Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
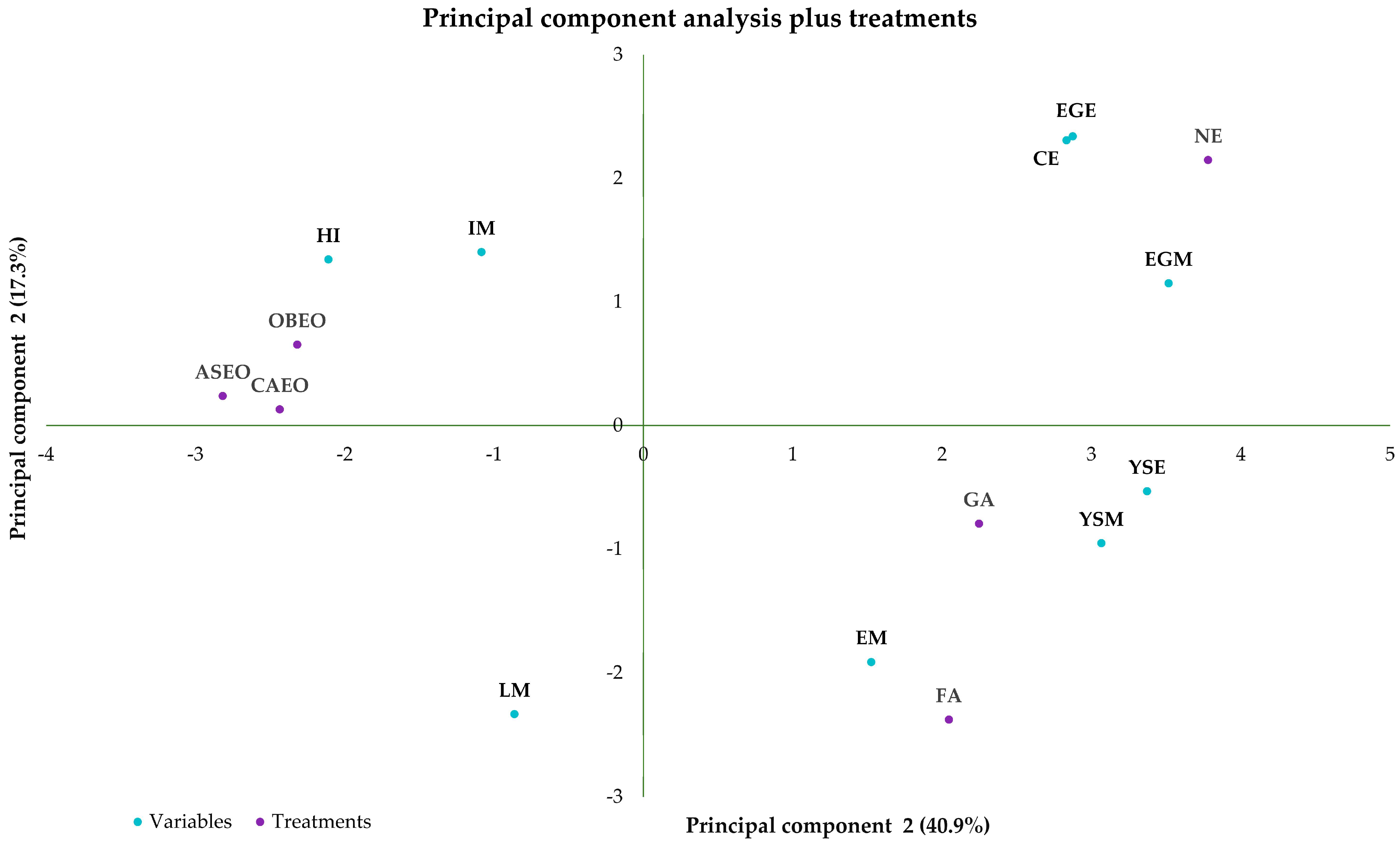
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, C.; Li, Q.; Lan, F.; Li, X.; Li, G.; Yan, Y.; Wu, G.; Yang, N.; Sun, C. Microbiota continuum along the chicken oviduct and its association with host genetics and egg formation. Poult. Sci. 2021, 100, 101104. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Pena, P.; Cervantes, R.; Dias, M.; Viegas, C. Microbial contamination of bedding material: One health in poultry production. Int. J. Environ. Res. Public Health 2022, 19, 16508. [Google Scholar] [CrossRef] [PubMed]
- Jahantigh, M.; Rashki, A.; Najimi, M. A study on bacterial flora and antibacterial resistance of yolk sac infection in Japanese quail (Coturnix japonica). Comp. Clin. Pathol. 2013, 22, 645–648. [Google Scholar] [CrossRef]
- Eltokhy, E.; Hefny, E.; Koraney, N.; Azam, A. Eggshell contamination with special reference to different methods of disinfection on the isolated bacteria. Benha Vet. Med. J. 2021, 41, 13–18. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of sanitizers on microbiological control of hatching eggshells and poultry health during embryogenesis and early stages after hatching in the last decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; de Araújo, M.V.; de Sousa, D.E.R.; de Macêdo, I.L.; de Castro, M.B.; dos Santos, V.M. Sanitizing hatching eggs with essential oils: Avian and microbiological safety. Microorganisms 2023, 11, 1890. [Google Scholar] [CrossRef]
- Ricke, S.C.; Richardson, K.; Dittoe, D.K. Formaldehydes in feed and their potential interaction with the poultry gastrointestinal tract microbial community—A review. Front. Vet. Sci. 2019, 6, 188. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Chen, X.; Cui, H.; Lin, L. The interference mechanism of basil essential oil on the cell membrane barrier and respiratory metabolism of Listeria monocytogenes. Front. Microbiol. 2022, 13, 855905. [Google Scholar] [CrossRef]
- Melo, E.F.; Clímaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; de Souza, M.R.; Baião, N.C.; Pompeu, M.A.; Lara, L.J.C. An evaluation of alternative methods for sanitizing hatching eggs. Poult. Sci. 2019, 98, 2466–2473. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove essential oil in the sanitation of fertile eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Taşdemir, A.N.; Onbaşılar, E.E.; Yalçın, S.; Boyalı, B.; Aygören, H.; Tülek, E.; Sarıçam, S.; Akan, M. Effects of oregano juice on eggshell microbial load, layer embryo development, hatching results, and growth at the first 2 weeks after hatch. Trop. Anim. Health Prod. 2021, 53, 404. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Aragh, M.K.; Linhoss, J.E.; Evans, J.D. Effects of various disinfectants on the bacterial load and microbiome of broiler hatching eggs using electrostatic spray. J. Appl. Poult. Res. 2022, 31, 100278. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Q.; Tang, K.; Han, K.; Bai, H.; Yin, Y.; Li, C.; Ma, C.; Teng, L.; Li, J.; et al. Effect of phage spray on hatchability and chick quality of eggs contaminated with Salmonella typhimurium. Viruses 2024, 16, 1338. [Google Scholar] [CrossRef] [PubMed]
- Shahein, E.H.A.; Sedeek, E.K. Role of spraying hatching eggs with natural disinfectants on hatching characteristics and eggshell bacterial counts. Egypt. Poult. Sci. J. 2014, 34, 213–230. [Google Scholar] [CrossRef]
- Fouad, W.; Kassab, A.Y.; Abd Elshahed, A.A.; El-damrawy, S.Z. Effect of spraying moringa oil on embryonic development, hatchability, physiological parameters, post-hatch chick growth and bacterial contamination of fertile quail eggs. Egypt. J. Nutr. Feeds 2023, 26, 203–221. [Google Scholar] [CrossRef]
- Mauldin, J.M. Factors affecting hatchability. In Commercial Chicken Meat and Egg Production; Springer: Boston, MA, USA, 2022; pp. 727–773. [Google Scholar]
- King’ori, A.M. Review of the factors that influence egg fertility and hatchability in poultry. Int. J. Poult. Sci. 2011, 10, 483–484. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Santos, P.H.G.D.S.; de Sousa, D.E.R.; Jivago, J.L.D.P.R.; de Castro, M.B.; dos Santos, V.M. Hatching egg sanitizers based on essential oils: Microbiological parameters, hatchability, and poultry health. Antibiotics 2024, 13, 1066. [Google Scholar] [CrossRef]
- Copur, G.; Arslan, M.; Duru, M.; Baylan, M.; Canogullari, S.; Aksan, E. Use of oregano (Origanum onites L.) essential oil as hatching egg disinfectant. Afr. J. Biotechnol. 2010, 9, 2531–2538. [Google Scholar]
- Wells, J.B.; Coufal, C.D.; Parker, H.M.; Kiess, A.S.; Purswell, J.L.; Young, K.M.; McDaniel, C.D. Hatchability of broiler breeder eggs following eggshell sanitization by repeated treatment with a combination of ultraviolet light and hydrogen peroxide. Int. J. Poult. Sci. 2011, 10, 421–425. [Google Scholar] [CrossRef]
- Mousa-Balabel, T.M.; Mohamed, R.A.; Al-Midani, S.A.; El-Samad, M.S.A. Impact of boiler breeders hatching eggs disinfection time on some hatchability parameters. Int. J. Sci. Basic Appl. Res. 2016, 30, 230–240. [Google Scholar]
