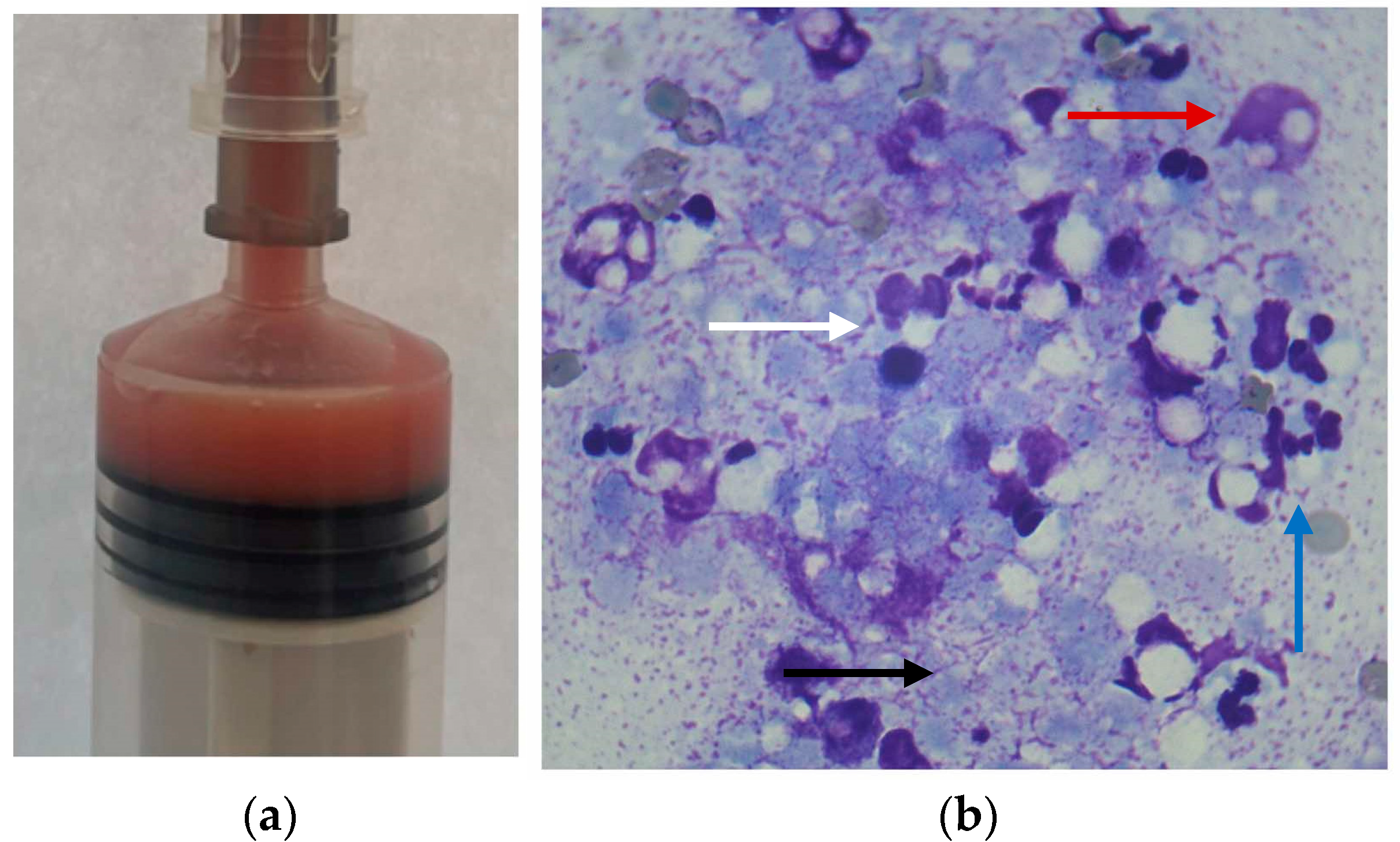
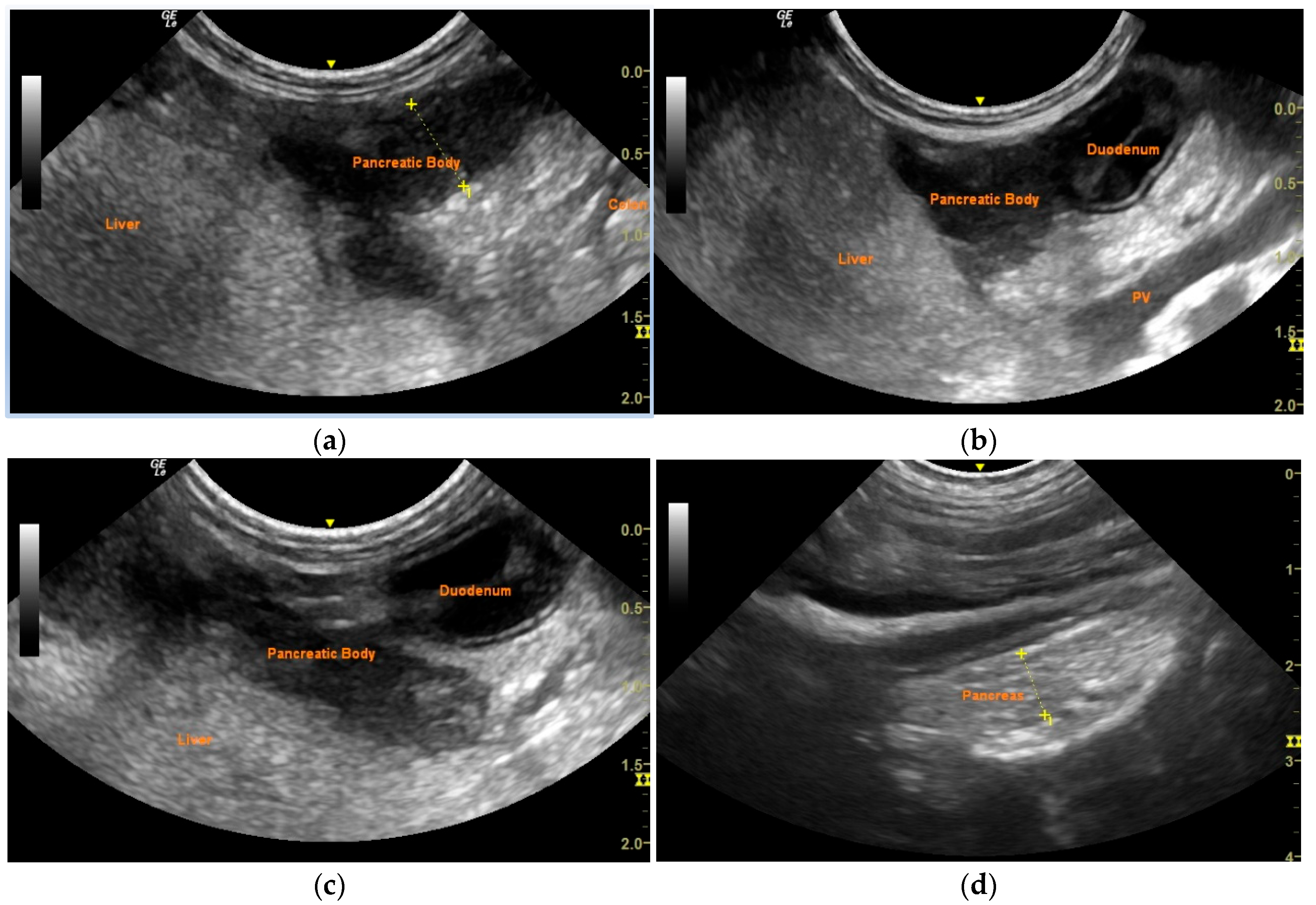
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| CRT | Capillary refill time |
| Spec cPL | Specific pancreatic lipase |
| cTLI | Canine trypsin-like immunoreactivity |
| EPI | Exocrine pancreatic insufficiency |
References
- Warshaw, A.L. Inflammatory Masses Following Acute Pancreatitis: Phlegmon, Pseudocyst, and Abscess. Surg. Clin. N. Am. 1974, 54, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, S.K.; Lantz, G.C.; Nelson, R.W.; Kazacos, E.A. Pancreatic Abscess in Dogs: Six Cases (1978–1986). J. Am. Vet. Med. Assoc. 1988, 193, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Bauer, M.; Walker, M.; Pardo, A.D.; McCracken, M.; Walker, T. Pancreatic Masses in Seven Dogs Following Acute Pancreatitis. J. Am. Anim. Hosp. Assoc. 1990, 26, 189–198. [Google Scholar]
- VanEnkevort, B.A.; O’Brien, R.T.; Young, K.M. Pancreatic Pseudocysts in 4 Dogs and 2 Cats: Ultrasonographic and Clinicopathologic Findings. J. Vet. Intern. Med. 1999, 13, 309–313. [Google Scholar] [CrossRef]
- Stimson, E.L.; Espada, Y.; Moon, M.; Troy, G.C. Pancreatic Abscess in Nine Dogs. J. Vet. Intern. Med. 1998, 9, 202. [Google Scholar]
- Coleman, M.; Robson, M. Pancreatic Masses Following Pancreatitis: Pancreatic Pseudocysts, Necrosis, and Abscesses. Contin. Educ. Vet. 2005, 27, 147–153. [Google Scholar]
- Johnson, M.D.; Mann, F.A. Treatment for Pancreatic Abscesses via Omentalization with Abdominal Closure versus Open Peritoneal Drainage in Dogs: 15 Cases (1994–2004). J. Am. Vet. Med. Assoc. 2006, 228, 397–402. [Google Scholar] [CrossRef]
- Anderson, J.R.; Cornell, K.K.; Parnell, N.K.; Salisbury, S.K. Pancreatic Abscess in 36 Dogs: A Retrospective Analysis of Prognostic Indicators. J. Am. Anim. Hosp. Assoc. 2008, 44, 171–179. [Google Scholar] [CrossRef]
