piRNAs as Potential Regulators of Mammary Gland Development and Pathology in Livestock
Simple Summary
Abstract
1. Introduction
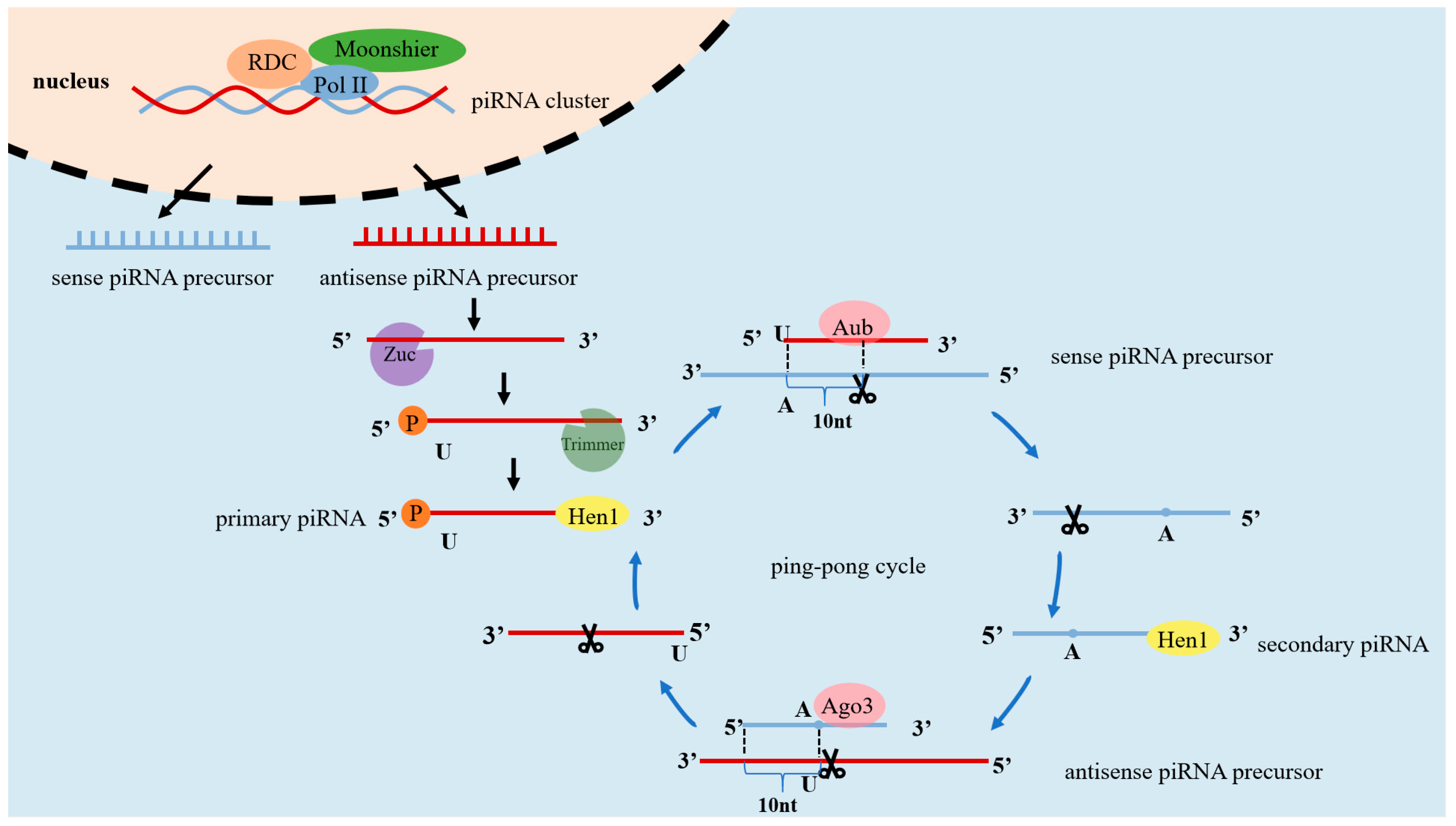
2. The Process of Generating piRNAs
2.1. Primary Processing
2.2. Secondary Amplification
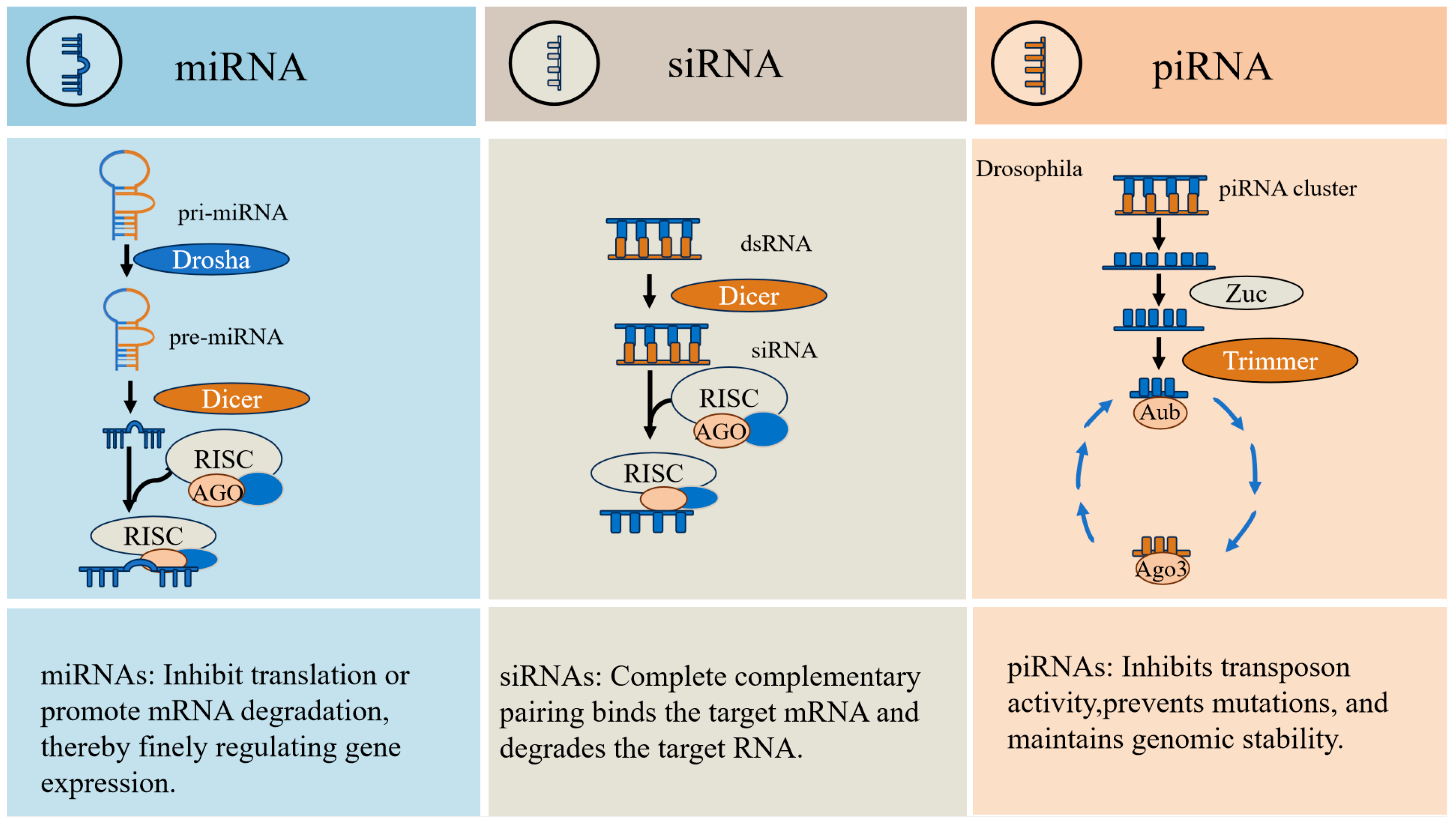
3. Characteristics and Functions of piRNAs
3.1. The Characteristics of piRNAs
3.2. The Role of piRNAs in Silencing Transposons and Stabilizing Genomes
3.3. Physiological Functions of piRNAs
3.4. Factors Regulating piRNAs
4. The Role of ncRNAs in Mammary Gland Development
5. Prospects for piRNAs in Livestock Animals
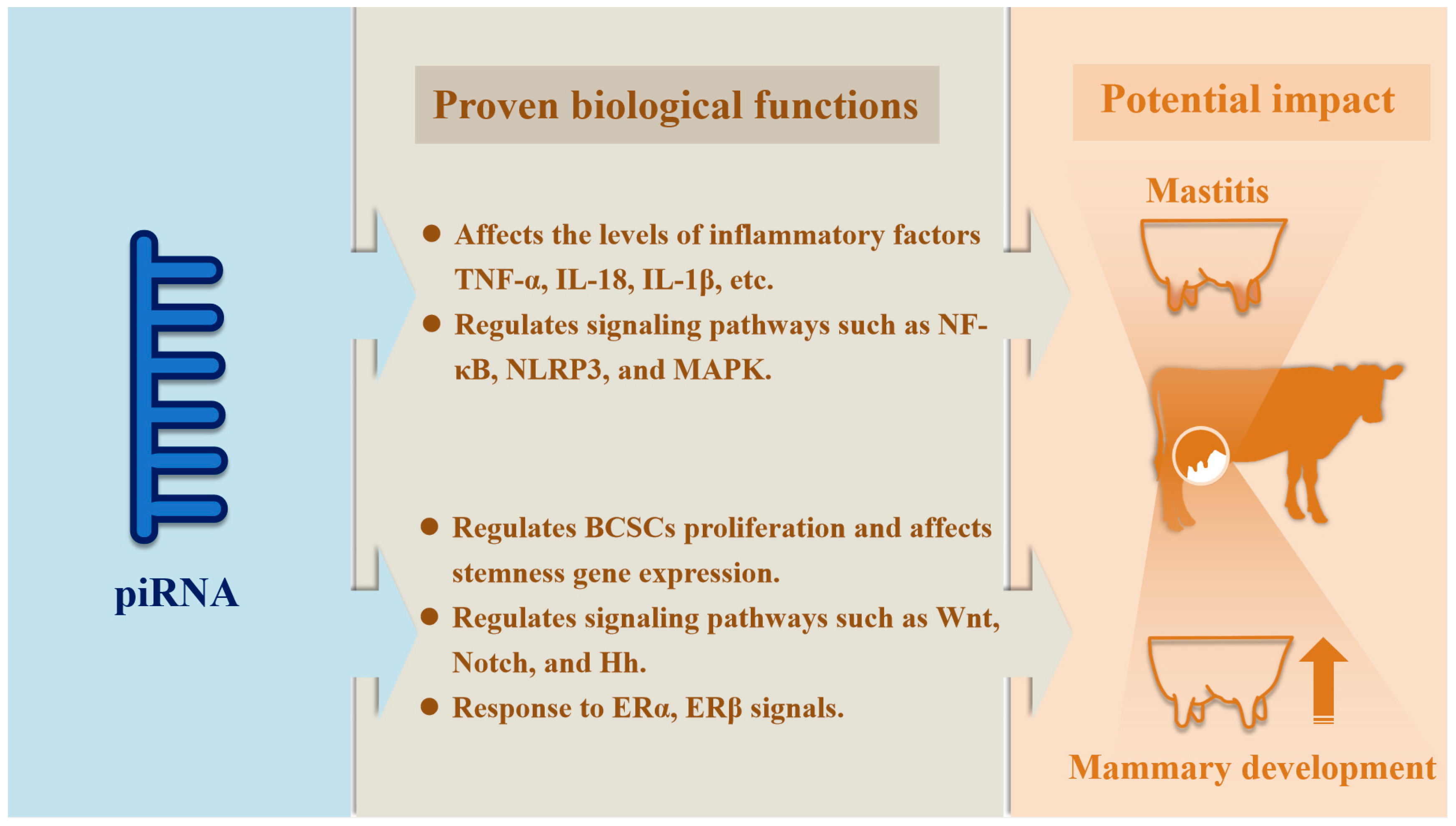
6. The Role of piRNAs in Breast Cancer
7. The Role of piRNAs in Inflammation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.P.; Nilsson, E.; Skinner, M.K. Environmental epigenetics and epigenetic inheritance in domestic farm animals. Anim. Reprod. Sci. 2020, 220, 106316. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Khatib, H. Epigenetics of livestock health, production, and breeding. In Handbook of Epigenetics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 569–610. [Google Scholar]
- Do, D.N.; Suravajhala, P. Editorial: Role of Non-Coding RNAs in Animals. Animals 2023, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Boucher, A.; Dahl, G.E.; Laporta, J. Consequences of maternal heat stress at different stages of embryonic and fetal development on dairy cows’ progeny. Anim. Front. 2021, 11, 48–56. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Hu, L.; Zhang, C.; Chen, G.; Hou, L.; Xu, Q.; Wang, Y.; Li, M. Molecular regulation of whole genome DNA methylation in heat stress response of dairy cows. BMC Genom. 2025, 26, 464. [Google Scholar] [CrossRef]
- Stefanon, B.; Cintio, M.; Sgorlon, S.; Scarsella, E.; Licastro, D.; Zecconi, A.; Colitti, M. Regulatory Role of microRNA of Milk Exosomes in Mastitis of Dairy Cows. Animals 2023, 13, 821. [Google Scholar] [CrossRef]
- Ghulam Mohyuddin, S.; Liang, Y.; Xia, Y.; Wang, M.; Zhang, H.; Li, M.; Yang, Z.; Niel, A.K.; Mao, Y. Identification and Classification of Long Non-Coding RNAs in the Mammary Gland of the Holstein Cow. Int. J. Mol. Sci. 2023, 24, 13585. [Google Scholar] [CrossRef]
- Vagin, V.V.; Sigova, A.; Li, C.; Seitz, H.; Gvozdev, V.; Zamore, P.D. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 2006, 313, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef]
- Lau, N.C.; Seto, A.G.; Kim, J.; Kuramochi-Miyagawa, S.; Nakano, T.; Bartel, D.P.; Kingston, R.E. Characterization of the piRNA complex from rat testes. Science 2006, 313, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, Y.; Xu, H.; Pang, D.; Duan, C.; Liu, C. The expression of stem cell protein Piwil2 and piR-932 in breast cancer. Surg. Oncol. 2013, 22, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Chuma, S.; Nakano, T. piRNA and spermatogenesis in mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110338. [Google Scholar] [CrossRef]
- Ahmad, A.; Bogoch, Y.; Shvaizer, G.; Guler, N.; Levy, K.; Elkouby, Y.M. The piRNA protein Asz1 is essential for germ cell and gonad development in zebrafish and exhibits differential necessities in distinct types of germ granules. PLoS Genet. 2025, 21, e1010868. [Google Scholar] [CrossRef]
- Chalbatani, G.M.; Dana, H.; Memari, F.; Gharagozlou, E.; Ashjaei, S.; Kheirandish, P.; Marmari, V.; Mahmoudzadeh, H.; Mozayani, F.; Maleki, A.R.; et al. Biological function and molecular mechanism of piRNA in cancer. Pract. Lab. Med. 2019, 13, e00113. [Google Scholar] [CrossRef]
- Trzybulska, D.; Vergadi, E.; Tsatsanis, C. miRNA and Other Non-Coding RNAs as Promising Diagnostic Markers. Ejifcc 2018, 29, 221–226. [Google Scholar] [PubMed]
- Zhao, Q.; Qian, L.; Guo, Y.; Lü, J.; Li, D.; Xie, H.; Wang, Q.; Ma, W.; Liu, P.; Liu, Y.; et al. IL11 signaling mediates piR-2158 suppression of cell stemness and angiogenesis in breast cancer. Theranostics 2023, 13, 2337–2349. [Google Scholar] [CrossRef]
- Jiao, A.; Liu, H.; Wang, H.; Yu, J.; Gong, L.; Zhang, H.; Fu, L. piR112710 attenuates diabetic cardiomyopathy through inhibiting Txnip/NLRP3-mediated pyroptosis in db/db mice. Cell. Signal. 2024, 122, 111333. [Google Scholar] [CrossRef]
- Gainetdinov, I.; Colpan, C.; Arif, A.; Cecchini, K.; Zamore, P.D. A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol. Cell 2018, 71, 775–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, R.; Gu, J.; Wu, P.; Peng, C.; Ma, J.; Wu, L.; Yu, Y.; Huang, Y. Structural insights into the sequence-specific recognition of Piwi by Drosophila Papi. Proc. Natl. Acad. Sci. USA 2018, 115, 3374–3379. [Google Scholar] [CrossRef]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its Biogenesis and Functions. Annu. Rev. Biochem. 2015, 84, 405–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, A.; Liu, Z.; He, Z.; Yuan, X.; Tuo, S. Prediction of cancer-associated piRNA–mRNA and piRNA–lncRNA interactions by integrated analysis of expression and sequence data. Tsinghua Sci. Technol. 2018, 23, 115–125. [Google Scholar] [CrossRef]
- Goriaux, C.; Desset, S.; Renaud, Y.; Vaury, C.; Brasset, E. Transcriptional properties and splicing of the flamenco piRNA cluster. EMBO Rep. 2014, 15, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ipsaro, J.J.; Haase, A.D.; Knott, S.R.; Joshua-Tor, L.; Hannon, G.J. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature 2012, 491, 279–283. [Google Scholar] [CrossRef]
- Zhang, R.; Tu, Y.X.; Ye, D.; Gu, Z.; Chen, Z.X.; Sun, Y. A Germline-Specific Regulator of Mitochondrial Fusion is Required for Maintenance and Differentiation of Germline Stem and Progenitor Cells. Adv. Sci. 2022, 9, 2203631. [Google Scholar] [CrossRef]
- Saito, K.; Ishizu, H.; Komai, M.; Kotani, H.; Kawamura, Y.; Nishida, K.M.; Siomi, H.; Siomi, M.C. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 2010, 24, 2493–2498. [Google Scholar] [CrossRef]
- Andersen, P.R.; Tirian, L.; Vunjak, M.; Brennecke, J. A heterochromatin-dependent transcription machinery drives piRNA expression. Nature 2017, 549, 54–59. [Google Scholar] [CrossRef]
- Pastore, B.; Hertz, H.L.; Tang, W. Comparative analysis of piRNA sequences, targets and functions in nematodes. RNA Biol. 2022, 19, 1276–1292. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef]
- Houwing, S.; Berezikov, E.; Ketting, R.F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 2008, 27, 2702–2711. [Google Scholar] [CrossRef]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, S.; Siomi, M.C. piRNA biogenesis in the germline: From transcription of piRNA genomic sources to piRNA maturation. Biochim. Biophys. Acta 2016, 1859, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Grivna, S.T.; Pyhtila, B.; Lin, H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 13415–13420. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeda, A.; Tsukiyama, T.; Mise, K.; Okuno, T.; Sasaki, H.; Minami, N.; Imai, H. Identification and characterization of two novel classes of small RNAs in the mouse germline: Retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006, 20, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, J.; Dong, K.; Midic, U.; Hess, R.A.; Xie, H.; Demireva, E.Y.; Chen, C. PNLDC1 is essential for piRNA 3′ end trimming and transposon silencing during spermatogenesis in mice. Nat. Commun. 2017, 8, 819. [Google Scholar] [CrossRef]