- Fouad, W.; Abdel-Hafez, M.S.; Abd El-Halim, H.A.H. Influence of spraying garlic oil on embryonic development, hatchability, physiological parameters, post-hatch chick growth and bacterial contamination of fertile quail eggs. Egypt. Poult. Sci. J. 2018, 38, 877–893. [Google Scholar] [CrossRef]
- Badran, A.M.M.; Osman, A.M.R.; Yassein, D.M.M. Comparative study of the effect of some disinfectants on embryonic mortality, hatchability, and some blood components. Egypt. Poult. Sci. J. 2018, 38, 1069–1081. [Google Scholar] [CrossRef]
- Ergun, F.; Taskin, A.; Ergun, D. Effects of spraying cemele pepper (Capsicum annuum L.) extracts onto hatching eggs of atak-s layer hens on hatching results, serum glutathione and malondialdehyde levels. J. Anim. Plant Sci. 2024, 34, 1210–1219. [Google Scholar] [CrossRef]
- Upadhyaya, I.; Yin, H.B.; Nair, M.S.; Chen, C.H.; Upadhyay, A.; Darre, M.J.; Venkitanarayanan, K. Efficacy of fumigation with trans-cinnamaldehyde and eugenol in reducing Salmonella enterica serovar enteritidis on embryonated eggshells. Poult. Sci. 2015, 94, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.D.S.; McManus, C.; dos Santos, V.M. Syzygium aromaticum Essential oil as a safe natural solution to control bacteria in hatching eggs. Pathogens 2025, 14, 422. [Google Scholar] [CrossRef]