- Akol, K.G.; Washabau, R.J.; Saunders, H.M.; Hendrick, M.J. Acute Pancreatitis in Cats with Hepatic Lipidosis. J. Vet. Intern. Med. 1993, 7, 205–209. [Google Scholar] [CrossRef]
- Saunders, H.M.; VanWinkle, T.J.; Drobatz, K.; Kimmel, S.E.; Washabau, R.J. Ultrasonographic Findings in Cats with Clinical, Gross Pathologic, and Histologic Evidence of Acute Pancreatic Necrosis: 20 Cases (1994–2001). J. Am. Vet. Med. Assoc. 2002, 221, 1724–1730. [Google Scholar] [CrossRef]
- Hecht, S.; Henry, G. Sonographic Evaluation of the Normal and Abnormal Pancreas. Clin. Tech. Small Anim. Pract. 2007, 22, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, N.; Ohlerth, S.; Scharf, G.; Laluhova, D.; Sieber-Ruckstuhl, N.; Alt, M.; Roos, M.; Grest, P.; Kaser-Hotz, B. Contrast-Enhanced Power and Color Doppler Ultrasonography of the Pancreas in Healthy and Diseased Cats. J. Vet. Intern. Med. 2008, 22, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.L. A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, Ga, September 11 through 12, 1992. Arch. Surg. 1993, 128, 586–590. [Google Scholar] [CrossRef]
- van Santvoort, H.C.; Besselink, M.G.; Bakker, O.J.; Hofker, H.S.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Schaapherder, A.F.; van Eijck, C.H.; Bollen, T.L.; et al. A Step-up Approach or Open Necrosectomy for Necrotizing Pancreatitis. N. Engl. J. Med. 2010, 362, 1491–1502. [Google Scholar] [CrossRef]
- Varadarajulu, S.; Bang, J.H.; Phadnis, M.A.; Christein, J.D.; Wilcox, C.M. Endoscopic Transmural Drainage of Peripancreatic Fluid Collections: Outcomes and Predictors of Treatment Success in 211 Consecutive Patients. J. Gastrointest. Surg. 2011, 15, 2080–2088. [Google Scholar] [CrossRef]