- Saito, K.; Sakaguchi, Y.; Suzuki, T.; Suzuki, T.; Siomi, H.; Siomi, M.C. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi- interacting RNAs at their 3’ ends. Genes Dev. 2007, 21, 1603–1608. [Google Scholar] [CrossRef]
- Gunawardane, L.S.; Saito, K.; Nishida, K.M.; Miyoshi, K.; Kawamura, Y.; Nagami, T.; Siomi, H.; Siomi, M.C. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 2007, 315, 1587–1590. [Google Scholar] [CrossRef]
- Olivieri, D.; Senti, K.A.; Subramanian, S.; Sachidanandam, R.; Brennecke, J. The cochaperone shutdown defines a group of biogenesis factors essential for all piRNA populations in Drosophila. Mol. Cell 2012, 47, 954–969. [Google Scholar] [CrossRef]
- Nagao, A.; Mituyama, T.; Huang, H.; Chen, D.; Siomi, M.C.; Siomi, H. Biogenesis pathways of piRNAs loaded onto AGO3 in the Drosophila testis. RNA 2010, 16, 2503–2515. [Google Scholar] [CrossRef]
- Horwich, M.D.; Li, C.; Matranga, C.; Vagin, V.; Farley, G.; Wang, P.; Zamore, P.D. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr. Biol. 2007, 17, 1265–1272. [Google Scholar] [CrossRef]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Papin, C.; Boureux, A.; Meunier, A.C.; Franco, B.; Robine, N.; Lai, E.C.; Pelisson, A.; Simonelig, M. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 2010, 467, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.T.; Dai, P.; Yang, J.H.; Xue, Y.; Hu, Y.P.; Zhou, Y.; Kang, J.Y.; Wang, X.; Li, H.; Hua, M.M.; et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2014, 24, 680–700. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Zhang, H.; Benson, M.; Han, X.; Li, D. Roles of piRNAs in microcystin-leucine-arginine (MC-LR) induced reproductive toxicity in testis on male offspring. Food Chem. Toxicol. 2017, 105, 177–185. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Santos, D.; Mingels, L.; Vogel, E.; Wang, L.; Christiaens, O.; Cappelle, K.; Wynant, N.; Gansemans, Y.; Van Nieuwerburgh, F.; Smagghe, G.; et al. Generation of Virus- and dsRNA-Derived siRNAs with Species-Dependent Length in Insects. Viruses 2019, 11, 738. [Google Scholar] [CrossRef]
- Liu, Q.; Ding, C.; Chu, Y.; Zhang, W.; Guo, G.; Chen, J.; Su, X. Pln24NT: A web resource for plant 24-nt siRNA producing loci. Bioinformatics 2017, 33, 2065–2067. [Google Scholar] [CrossRef]
- Swarts, D.C.; Makarova, K.; Wang, Y.; Nakanishi, K.; Ketting, R.F.; Koonin, E.V.; Patel, D.J.; van der Oost, J. The evolutionary journey of Argonaute proteins. Nat. Struct. Mol. Biol. 2014, 21, 743–753. [Google Scholar] [CrossRef]
- Tolia, N.H.; Joshua-Tor, L. Slicer and the argonautes. Nat. Chem. Biol. 2007, 3, 36–43. [Google Scholar] [CrossRef]
- Peters, L.; Meister, G. Argonaute proteins: Mediators of RNA silencing. Mol. Cell 2007, 26, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ke, A. PIWI Takes a Giant Step. Cell 2016, 167, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef]
- Nishida, K.M.; Saito, K.; Mori, T.; Kawamura, Y.; Nagami-Okada, T.; Inagaki, S.; Siomi, H.; Siomi, M.C. Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad. RNA 2007, 13, 1911–1922. [Google Scholar] [CrossRef]
- Han, H.; Fan, G.; Song, S.; Jiang, Y.; Qian, C.; Zhang, W.; Su, Q.; Xue, X.; Zhuang, W.; Li, B. piRNA-30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood 2021, 137, 1603–1614. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Lee, H.C.; Gu, W.; Shirayama, M.; Youngman, E.; Conte, D., Jr.; Mello, C.C. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell 2012, 150, 78–87. [Google Scholar] [CrossRef]
- Batista, P.J.; Ruby, J.G.; Claycomb, J.M.; Chiang, R.; Fahlgren, N.; Kasschau, K.D.; Chaves, D.A.; Gu, W.; Vasale, J.J.; Duan, S.; et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell 2008, 31, 67–78. [Google Scholar] [CrossRef]
- Suen, K.M.; Braukmann, F.; Butler, R.; Bensaddek, D.; Akay, A.; Lin, C.C.; Milonaitytė, D.; Doshi, N.; Sapetschnig, A.; Lamond, A.; et al. DEPS-1 is required for piRNA-dependent silencing and PIWI condensate organisation in Caenorhabditis elegans. Nat. Commun. 2020, 11, 4242. [Google Scholar] [CrossRef]
- Sienski, G.; Dönertas, D.; Brennecke, J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell 2012, 151, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi-Miyagawa, S.; Watanabe, T.; Gotoh, K.; Totoki, Y.; Toyoda, A.; Ikawa, M.; Asada, N.; Kojima, K.; Yamaguchi, Y.; Ijiri, T.W.; et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008, 22, 908–917. [Google Scholar] [CrossRef]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef]
- Ashe, A.; Sapetschnig, A.; Weick, E.M.; Mitchell, J.; Bagijn, M.P.; Cording, A.C.; Doebley, A.L.; Goldstein, L.D.; Lehrbach, N.J.; Le Pen, J.; et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 2012, 150, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Klenov, M.S.; Sokolova, O.A.; Yakushev, E.Y.; Stolyarenko, A.D.; Mikhaleva, E.A.; Lavrov, S.A.; Gvozdev, V.A. Separation of stem cell maintenance and transposon silencing functions of Piwi protein. Proc. Natl. Acad. Sci. USA 2011, 108, 18760–18765. [Google Scholar] [CrossRef] [PubMed]
- Le Thomas, A.; Rogers, A.K.; Webster, A.; Marinov, G.K.; Liao, S.E.; Perkins, E.M.; Hur, J.K.; Aravin, A.A.; Tóth, K.F. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev. 2013, 27, 390–399. [Google Scholar] [CrossRef]
- Pezic, D.; Manakov, S.A.; Sachidanandam, R.; Aravin, A.A. piRNA pathway targets active LINE1 elements to establish the repressive H3K9me3 mark in germ cells. Genes Dev. 2014, 28, 1410–1428. [Google Scholar] [CrossRef]
- Khurana, J.S.; Xu, J.; Weng, Z.; Theurkauf, W.E. Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet. 2010, 6, e1001246. [Google Scholar] [CrossRef]
- Pek, J.W.; Kai, T. DEAD-box RNA helicase Belle/DDX3 and the RNA interference pathway promote mitotic chromosome segregation. Proc. Natl. Acad. Sci. USA 2011, 108, 12007–12012. [Google Scholar] [CrossRef]
- Cox, D.N.; Chao, A.; Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 2000, 127, 503–514. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Q.; Peng, X.; Choi, S.Y.; Sarma, K.; Ren, H.; Morris, A.J.; Frohman, M.A. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 2011, 20, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Loubalova, Z.; Fulka, H.; Horvat, F.; Pasulka, J.; Malik, R.; Hirose, M.; Ogura, A.; Svoboda, P. Formation of spermatogonia and fertile oocytes in golden hamsters requires piRNAs. Nat. Cell Biol. 2021, 23, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Casier, K.; Delmarre, V.; Gueguen, N.; Hermant, C.; Viodé, E.; Vaury, C.; Ronsseray, S.; Brasset, E.; Teysset, L.; Boivin, A. Environmentally-induced epigenetic conversion of a piRNA cluster. Elife 2019, 8, e39842. [Google Scholar] [CrossRef]