- El-Soufi, A.; Al Khatib, A.; Khazaal, S.; El Darra, N.; Raafat, K. Evaluation of essential oils as natural antibacterial agents for eggshell sanitization and quality preservation. Processes 2025, 13, 224. [Google Scholar] [CrossRef]
- Hassan, A.S.I.; Morsy, E.A.; El Moustafa, K.M.; Ibrahim, F.A.; Elmenawey, M.A. Effects of clove essential oil on eggshell bacterial load, antibacterial sensitivity, and hatchability. Egypt. Pharm. J. 2023, 22, 650–658. [Google Scholar] [CrossRef]
- Bekhet, G.M. Impact of hatching egg disinfection on hatching characteristics and chick embryos. Indian J. Anim. Res. 2021, 55, 353–358. [Google Scholar] [CrossRef]
- Bekhet, G.; Khalifa, A.Y.Z. Essential oil sanitizers to sanitize hatching eggs. J. Appl. Anim. Res. 2022, 50, 695–701. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Mirza, R.A.; Aziz, H.I. Lavender essential oil in sanitation on fertile egg. Passer J. Basic Appl. Sci. 2023, 5, 377–381. [Google Scholar] [CrossRef]
- Iraqi, E.E.; El-Sahn, A.A.; El-Barbary, A.M.; Ahmed, M.M.; Elkomy, A.E. Antimicrobial activity of tea tree and lavender essential oils and their effects on hatching performance and eggshell bacterial count of Japanese quail eggs. BMC Vet. Res. 2025, 21, 176. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, O.C.; Oguike, M.A. Hatchability of fertile eggs in poultry industry. J. Agric. Sustain. 2019, 12, 107–123. [Google Scholar]
- Thermote, L. Effective Hygiene within the Hatchery. Int. Hatch. Pract. 2006, 20, 18–21. [Google Scholar]
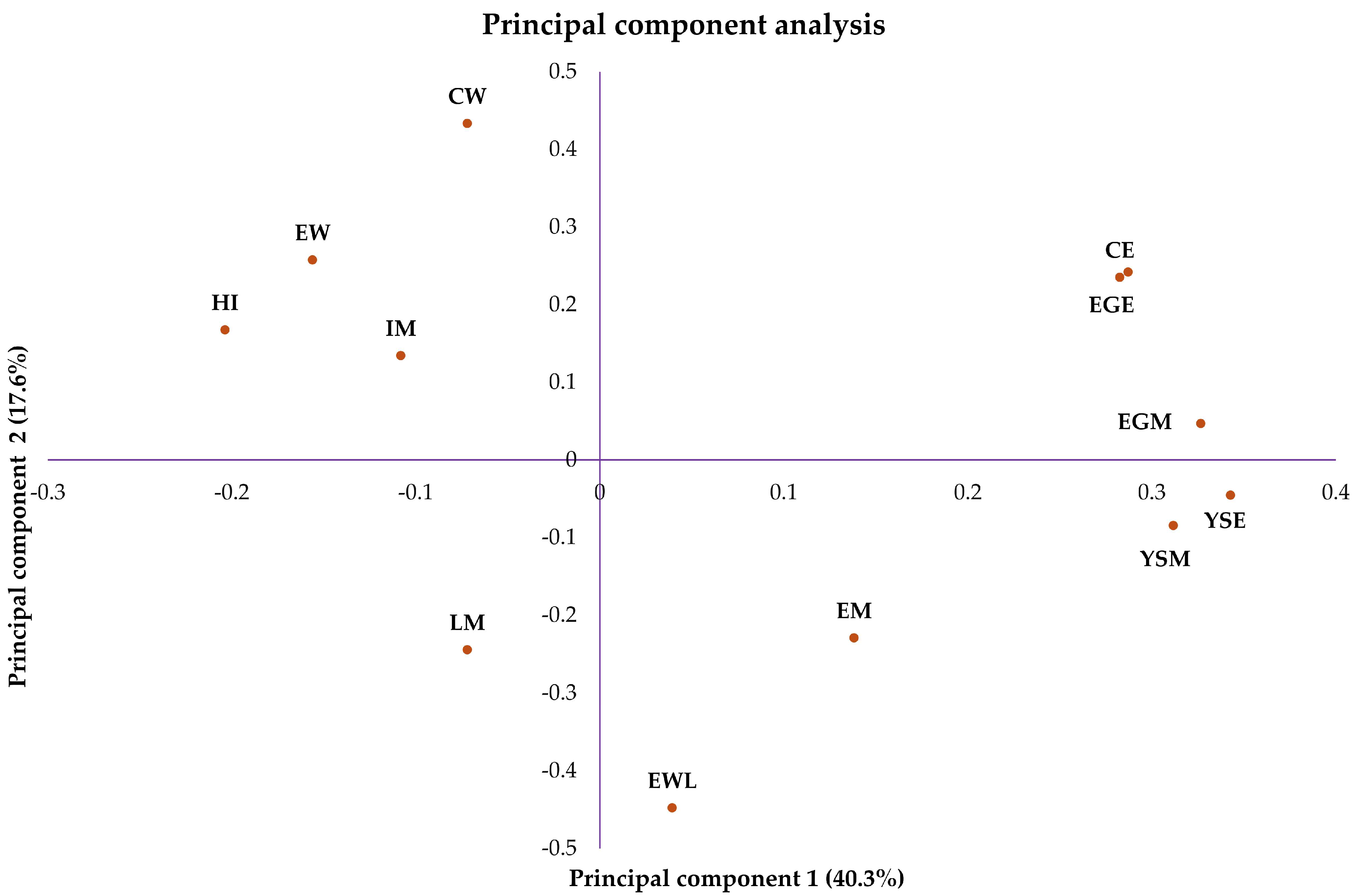
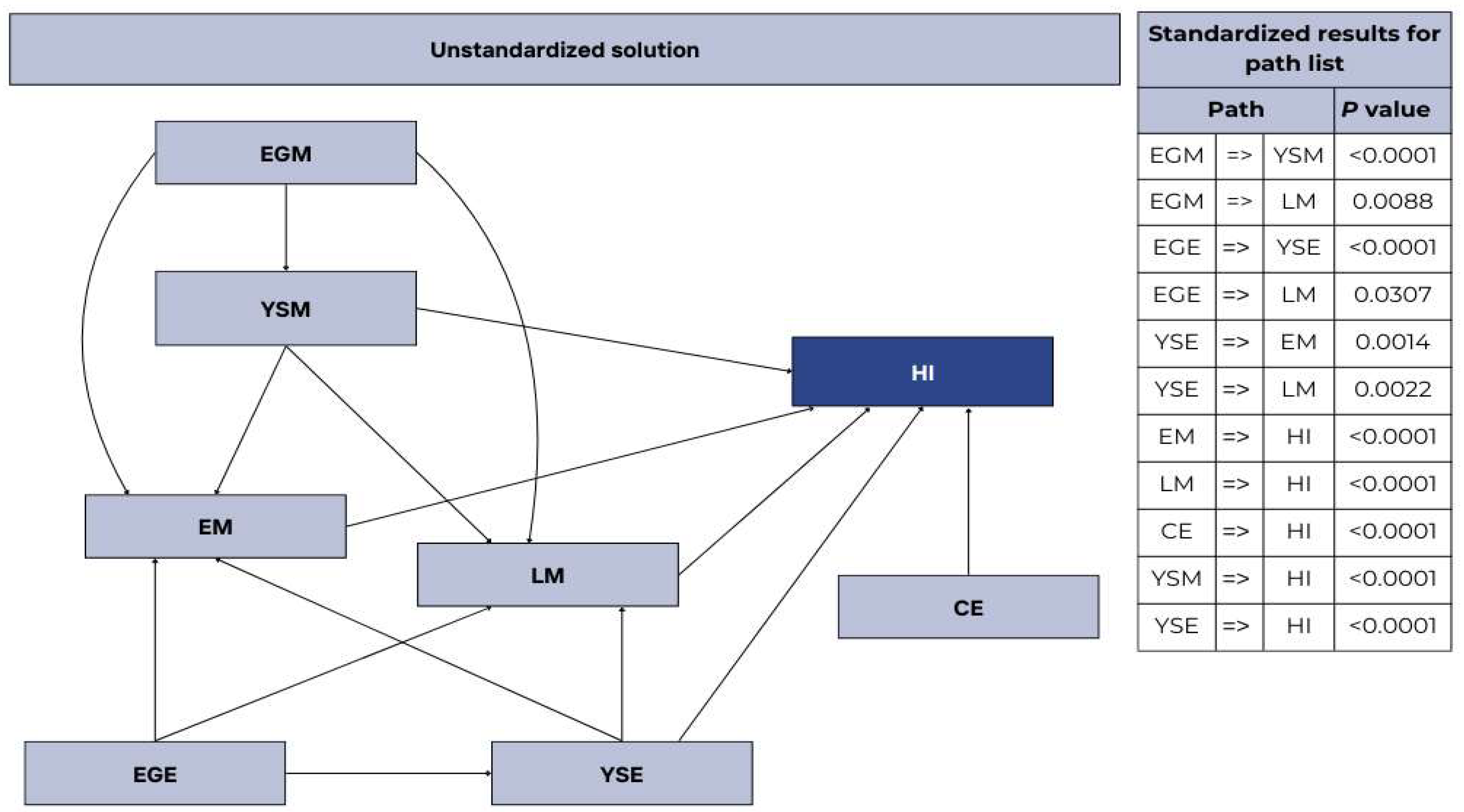
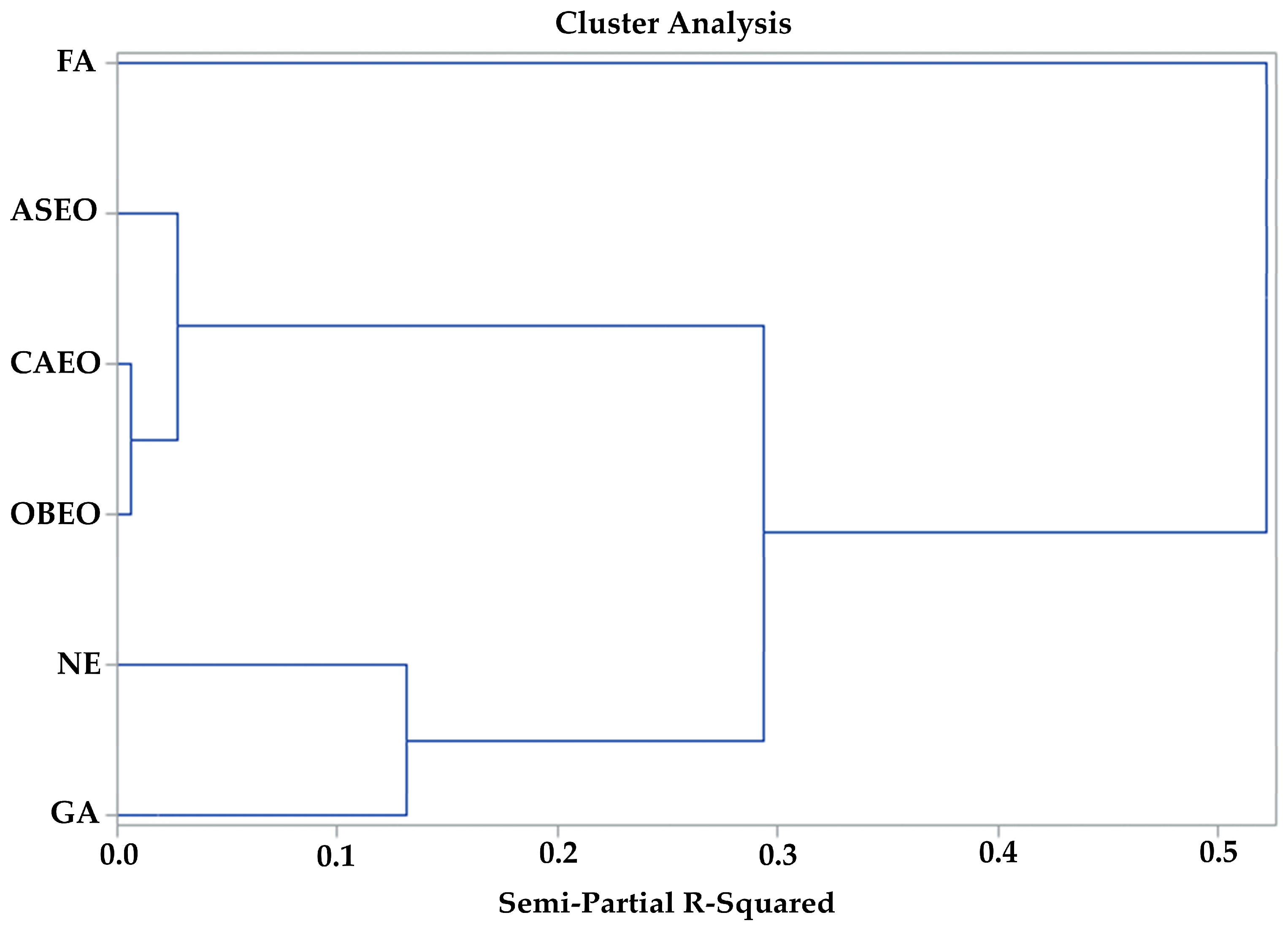
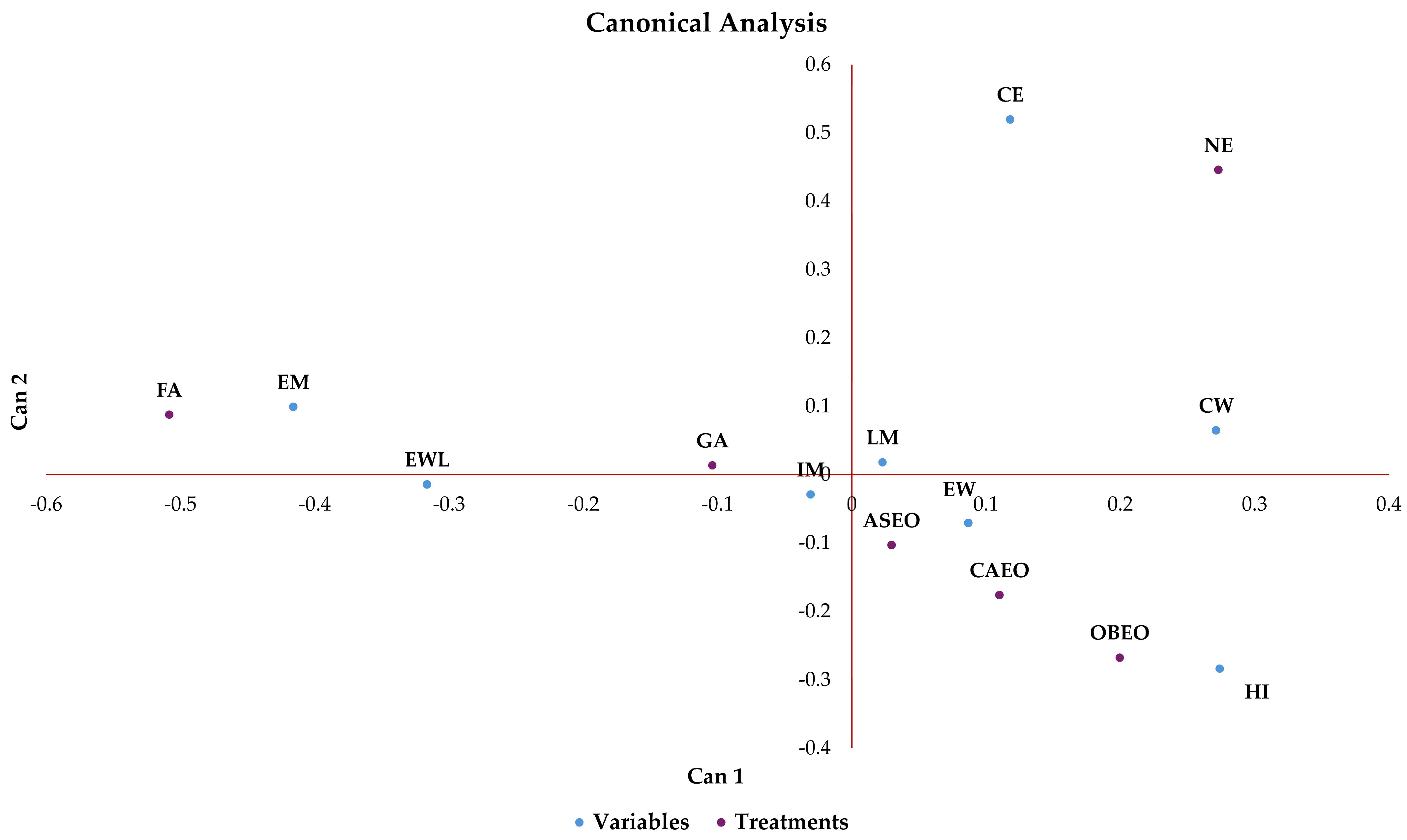
| CE | EM | IM | LM | EW | EWL | CW | YSM | YSE | EGM | EGE | |
| HI | −0.35 ns | −0.79 **** | −0.10 ns | −0.25 ns | 0.03 ns | −0.18 ns | 0.08 ns | −0.30 ns | −0.47 ns | −0.37 ns | −0.17 ns |
| CE | 0.11 ns | −0.08 ns | −0.31 ns | −0.03 ns | −0.25 ns | 0.30 ns | 0.49 * | 0.71 *** | 0.70 ** | 0.61 ** | |
| EM | −0.04 ns | −0.19 ns | −0.15 ns | 0.24 ns | −0.35 ns | 0.18 ns | 0.35 ns | 0.04 ns | 0.04 ns | ||
| IM | −0.12 ns | 0.15 ns | 0.09 ns | 0.23 ns | −0.23 ns | −0.22 ns | −0.17 ns | −0.11 ns | |||
| LM | 0.14 ns | 0.11 ns | 0.06 ns | −0.11 ns | −0.31 ns | 0.03 ns | −0.29 ns | ||||
| EW | 0.18 ns | 0.63 *** | −0.48 * | −0.42 ns | −0.35 ns | −0.38 ns | |||||
| EWL | −0.37 ns | 0.30 ns | 0.20 ns | 0.05 ns | −0.35 ns | ||||||
| CW | −0.37 ns | −0.16 ns | −0.13 ns | 0.08 ns | |||||||