- Hollemans, R.A.; Bakker, O.J.; Boermeester, M.A.; Bollen, T.L.; Bosscha, K.; Bruno, M.J.; Buskens, E.; Dejong, C.H.; van Duijvendijk, P.; van Eijck, C.H.; et al. Superiority of Step-up Approach vs Open Necrosectomy in Long-Term Follow-up of Patients with Necrotizing Pancreatitis. Gastroenterology 2019, 156, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Goyal, J.; Ramesh, J. Endoscopic Management of Peripancreatic Fluid Collections. Frontline Gastroenterol. 2014, 6, 199–207. [Google Scholar] [CrossRef]
- Talbot, C.T.; Cheung, R.; Holmes, E.J.; Cook, S.D. Medical and Surgical Management of Pancreatic Fluid Accumulations in Dogs: A Retrospective Study of 15 Cases. J. Vet. Intern. Med. 2022, 36, 919–926. [Google Scholar] [CrossRef]
- Nemoto, Y.; Haraguchi, T.; Shimokawa Miyama, T.; Kobayashi, K.; Hama, K.; Kurogouchi, Y.; Fujiki, N.; Baba, K.; Okuda, M.; Mizuno, T. Pancreatic Abscess in a Cat Due to Staphylococcus aureus Infection. J. Vet. Med. Sci. 2017, 79, 1146–1150. [Google Scholar] [CrossRef]
- Lee, M.; Kang, J.-H.; Chang, D.; Na, K.-J.; Yang, M.-P. Pancreatic Abscess in a Cat with Diabetes Mellitus. J. Am. Anim. Hosp. Assoc. 2015, 51, 180–184. [Google Scholar] [CrossRef]
- Smith, S.; Biller, D. Resolution of a Pancreatic Pseudocyst in a Dog Following Percutaneous Ultrasonographic-Guided Drainage. J. Am. Anim. Hosp. Assoc. 1998, 34, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.S.; Chastain, C.B. Endocrine and Metabolic System. In Veterinary Pediatrics: Dogs and Cats from Birth to Six Months, 3rd ed.; Hoskins, J.D., Ed.; Saunders: Philadelphia, PA, USA, 2001; pp. 344–370. [Google Scholar]
- Greco, D.S. Pediatric Endocrinology. Vet. Clin. Small Anim. Pract. 2006, 36, 549–556. [Google Scholar] [CrossRef]