- Belicard, T.; Jareosettasin, P.; Sarkies, P. The piRNA pathway responds to environmental signals to establish intergenerational adaptation to stress. BMC Biol. 2018, 16, 103. [Google Scholar] [CrossRef]
- Donkin, I.; Versteyhe, S.; Ingerslev, L.R.; Qian, K.; Mechta, M.; Nordkap, L.; Mortensen, B.; Appel, E.V.; Jørgensen, N.; Kristiansen, V.B.; et al. Obesity and Bariatric Surgery Drive Epigenetic Variation of Spermatozoa in Humans. Cell Metab. 2016, 23, 369–378. [Google Scholar] [CrossRef]
- Grandjean, V.; Fourré, S.; De Abreu, D.A.; Derieppe, M.A.; Remy, J.J.; Rassoulzadegan, M. RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders. Sci. Rep. 2015, 5, 18193. [Google Scholar] [CrossRef]
- de Castro Barbosa, T.; Ingerslev, L.R.; Alm, P.S.; Versteyhe, S.; Massart, J.; Rasmussen, M.; Donkin, I.; Sjögren, R.; Mudry, J.M.; Vetterli, L.; et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol. Metab. 2016, 5, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, L.R.; Donkin, I.; Fabre, O.; Versteyhe, S.; Mechta, M.; Pattamaprapanont, P.; Mortensen, B.; Krarup, N.T.; Barrès, R. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin. Epigenetics 2018, 10, 12. [Google Scholar] [CrossRef]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef]
- Han, R.; Zhang, L.; Gan, W.; Fu, K.; Jiang, K.; Ding, J.; Wu, J.; Han, X.; Li, D. piRNA-DQ722010 contributes to prostate hyperplasia of the male offspring mice after the maternal exposed to microcystin-leucine arginine. Prostate 2019, 79, 798–812. [Google Scholar] [CrossRef]
- Davis, S.R. TRIENNIAL LACTATION SYMPOSIUM/BOLFA: Mammary growth during pregnancy and lactation and its relationship with milk yield. J. Anim. Sci. 2017, 95, 5675–5688. [Google Scholar] [CrossRef] [PubMed]
- Hue-Beauvais, C.; Faulconnier, Y.; Charlier, M.; Leroux, C. Nutritional Regulation of Mammary Gland Development and Milk Synthesis in Animal Models and Dairy Species. Genes 2021, 12, 523. [Google Scholar] [CrossRef]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L. Review: Mammary gland development in swine: Embryo to early lactation. Animal 2019, 13, s11–s19. [Google Scholar] [CrossRef]
- Akers, R.M.; Nickerson, S.C. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland. Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Wang, Y.; Cao, X.; Lv, X.; Yang, Z.; Chen, Z. Progress in Research on Key Factors Regulating Lactation Initiation in the Mammary Glands of Dairy Cows. Genes 2023, 14, 1163. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, M.; Kong, L.; Liu, J.; Wang, Y.; Song, C.; Chen, X.; Lai, M.; Fang, X.; Chen, H.; et al. MiR-204-5p promotes lipid synthesis in mammary epithelial cells by targeting SIRT1. Biochem. Biophys. Res. Commun. 2020, 533, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Lanz, R.B.; Chua, S.S.; Barron, N.; Söder, B.M.; DeMayo, F.; O’Malley, B.W. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol. Cell Biol. 2003, 23, 7163–7176. [Google Scholar] [CrossRef]
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Le Guillou, S.; Sdassi, N.; Laubier, J.; Passet, B.; Vilotte, M.; Castille, J.; Laloë, D.; Polyte, J.; Bouet, S.; Jaffrézic, F.; et al. Overexpression of miR-30b in the developing mouse mammary gland causes a lactation defect and delays involution. PLoS ONE 2012, 7, e45727. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Li, F.; Li, X.; Tian, Y.; Zhang, Y.; Sheng, X.; Song, Y.; Meng, Q.; Yuan, S.; Luan, L.; et al. Author Correction: MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat. Commun. 2020, 11, 5308. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Luo, J.; Zhang, L.; Wang, W.; Gou, D. MiR-103 controls milk fat accumulation in goat (Capra hircus) mammary gland during lactation. PLoS ONE 2013, 8, e79258. [Google Scholar] [CrossRef]
- Ibarra, I.; Erlich, Y.; Muthuswamy, S.K.; Sachidanandam, R.; Hannon, G.J. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007, 21, 3238–3243. [Google Scholar] [CrossRef]
- Feuermann, Y.; Kang, K.; Shamay, A.; Robinson, G.W.; Hennighausen, L. MiR-21 is under control of STAT5 but is dispensable for mammary development and lactation. PLoS ONE 2014, 9, e85123. [Google Scholar] [CrossRef]
- Bonetti, P.; Climent, M.; Panebianco, F.; Tordonato, C.; Santoro, A.; Marzi, M.J.; Pelicci, P.G.; Ventura, A.; Nicassio, F. Correction: Dual role for miR-34a in the control of early progenitor proliferation and commitment in the mammary gland and in breast cancer. Oncogene 2020, 39, 2228. [Google Scholar] [CrossRef]
- Tanaka, T.; Haneda, S.; Imakawa, K.; Sakai, S.; Nagaoka, K. A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 2009, 77, 181–187. [Google Scholar] [CrossRef]
- Ucar, A.; Vafaizadeh, V.; Jarry, H.; Fiedler, J.; Klemmt, P.A.; Thum, T.; Groner, B.; Chowdhury, K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat. Genet. 2010, 42, 1101–1108. [Google Scholar] [CrossRef]
- Lee, J.M.; Cho, K.W.; Kim, E.J.; Tang, Q.; Kim, K.S.; Tickle, C.; Jung, H.S. A contrasting function for miR-137 in embryonic mammogenesis and adult breast carcinogenesis. Oncotarget 2015, 6, 22048–22059. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Li, Q.; Li, Y. miR-138 function and its targets on mouse mammary epithelial cells. Progress Biochem. Biophys. 2006. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-592163 (accessed on 1 May 2025).
- Cui, Y.; Sun, X.; Jin, L.; Yu, G.; Li, Q.; Gao, X.; Ao, J.; Wang, C. MiR-139 suppresses β-casein synthesis and proliferation in bovine mammary epithelial cells by targeting the GHR and IGF1R signaling pathways. BMC Vet. Res. 2017, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wu, C.; Li, Y.; Liu, J.; Yuan, Y.; Shi, H. Identification and profiling of microRNAs involved in the regenerative involution of mammary gland. Genomics 2022, 114, 110442. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Kang, K.; Feuermann, Y.; Jang, S.J.; Robinson, G.W.; Hennighausen, L. The STAT5-regulated miR-193b locus restrains mammary stem and progenitor cell activity and alveolar differentiation. Dev. Biol. 2014, 395, 245–254. [Google Scholar] [CrossRef]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cao, J.; Napoli, M.; Xia, Z.; Zhao, N.; Creighton, C.J.; Li, W.; Chen, X.; Flores, E.R.; McManus, M.T.; et al. miR-205 Regulates Basal Cell Identity and Stem Cell Regenerative Potential During Mammary Reconstitution. Stem Cells 2018, 36, 1875–1889. [Google Scholar] [CrossRef]