| YSM | 0.82 **** | 0.76 *** | 0.62 ** | ||||||||
| YSE | 0.73 *** | 0.64 ** | |||||||||
| EGM | 0.79 **** |
| Cluster 1 vs. Clusters 2 and 3 | ||||
| Variables | R2 Partial | p < F | p < Lambda | p < ASCC |
| EGE | 0.8086 | 0.0011 | <0.0001 | <0.0001 |
| EGM | 0.8068 | 0.0024 | <0.0001 | 0.0017 |
| CE | 0.7918 | 0.0067 | <0.0001 | <0.0001 |
| EM | 0.7694 | 0.0188 | <0.0001 | 0.0002 |
| EWL | 0.7513 | 0.0432 | <0.0001 | <0.0001 |
| Cluster 2 vs. cluster 3 | ||||
| EGM | 0.8813 | 0.0005 | 0.0005 | 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils. Vet. Sci. 2025, 12, 600. https://doi.org/10.3390/vetsci12070600
Oliveira GdS, McManus C, dos Santos VM. Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils. Veterinary Sciences. 2025; 12(7):600. https://doi.org/10.3390/vetsci12070600
Chicago/Turabian StyleOliveira, Gabriel da Silva, Concepta McManus, and Vinícius Machado dos Santos. 2025. "Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils" Veterinary Sciences 12, no. 7: 600. https://doi.org/10.3390/vetsci12070600
APA StyleOliveira, G. d. S., McManus, C., & dos Santos, V. M. (2025). Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils. Veterinary Sciences, 12(7), 600. https://doi.org/10.3390/vetsci12070600