- Gilor, C.; Niessen, S.J.M.; Furrow, E.; DiBartola, S.P. What’s in a Name? Classification of Diabetes Mellitus in Veterinary Medicine and Why It Matters. J. Vet. Intern. Med. 2016, 30, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, A.L.; Richter, J.M. A Practical Guide to Pancreatitis. Curr. Probl. Surg. 1984, 21, 6–79. [Google Scholar] [CrossRef]
- Warshaw, A.L.; Jin, G.L. Improved Survival in 45 Patients with Pancreatic Abscess. Ann. Surg. 1985, 202, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Saxon, A.; Reynolds, J.T.; Doolas, A. Management of Pancreatic Abscesses. Ann. Surg. 1981, 194, 545–552. [Google Scholar] [CrossRef]
- Amano, H.; Takada, T.; Isaji, S.; Takeyama, Y.; Hirata, K.; Yoshida, M.; Mayumi, T.; Yamanouchi, E.; Gabata, T.; Kadoya, M.; et al. Therapeutic Intervention and Surgery of Acute Pancreatitis. J. Hepatobiliary Pancreat. Sci. 2009, 17, 53–59. [Google Scholar] [CrossRef]
- Schaer, M. Abscess, Necrosis, Pseudocyst, Phlegmon, and Infection. In Canine & Feline Gastroenterology; Washabau, R.J., Day, M.J., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2013; pp. 829–834. [Google Scholar]
- Kang, J.-H.; Na, K.-J.; Mo, I.-P.; Chang, D.; Yang, M.-P. Juvenile Diabetes Mellitus Accompanied by Exocrine Pancreatic Insufficiency in a Dog. J. Vet. Med. Sci. 2008, 70, 1337–1340. [Google Scholar] [CrossRef]
- Mamom, T.; Rungpupradit, J. Diabetes Mellitus Concurrent with Exocrine Pancreatic Insufficiency in a Young Golden Retriever Dog: A Clinicopathological Report. J. Mahanakorn Vet. Med. 2010, 5, 51–60. [Google Scholar]
- Neiger, R.; Jaunin, V.B.; Boujon, C.E. Exocrine Pancreatic Insufficiency Combined with Insulin-Dependent Diabetes Mellitus in a Juvenile German Shepherd Dog. J. Small Anim. Pract. 1996, 37, 344–349. [Google Scholar] [CrossRef]