- Patel, Y.; Soni, M.; Awgulewitsch, A.; Kern, M.J.; Liu, S.; Shah, N.; Singh, U.P.; Chen, H. Correction: Overexpression of miR-489 derails mammary hierarchy structure and inhibits HER2/neu-induced tumorigenesis. Oncogene 2019, 38, 454. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Yu, S.; Hao, Y.; Zhang, P.; Feng, Y.; Zhang, H.; Ma, D.; Liu, J.; Cheng, M.; et al. miR-15b negatively correlates with lipid metabolism in mammary epithelial cells. Am. J. Physiol. Cell Physiol. 2018, 314, C43–C52. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Sun, S.; Cao, D.; Shi, H.; Loor, J.J. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. RNA Biol. 2017, 14, 326–338. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Chen, Z.; Cao, W.T.; Xu, H.F.; Gou, D.M.; Zhu, J.J. MicroRNA-24 can control triacylglycerol synthesis in goat mammary epithelial cells by targeting the fatty acid synthase gene. J. Dairy Sci. 2015, 98, 9001–9014. [Google Scholar] [CrossRef]
- Ma, L.; Qiu, H.; Chen, Z.; Li, L.; Zeng, Y.; Luo, J.; Gou, D. miR-25 modulates triacylglycerol and lipid accumulation in goat mammary epithelial cells by repressing PGC-1beta. J. Anim. Sci. Biotechnol. 2018, 9, 48. [Google Scholar] [CrossRef]
- Lin, X.Z.; Luo, J.; Zhang, L.P.; Wang, W.; Shi, H.B.; Zhu, J.J. MiR-27a suppresses triglyceride accumulation and affects gene mRNA expression associated with fat metabolism in dairy goat mammary gland epithelial cells. Gene 2013, 521, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Lei, Y.; Wang, C.; Wang, J.; Wang, L.; Liu, L.; Liu, L.; Gao, X.; Li, Q. Epigenetic Regulation of miR-29s Affects the Lactation Activity of Dairy Cow Mammary Epithelial Cells. J. Cell Physiol. 2015, 230, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, Q.; Feng, L.; Ding, W. MiR-126-3p regulates progesterone receptors and involves development and lactation of mouse mammary gland. Mol. Cell Biochem. 2011, 355, 17–25. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Feng, Y.; Zhang, H.; Liu, J.; Cheng, M.; Li, L.; Shen, W.; Cao, H.; Li, Q.; et al. MicroRNA-126 participates in lipid metabolism in mammary epithelial cells. Mol. Cell Endocrinol. 2017, 454, 77–86. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, L.; Cui, Y.; Li, H.; Xie, X.; Li, Y.; Wang, C. miR-142-3p Regulates Milk Synthesis and Structure of Murine Mammary Glands via PRLR-Mediated Multiple Signaling Pathways. J. Agric. Food Chem. 2019, 67, 9532–9542. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, H.; Luo, J.; Yi, Y.; Yao, D.; Zhang, X.; Ma, G.; Loor, J.J. MiR-145 Regulates Lipogenesis in Goat Mammary Cells Via Targeting INSIG1 and Epigenetic Regulation of Lipid-Related Genes. J. Cell Physiol. 2017, 232, 1030–1040. [Google Scholar] [CrossRef]
- Heinz, R.E.; Rudolph, M.C.; Ramanathan, P.; Spoelstra, N.S.; Butterfield, K.T.; Webb, P.G.; Babbs, B.L.; Gao, H.; Chen, S.; Gordon, M.A.; et al. Constitutive expression of microRNA-150 in mammary epithelium suppresses secretory activation and impairs de novo lipogenesis. Development 2016, 143, 4236–4248. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, H.; Sun, S.; Xu, H.; Cao, D.; Luo, J. MicroRNA-181b suppresses TAG via target IRS2 and regulating multiple genes in the Hippo pathway. Exp. Cell Res. 2016, 348, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Aydoğdu, E.; Mukhopadhyay, S.; Helguero, L.A.; Williams, C. A miR-206 regulated gene landscape enhances mammary epithelial differentiation. J. Cell Physiol. 2019, 234, 22220–22233. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, Y.; Yu, S.; Hao, Y.; Zhang, P.; Feng, Y.; Zhang, H.; Ma, D.; Liu, J.; Cheng, M.; et al. MicroRNA-221 may be involved in lipid metabolism in mammary epithelial cells. Int. J. Biochem. Cell Biol. 2018, 97, 118–127. [Google Scholar] [CrossRef]
- Li, D.; Xie, X.; Wang, J.; Bian, Y.; Li, Q.; Gao, X.; Wang, C. MiR-486 regulates lactation and targets the PTEN gene in cow mammary glands. PLoS ONE 2015, 10, e0118284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, J.; Li, D.; Lai, L.; Siwko, S.; Li, Y.; Liu, M. Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells 2013, 31, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Askarian-Amiri, M.E.; Crawford, J.; French, J.D.; Smart, C.E.; Smith, M.A.; Clark, M.B.; Ru, K.; Mercer, T.R.; Thompson, E.R.; Lakhani, S.R.; et al. SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 2011, 17, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.; Lottin, S.; Dugimont, T.; Fauquette, W.; Coll, J.; Dupouy, J.P.; Boilly, B.; Curgy, J.J. Steroid hormones modulate H19 gene expression in both mammary gland and uterus. Oncogene 1999, 18, 4460–4473. [Google Scholar] [CrossRef][Green Version]
- Ginger, M.R.; Shore, A.N.; Contreras, A.; Rijnkels, M.; Miller, J.; Gonzalez-Rimbau, M.F.; Rosen, J.M. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc. Natl. Acad. Sci. USA 2006, 103, 5781–5786. [Google Scholar] [CrossRef]
- Shore, A.N.; Kabotyanski, E.B.; Roarty, K.; Smith, M.A.; Zhang, Y.; Creighton, C.J.; Dinger, M.E.; Rosen, J.M. Pregnancy-induced noncoding RNA (PINC) associates with polycomb repressive complex 2 and regulates mammary epithelial differentiation. PLoS Genet. 2012, 8, e1002840. [Google Scholar] [CrossRef]
- Ma, X.; Niu, X.; Huang, S.; Li, S.; Ran, X.; Wang, J.; Dai, X. The piRNAs present in the developing testes of Chinese indigenous Xiang pigs. Theriogenology 2022, 189, 92–106. [Google Scholar] [CrossRef]
- Gebert, D.; Ketting, R.F.; Zischler, H.; Rosenkranz, D. piRNAs from Pig Testis Provide Evidence for a Conserved Role of the Piwi Pathway in Post-Transcriptional Gene Regulation in Mammals. PLoS ONE 2015, 10, e0124860. [Google Scholar] [CrossRef]
- Zhang, G.W.; Wang, L.; Chen, H.; Guan, J.; Wu, Y.; Zhao, J.; Luo, Z.; Huang, W.; Zuo, F. Promoter hypermethylation of PIWI/piRNA pathway genes associated with diminished pachytene piRNA production in bovine hybrid male sterility. Epigenetics 2020, 15, 914–931. [Google Scholar] [CrossRef]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.M.; Fojadelli, I.; Stella, A.; Pizzi, F. Small RNA sequencing of cryopreserved semen from single bull revealed altered miRNAs and piRNAs expression between High- and Low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Zhang, Z.; Ren, C.; Wang, X.; He, X.; Mwacharo, J.M.; Zhang, X.; Zhang, J.; Di, R.; et al. Expression characteristics of piRNAs in ovine luteal phase and follicular phase ovaries. Front. Vet. Sci. 2022, 9, 921868. [Google Scholar] [CrossRef] [PubMed]
- Testroet, E.D.; Shome, S.; Testroet, A.; Reecy, J.; Jernigan, R.L.; Zhu, M.; Du, M.; Clark, S.; Beitz, D. Profiling of the Exosomal Cargo of Bovine Milk Reveals the Presence of Immune-and Growth-modulatory ncRNAs. FASEB J. 2018, 32, 747.25. [Google Scholar] [CrossRef]
- Ablondi, M.; Gòdia, M.; Rodriguez-Gil, J.E.; Sánchez, A.; Clop, A. Characterisation of sperm piRNAs and their correlation with semen quality traits in swine. Anim. Genet. 2021, 52, 114–120. [Google Scholar] [CrossRef]
- Kowalczykiewicz, D.; Pawlak, P.; Lechniak, D.; Wrzesinski, J. Altered expression of porcine Piwi genes and piRNA during development. PLoS ONE 2012, 7, e43816. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Yang, X.; Du, Y.; Xu, Z.; Zhou, Y.; Yang, X.; Wang, X.; Zhang, C.; Li, S.; et al. The porcine piRNA transcriptome response to Senecavirus a infection. Front. Vet. Sci. 2023, 10, 1126277. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Ran, M.; Chen, B.; Wu, M.; Peng, F.; Dong, L.; He, C.; Zhang, S.; Li, Z. Systematic identification and characterization of miRNAs and piRNAs from porcine testes. Genes Genom. 2017, 39, 1047–1057. [Google Scholar] [CrossRef]
- Yang, C.X.; Du, Z.Q.; Wright, E.C.; Rothschild, M.F.; Prather, R.S.; Ross, J.W. Small RNA profile of the cumulus-oocyte complex and early embryos in the pig. Biol. Reprod. 2012, 87, 117. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G.; et al. Expression Pattern of Seminal Plasma Extracellular Vesicle Small RNAs in Boar Semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef]
- Russell, S.; Patel, M.; Gilchrist, G.; Stalker, L.; Gillis, D.; Rosenkranz, D.; LaMarre, J. Bovine piRNA-like RNAs are associated with both transposable elements and mRNAs. Reproduction 2017, 153, 305–318. [Google Scholar] [CrossRef]
- Spornraft, M.; Kirchner, B.; Pfaffl, M.W.; Riedmaier, I. Comparison of the miRNome and piRNome of bovine blood and plasma by small RNA sequencing. Biotechnol. Lett. 2015, 37, 1165–1176. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, J.; Chai, Z.; Zhu, J.; Xin, J. Comparative expression profile of microRNAs and piRNAs in three ruminant species testes using next-generation sequencing. Reprod. Domest. Anim. 2018, 53, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Sellem, E.; Marthey, S.; Rau, A.; Jouneau, L.; Bonnet, A.; Perrier, J.P.; Fritz, S.; Le Danvic, C.; Boussaha, M.; Kiefer, H.; et al. A comprehensive overview of bull sperm-borne small non-coding RNAs and their diversity across breeds. Epigenetics Chromatin 2020, 13, 19. [Google Scholar] [CrossRef]
- Roovers, E.F.; Rosenkranz, D.; Mahdipour, M.; Han, C.T.; He, N.; Chuva de Sousa Lopes, S.M.; van der Westerlaken, L.A.; Zischler, H.; Butter, F.; Roelen, B.A.; et al. Piwi proteins and piRNAs in mammalian oocytes and early embryos. Cell Rep. 2015, 10, 2069–2082. [Google Scholar] [CrossRef] [PubMed]
- Sellem, E.; Marthey, S.; Rau, A.; Jouneau, L.; Bonnet, A.; Le Danvic, C.; Guyonnet, B.; Kiefer, H.; Jammes, H.; Schibler, L. Dynamics of cattle sperm sncRNAs during maturation, from testis to ejaculated sperm. Epigenetics Chromatin 2021, 14, 24. [Google Scholar] [CrossRef]
- Shome, S.; Jernigan, R.L.; Beitz, D.C.; Clark, S.; Testroet, E.D. Non-coding RNA in raw and commercially processed milk and putative targets related to growth and immune-response. BMC Genom. 2021, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; Goel, B.M.; Arora, R. Analysis of differentially expressed diverse non-coding rnas in different stages of lactation of murrah buffalo. BIOINFOLET Q. J. Life Sci. 2023, 20, 524–530. [Google Scholar]
- Li, B.; He, X.; Zhao, Y.; Bai, D.; Bou, G.; Zhang, X.; Su, S.; Dao, L.; Liu, R.; Wang, Y.; et al. Identification of piRNAs and piRNA clusters in the testes of the Mongolian horse. Sci. Rep. 2019, 9, 5022. [Google Scholar] [CrossRef]
- Di, R.; Zhang, R.; Mwacharo, J.M.; Wang, X.; He, X.; Liu, Y.; Zhang, J.; Gong, Y.; Zhang, X.; Chu, M. Characteristics of piRNAs and their comparative profiling in testes of sheep with different fertility. Front. Genet. 2022, 13, 1078049. [Google Scholar] [CrossRef]
- He, X.; Li, B.; Fu, S.; Wang, B.; Qi, Y.; Da, L.; Te, R.; Sun, S.; Liu, Y.; Zhang, W. Identification of piRNAs in the testes of Sunite and Small-tailed Han sheep. Anim. Biotechnol. 2021, 32, 13–20. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Ma, K.; Wu, Y.; Qi, X.; Liu, Z.; Li, Q.; Zhang, Y.; Ma, Y. Identification and functional characterization of developmental-stage-dependent piRNAs in Tibetan sheep testes. J. Anim. Sci. 2023, 101, skad189. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, J.M.; Xiao, B.X.; Miao, Y.; Jiang, Z.; Zhou, H.; Li, Q.N. piRNA, the new non-coding RNA, is aberrantly expressed in human cancer cells. Clin. Chim. Acta 2011, 412, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Hu, H.; Xue, X.; Shen, S.; Gao, E.; Guo, G.; Shen, X.; Zhang, X. Altered expression of piRNAs and their relation with clinicopathologic features of breast cancer. Clin. Transl. Oncol. 2013, 15, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.; Rizzo, F.; Marchese, G.; Ravo, M.; Tarallo, R.; Nassa, G.; Giurato, G.; Santamaria, G.; Cordella, A.; Cantarella, C.; et al. RNA sequencing identifies specific PIWI-interacting small non-coding RNA expression patterns in breast cancer. Oncotarget 2014, 5, 9901–9910. [Google Scholar] [CrossRef]
- Koduru, S.V.; Tiwari, A.K.; Leberfinger, A.; Hazard, S.W.; Kawasawa, Y.I.; Mahajan, M.; Ravnic, D.J. A Comprehensive NGS Data Analysis of Differentially Regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in Triple Negative Breast Cancer. J. Cancer 2017, 8, 578–596. [Google Scholar] [CrossRef]
- Kärkkäinen, E.; Heikkinen, S.; Tengström, M.; Kosma, V.M.; Mannermaa, A.; Hartikainen, J.M. The debatable presence of PIWI-interacting RNAs in invasive breast cancer. Cancer Med. 2021, 10, 3593–3603. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Sun, L.; Li, M.; He, X.; Jiang, J.; Zhou, Q. Piwi-interacting RNA-651 promotes cell proliferation and migration and inhibits apoptosis in breast cancer by facilitating DNMT1-mediated PTEN promoter methylation. Cell Cycle 2021, 20, 1603–1616. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Lü, J.; Zhao, Q.; Guo, Y.; Lu, Z.; Ma, W.; Liu, P.; Pestell, R.G.; Liang, C.; et al. piRNA-823 Is Involved in Cancer Stem Cell Regulation Through Altering DNA Methylation in Association with Luminal Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 641052. [Google Scholar] [CrossRef]
- Oner, C.; Colak, E. PIWI interacting RNA-823: Epigenetic regulator of the triple negative breast cancer cells proliferation. Eurasian J. Med. Oncol. 2022, 6, 339–344. [Google Scholar] [CrossRef]
- Ou, B.; Liu, Y.; Gao, Z.; Xu, J.; Yan, Y.; Li, Y.; Zhang, J. Senescent neutrophils-derived exosomal piRNA-17560 promotes chemoresistance and EMT of breast cancer via FTO-mediated m6A demethylation. Cell Death Dis. 2022, 13, 905. [Google Scholar] [CrossRef]
- Huang, S.; Chen, B.; Qiu, P.; Yan, Z.; Liang, Z.; Luo, K.; Huang, B.; Jiang, H. In vitro study of piwi interaction RNA-31106 promoting breast carcinogenesis by regulating METTL3-mediated m6A RNA methylation. Transl. Cancer Res. 2023, 12, 1588–1601. [Google Scholar] [CrossRef]
- Du, S.; Liu, J.; Ning, Y.; Yin, M.; Xu, M.; Liu, Z.; Liu, K. The piR-31115-PIWIL4 complex promotes the migration of the triple-negative breast cancer cell lineMDA-MB-231 by suppressing HSP90AA1 degradation. Gene 2025, 942, 149255. [Google Scholar] [CrossRef]