- Chen, J.; Fukami, N.; Li, Z. Endoscopic Approach to Pancreatic Pseudocyst, Abscess and Necrosis: Review on Recent Progress. Dig. Endosc. 2012, 24, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES Guidelines for the Management of Severe Acute Pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Seshadri, R.; Raffe, M.R. Characteristics and Outcomes in Surgical Management of Severe Acute Pancreatitis: 37 Dogs (2001–2007). J. Vet. Emerg. Crit. Care 2009, 19, 165–173. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daravigka, A.; Ninis, S.; Bourdekas, P.; Konstantinidis, A.O.; Ginoudis, A.; Adamama-Moraitou, K.K.; Lyraki, M.; Soubasis, N. Successful Management of a Pancreatic Abscess in a Dog with Juvenile Diabetes Mellitus Through Ultrasound-Guided Drainage and Medical Therapy. Vet. Sci. 2025, 12, 604. https://doi.org/10.3390/vetsci12070604
Daravigka A, Ninis S, Bourdekas P, Konstantinidis AO, Ginoudis A, Adamama-Moraitou KK, Lyraki M, Soubasis N. Successful Management of a Pancreatic Abscess in a Dog with Juvenile Diabetes Mellitus Through Ultrasound-Guided Drainage and Medical Therapy. Veterinary Sciences. 2025; 12(7):604. https://doi.org/10.3390/vetsci12070604
Chicago/Turabian StyleDaravigka, Alexandra, Stefanos Ninis, Panagiotis Bourdekas, Alexandros O. Konstantinidis, Argyrios Ginoudis, Katerina K. Adamama-Moraitou, Maria Lyraki, and Nektarios Soubasis. 2025. "Successful Management of a Pancreatic Abscess in a Dog with Juvenile Diabetes Mellitus Through Ultrasound-Guided Drainage and Medical Therapy" Veterinary Sciences 12, no. 7: 604. https://doi.org/10.3390/vetsci12070604
APA StyleDaravigka, A., Ninis, S., Bourdekas, P., Konstantinidis, A. O., Ginoudis, A., Adamama-Moraitou, K. K., Lyraki, M., & Soubasis, N. (2025). Successful Management of a Pancreatic Abscess in a Dog with Juvenile Diabetes Mellitus Through Ultrasound-Guided Drainage and Medical Therapy. Veterinary Sciences, 12(7), 604. https://doi.org/10.3390/vetsci12070604