- Alexandrova, E.; Lamberti, J.; Saggese, P.; Pecoraro, G.; Memoli, D.; Cappa, V.M.; Ravo, M.; Iorio, R.; Tarallo, R.; Rizzo, F.; et al. Small Non-Coding RNA Profiling Identifies miR-181a-5p as a Mediator of Estrogen Receptor Beta-Induced Inhibition of Cholesterol Biosynthesis in Triple-Negative Breast Cancer. Cells 2020, 9, 874. [Google Scholar] [CrossRef]
- Tan, L.; Mai, D.; Zhang, B.; Jiang, X.; Zhang, J.; Bai, R.; Ye, Y.; Li, M.; Pan, L.; Su, J.; et al. PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA. Mol. Cancer 2019, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Zhao, Q.; Ding, X.; Guo, Y.; Li, Y.; Xu, Z.; Li, S.; Wang, Z.; Shen, L.; Chen, H.W.; et al. Cyclin D1 promotes secretion of pro-oncogenic immuno-miRNAs and piRNAs. Clin. Sci. 2020, 134, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Jacobs, D.I.; Hoffman, A.E.; Zheng, T.; Zhu, Y. PIWI-interacting RNA 021285 is involved in breast tumorigenesis possibly by remodeling the cancer epigenome. Carcinogenesis 2015, 36, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, X.; Zhang, X.; Duan, X.; Pan, T.; Hu, Q.; Zhang, Y.; Zhong, F.; Liu, J.; Zhang, H.; et al. An Lnc RNA (GAS5)/SnoRNA-derived piRNA induces activation of TRAIL gene by site-specifically recruiting MLL/COMPASS-like complexes. Nucleic Acids Res. 2015, 43, 3712–3725. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Z.; Wang, Z.; Tan, X.; Wang, Z.; Shen, L.; Long, C.; Wei, G.; He, D. Novel piRNA MW557525 regulates the growth of Piwil2-iCSCs and maintains their stem cell pluripotency. Mol. Biol. Rep. 2022, 49, 6957–6969. [Google Scholar] [CrossRef]
- Balaratnam, S.; West, N.; Basu, S. A piRNA utilizes HILI and HIWI2 mediated pathway to down-regulate ferritin heavy chain 1 mRNA in human somatic cells. Nucleic Acids Res. 2018, 46, 10635–10648. [Google Scholar] [CrossRef]
- Wu, L.; Huang, S.; Tian, W.; Liu, P.; Xie, Y.; Qiu, Y.; Li, X.; Tang, Y.; Zheng, S.; Sun, Y.; et al. PIWI-interacting RNA-YBX1 inhibits proliferation and metastasis by the MAPK signaling pathway via YBX1 in triple-negative breast cancer. Cell Death Discov. 2024, 10, 7. [Google Scholar] [CrossRef]
- Ma, X.; Wang, S.; Do, T.; Song, X.; Inaba, M.; Nishimoto, Y.; Liu, L.P.; Gao, Y.; Mao, Y.; Li, H.; et al. Piwi is required in multiple cell types to control germline stem cell lineage development in the Drosophila ovary. PLoS ONE 2014, 9, e90267. [Google Scholar] [CrossRef]
- Cox, D.N.; Chao, A.; Baker, J.; Chang, L.; Qiao, D.; Lin, H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998, 12, 3715–3727. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009, 23, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Clevers, H. Tissue-specific designs of stem cell hierarchies. Nat. Cell Biol. 2016, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M. Normal stem cells and cancer stem cells: Similar and different. Semin. Cancer Biol. 2010, 20, 85–92. [Google Scholar] [CrossRef]
- Yu, Q.C.; Verheyen, E.M.; Zeng, Y.A. Mammary Development and Breast Cancer: A Wnt Perspective. Cancers 2016, 8, 65. [Google Scholar] [CrossRef]
- Chen, W.; Wei, W.; Yu, L.; Ye, Z.; Huang, F.; Zhang, L.; Hu, S.; Cai, C. Mammary Development and Breast Cancer: A Notch Perspective. J. Mammary Gland. Biol. Neoplasia 2021, 26, 309–320. [Google Scholar] [CrossRef]
- Visbal, A.P.; Lewis, M.T. Hedgehog signaling in the normal and neoplastic mammary gland. Curr. Drug Targets 2010, 11, 1103–1111. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martínez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef]
- Liao, Z.; Yang, L.; Cheng, X.; Huang, X.; Zhang, Q.; Wen, D.; Song, Z.; Li, Y.; Wen, S.; Li, Y.; et al. pir-hsa-216911 inhibit pyroptosis in hepatocellular carcinoma by suppressing TLR4 initiated GSDMD activation. Cell Death Discov. 2025, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Radmehr, E.; Yazdanpanah, N.; Rezaei, N. Non-coding RNAs affecting NLRP3 inflammasome pathway in diabetic cardiomyopathy: A comprehensive review of potential therapeutic options. J. Transl. Med. 2025, 23, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Yu, F.; Wang, C.; Peng, J.; Wang, C.; Chen, X. PiRNA hsa_piR_019949 promotes chondrocyte anabolic metabolism by inhibiting the expression of lncRNA NEAT1. J. Orthop. Surg. Res. 2024, 19, 31. [Google Scholar] [CrossRef]
- Zhong, N.; Nong, X.; Diao, J.; Yang, G. piRNA-6426 increases DNMT3B-mediated SOAT1 methylation and improves heart failure. Aging 2022, 14, 2678–2694. [Google Scholar] [CrossRef]
- Liu, P.; Hu, L.; Shi, Y.; Liu, Y.; Yu, G.; Zhou, Y.; An, Q.; Zhu, W. Changes in the Small RNA Expression in Endothelial Cells in Response to Inflammatory Stimulation. Oxid. Med. Cell Longev. 2021, 2021, 8845520. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, X.; Wang, T.; Yue, X.; Wang, Y.; Cai, B.; Wang, C.; Lu, S. piRNA mmu_piR_037459 suppression alleviated the degeneration of chondrocyte and cartilage. Int. Immunopharmacol. 2024, 128, 111473. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Chakravarty, S.; Ray, S.; Saha, H.; Das, K.; Ghosh, I.; Mallick, B.; Biswas, N.K.; Goswami, S. Correlating tissue and plasma-specific piRNA changes to predict their possible role in pancreatic malignancy and chronic inflammation. Biomed. Rep. 2024, 21, 186. [Google Scholar] [CrossRef]
- Ren, R.; Tan, H.; Huang, Z.; Wang, Y.; Yang, B. Differential expression and correlation of immunoregulation related piRNA in rheumatoid arthritis. Front. Immunol. 2023, 14, 1175924. [Google Scholar] [CrossRef]
- Samir, M.; Vidal, R.O.; Abdallah, F.; Capece, V.; Seehusen, F.; Geffers, R.; Hussein, A.; Ali, A.A.H.; Bonn, S.; Pessler, F. Organ-specific small non-coding RNA responses in domestic (Sudani) ducks experimentally infected with highly pathogenic avian influenza virus (H5N1). RNA Biol. 2020, 17, 112–124. [Google Scholar] [CrossRef]
- Nathan, C. Nonresolving inflammation redux. Immunity 2022, 55, 592–605. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, M.; O’Neill, L.A. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev. 2015, 265, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Godber, O.F.; Wall, R. Livestock and food security: Vulnerability to population growth and climate change. Glob. Chang. Biol. 2014, 20, 3092–3102. [Google Scholar] [CrossRef]
- Klous, G.; Huss, A.; Heederik, D.J.J.; Coutinho, R.A. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health 2016, 2, 65–76. [Google Scholar] [CrossRef]
| Species | 5′-Terminal Enzyme | 3′-Terminal Enzyme | Key PIWI Protein | Reference |
|---|---|---|---|---|
| Nematodes | Uncertainty | Uncertainty | Plasticity-related gene 1 (PRG-1), plasticity-related gene 2 (PRG-2) | [29] |
| Zebrafish | Phospholipase D family member 6 (PLD6) | Uncertainty | ZIWI, ZILI | [30,31] |
| Drosophila | Zuc | Trimmer | PIWI, Argonaute 3 (Ago3), Aubergin(Aub) | [32,33] |
| Mice | PLD6 | Poly(A)-specific ribonuclease-like domain containing 1 (PNLDC1) | MIWI, MIWI2, MILI | [34,35,36] |
| Particular Year | miRNA | Research Target | Model Type | Outcomes | References |
|---|---|---|---|---|---|
| Mammary gland development | |||||
| 2007 | let-7 | Comma-Dβ | In vitro | Inhibited self-renewal capacity of progenitor cells and promoted differentiation. | [96] |
| 2014 | miR-21 | HC11, mice | In vivo and in vitro | Regulated mammary gland development and lactation. | [97] |
| 2012 | miR-30b | Mice | In vivo | Inhibited normal mammary gland development and lipid droplet accumulation. | [93] |
| 2020 | miR-31 | HC11, mice | In vivo and in vitro | Promoted MaSCS self-renewal, alveogenesis, and lipid droplet accumulation. | [94] |
| 2020 | miR-34a | Comma-Dβ, SUM159pt, mice | In vivo and in vitro | Inhibited MaSCs self-renewal, terminal end bud (TEB) development. | [98] |
| 2009 | miR-101a | HC11, mice | In vivo and in vitro | Inhibited HC11 proliferation and β-casein expression, affected mammary gland development and degeneration. | [99] |
| 2010 | miR-132, miR-212 | Mice | In vivo | Promoted ducts growth and modulated epithelial–stromal interactions. | [100] |
| 2015 | miR-137 | MDA-MB-231, 293T, mice | In vivo and in vitro | Promoted thickening of the mammary substrate. | [101] |
| 2006 | miR-138 | Mouse mammary epithelial cells, mice | In vivo and in vitro | Regulated mammary epithelial cell proliferation and mammary gland development, promoted β-casein expression. | [102] |
| 2017 | miR-139 | BMEC, Holstein cows | In vivo and in vitro | Inhibited β-casein synthesis and BMEC proliferation. | [103] |
| 2022 | miR-142-5p, miR-148C, miR-152, miR-218, | Goats | In vivo | Regulated mammary gland regenerative degeneration. | [104] |
| 2014 | miR-193b | MEC, mice | In vivo and in vitro | Inhibited mammary stem/progenitor cell activity and alveolar differentiation. | [105] |
| 2009 | miR-200c | 293T, Tera-2, mice | In vivo and in vitro | Inhibited mammary duct formation. | [106] |
| 2018 | miR-205 | MEC, mice | In vivo and in vitro | Impacted mammary regenerative capacity and mammary homeostasis. | [107] |
| 2019 | miR-489 | Mouse mammary epithelial cells, mice | In vivo and in vitro | Inhibited duct growth and TEB formation. | [108] |
| Milk component synthesis | |||||
| 2018 | miR-15b | MCF-10A, mice, goats | In vivo and in vitro | Inhibited lipid synthesis and metabolism. | [109] |
| 2017 | miR-17-5p miR-148a | GMEC, goats | In vivo and in vitro | Promoted triacylglycerol (TAG) synthesis and milk fat droplet accumulation. | [110] |
| 2015 | miR-24 | GMEC, goats | In vivo and in vitro | Increased unsaturated fatty acid concentrations, TAG levels, and milk fat droplet accumulation. | [111] |
| 2018 | miR-25 | GMEC, goats | In vivo and in vitro | Inhibited TAG synthesis and lipid droplet accumulation. | [112] |
| 2013 | miR-27a | GMEC, goats | In vivo and in vitro | Inhibited TAG synthesis and reduced the ratio of unsaturated/saturated fatty acids. | [113] |
| 2015 | miR-29s | DCMEC, 293T, Chinese Holstein cows | In vivo and in vitro | Inhibited triglyceride, protein, and lactose secretion. | [114] |
| 2013 | miR-103 | GMEC, goats | In vivo and in vitro | Promoted lipid droplet accumulation and TAG accumulation. | [95] |
| 2011/2017 | miR-126-3p | MCF-10A, mice | In vivo and in vitro | Inhibited β-casein secretion and lipid synthesis. | [115,116] |
| 2019 | miR-142-3p | MMGEC, mice | In vivo and in vitro | Inhibited secretion of β-casein and TAG. | [117] |
| 2017 | miR-145 | GMEC, goats | In vivo and in vitro | Promoted lipid droplet enlargement and TAG accumulation, increased the relative content of unsaturated fatty acids. | [118] |
| 2016 | miR-150-5p | Mice | In vivo | Inhibited the de novo synthesis of lipids and fatty acids. | [119] |
| 2016 | miR-181b | GMEC, goats | In vivo and in vitro | Increased TAG levels and cream droplet accumulation. | [120] |
| 2020 | miR-204 | HC11, mice | In vivo and in vitro | Promoted β-casein and milk fat synthesis. | [88] |
| 2019 | miR-206 | HC11, mice | In vivo and in vitro | Promoted lipid accumulation. | [121] |
| 2018 | miR-221 | MEC, MCF-10A, mice | In vivo and in vitro | Promoted lipid synthesis. | [122] |
| 2015 | miR-486 | BMEC, Holstein cows | In vivo and in vitro | Promoted beta-casein, lactose, and lipid secretion. | [123] |
| Particular Year | Detection Methods | Species | Outcomes | References |
|---|---|---|---|---|
| 2021 | Small RNA-seq | Porcine | Characterization of the composition of piRNAs in spermatozoa suggests that piRNAs may be potential negative regulatory markers of sperm quality. | [135] |
| 2012 | Small RNA-seq, qRT-PCR | Porcine | It was demonstrated that piRNAs were predominantly enriched in the mature gonads and were expressed more in the testis than in the ovary. | [136] |
| 2023 | Small RNA-seq | Porcine | Expression of piRNAs is regulated by Senecavirus A (SVA) and promotes apoptosis. | [137] |
| 2015 | Small RNA-seq | Porcine | Characterization of the composition of piRNAs in testis suggests that mammalian piRNAs exist in the ping-pong cycle and have a role in the post-transcriptional regulation of protein-coding genes. | [130] |
| 2022 | Small RNA-seq | Xiang pigs | Identification of the composition of piRNAs in testicular tissues at different stages demonstrates that piRNAs regulate spermatogenesis. | [129] |
| 2017 | Small RNA-seq, qRT-PCR | Porcine | Characterization of the expression profiles of testicular piRNAs at different stages of sexual maturation demonstrated that piRNAs regulate testicular development and spermatogenesis. | [138] |
| 2012 | Small RNA-seq, qRT-PCR | Porcine | Evidence for a potential role of piRNAs in female germ cell development. | [139] |
| 2020 | Small RNA-seq, qRT-PCR | Porcine | Characterization of the expression profile of sperm plasma extracellular vesicles (SP-EVs) piRNAs suggests that piRNAs play a role in the physiological function of spermatozoa. | [140] |
| 2017 | Small RNA-seq, qRT-PCR | Bovids | The piRNAs in the testis were identified as longer than the piIRNAs in oocytes and embryos. | [141] |
| 2020 | Small RNA-seq | Yattle, cattle, yaks, | Promoter hypermethylation of PIWI/piRNA pathway genes leading to gene silencing and reduction in testis-thick piRNAs is a driver of bovine HMS. | [131] |
| 2015 | Small RNA-seq | Calves | Expression of piRNAs in bovine blood and plasma was revealed, suggesting that they may originate from tissues other than blood cells and thus enter the circulation. | [142] |
| 2018 | Small RNA-seq | Cattle, yaks, dzo | Comparison of the expression characteristics of three ruminant piRNAs provides theoretical references for exploring their regulatory mechanisms in spermatogenesis and dzo reproductive therapy. | [143] |
| 2020 | Small RNA-seq, qRT-PCR | Bulls | Expression of piRNAs in spermatozoa was detected, suggesting that they may play a role in embryonic development and may serve as biomarkers of semen fertility. | [144] |
| 2017 | Small RNA-seq | Bulls | Characterization of the composition of piRNAs in frozen spermatozoa suggests a role in sperm development and fertility. | [132] |
| 2015 | Small RNA-seq | Bovine | Detection of the composition of mature testicular and ovarian piRNAs revealed that ovarian piRNAs were very similar to spermatogenesis thick-walled stage piRNAs. | [145] |
| 2018 | Small RNA-seq | Bovids | Detection of milk exosomal piRNAs expression suggests that they may be related to immune and developmental functions. | [134] |
| 2021 | Small RNA-seq | Cattle | The presence of piRNAs in ejaculated sperm was confirmed, suggesting that they may regulate sperm maturation, fertilization process, and embryonic genome activation. | [146] |
| 2021 | Small RNA-seq | Bovids | Expression of piRNAs was detected separately in both milks, suggesting a possible regulatory role in calf immunity and development. | [147] |
| 2023 | Small RNA-seq | Murrah buffalo | Characterization of the composition of piRNAs at different stages of lactation implies that piRNAs can serve as potential targets for the regulation of lactation. | [148] |
| 2019 | Small RNA-seq | Mongolian horse | Characterization of piRNAs composition in sexually mature and immature testes suggests that piRNAs may regulate testicular development and spermatogenesis. | [149] |
| 2022 | Small RNA-seq | Sheep | Expression profiles of piRNAs in LP and FP ovaries were characterized to facilitate understanding of the role of piRNAs in the estrous cycle. | [133] |
| 2022 | Small RNA-seq | Sheep | Characterization of the composition of testicular piRNAs demonstrates that piRNAs may mediate blood–testis barrier stability and spermatogonial stem cell differentiation. | [150] |
| 2021 | Small RNA-seq | Sunite (SN), Small-tailed Han (STH) | Identification of differential expression of testicular piRNAs in different breeds suggests that piRNAs may be associated with male fecundity. | [151] |
| 2023 | Small RNA-seq, qRT-PCR | Tibetan sheep | Characterization of piRNAs expression profiles in different stages of testis suggests that piRNAs regulate male fertility and spermatogenesis. | [152] |
| Particular Year | piRNA | Expression | Model Type | Species | Finding | References |
|---|---|---|---|---|---|---|
| 2021 | piR-651 | Upregulation | In vivo and in vitro | Human | Bound to PIWIL2, promoted cell proliferation and migration through DNMT1-mediated methylation of the PTEN promoter. | [158] |
| 2021 | piR-823 | Upregulation | In vivo and in vitro | Human and mice | Increased the expression of DNMT1, DNMT3A, and DNMT3B genes to promote DNA methylation of APC genes to activate the Wnt signaling pathway. | [159] |
| 2022 | piR-823 | Upregulation | In vivo and in vitro | Human and mice | Inhibited piR-823 expression inhibited cell proliferation, PI3K/Akt/mTOR gene expression, and increased gene and protein expression of ERα. | [160] |
| 2013 | piR-932 | Upregulation | In vivo and in vitro | Human and mice | Bound to PIWIL2, promoted methylation of promoter CpG islands to repress Latexin expression. | [13] |
| 2023 | piR-2158 | Downregulation | In vivo and in vitro | Human and mice | Competed with FOSL1 to inhibit IL-11 expression and secretion, inactivating JAK/STAT signaling and thereby inhibiting breast cancer. | [18] |
| 2022 | piR-17560 | Upregulation | In vivo and in vitro | Human | Targeted FTO-mediated m6A demethylation enhances ZEB1 expression, thereby promoting chemotherapy resistance and EMT. | [161] |
| 2013 | piR-4987, piR-20365, piR-20485, piR-20582 | Upregulation | In vivo | Human | Influenced cancer development and lymph node metastasis. | [154] |
| 2017 | piR-1282, piR-21131, piR-23672, piR-26526, piR-26527, piR-26528, piR-30293, piR-32745 | Upregulation | In vivo | Human | Can be used as a biomarker for breast cancer and provided a therapeutic target. | [156] |
| piR-23662 | Downregulation | |||||
| 2014 | piR-31106 | Upregulation | In vivo and in vitro | Human | Responded to cell growth, cell cycle progression, and hormonal signaling. | [155] |
| 2021 | piR-31106, piR-34998, piR-40067 | Upregulation | In vivo | Human | Can be used as a prognostic and therapeutic marker for breast cancer. | [157] |
| 2023 | piR-31106 | Upregulation | In vivo and in vitro | Human | Promoted cell proliferation and migration as well as oncogene expression and METTL3-mediated m6A methylation. | [162] |
| 2025 | piR-31115 | Upregulation | In vivo and in vitro | Human | Bound to PIWIL4 and inhibits the degradation of HSP90AA1 protein, thereby promoting cell migration. | [163] |
| 2020 | piR-31143 | Upregulation | In vivo and in vitro | Human | Can modulation of TNBC behavior through ERβ. | [164] |
| 2014 | piR-34377, piR-35407, piR-36743 | Upregulation | In vivo and in vitro | Human | Responded to cell growth, cell cycle progression, and hormonal signals. | [155] |
| piR-36026, piR-36249, piR-36318, piR-36712 | Downregulation | |||||
| 2019 | piR-36712 | Downregulation | In vivo and in vitro | Human and mice | Knockdown of piR-36712 inhibits p53 activity through SEPW1, upregulates Slug/p21, and decreases E-calmodulin levels, ultimately inhibiting cell proliferation, migration, and invasion. | [165] |
| 2020 | piR-016658 | Upregulation | In vivo and in vitro | Human | Regulated by cell Cyclin D1, affects stem cell function. | [166] |
| piR-016975 | Downregulation | |||||
| 2015 | piR-021285 | Upregulation | In vivo and in vitro | Human | Increases the methylation level of the ARHGAP11A gene, which promotes cell invasion and inhibits cell apoptosis. | [167] |
| 2015 | piR-sno75 | Upregulation | In vivo and in vitro | Human and mice | Binding to WDR5 recruits the MLL3/UTX complex to the TRAIL promoter region, thereby inducing H3K4 methylation and H3K27 demethylation. | [168] |
| 2022 | piR-MW557525 | Upregulation | In vivo and in vitro | Human | Promotes the proliferation, migration, and invasion of Piwil2-iCSCs, promotes the expression of CD24, CD133, KLF4, and SOX2, and inhibits apoptosis. | [169] |
| 2018 | piR-FTH1 | Downregulation | In vivo and in vitro | Human | Binds to HILI/HIWI2 and down-regulates FTH1, increasing sensitivity to chemotherapy. | [170] |
| 2024 | piR-YBX1 | Downregulation | In vivo and in vitro | Human and mice | Inhibition of YBX1 expression leads to inhibition of MEK and ERK1/2 MAPK signaling pathways, ultimately inhibiting cell proliferation and migration. | [171] |
| Particular Year | piRNA | Expression | Research Target | Finding | References |
|---|---|---|---|---|---|
| 2024 | hsa-piR-3411, hsa-piR-24541, hsa-piR-27080, hsa-piR-28104, hsa-piR-32157 and 10 others | Upregulation | Human peripheral venous blood | Identification of piRNAs in the plasma of CP patients demonstrated that piRNAs are associated with inflammation. | [189] |
| hsa-piR-32835, hsa-piR-32836, hsa-piR-32986, hsa-piR-33168 | Downregulation | ||||
| 2022 | piRNA-6426 | Downregulation | Rat cardiomyocytes, rats | Inhibits secretion of inflammatory factors IL-1β and TNF-α, cardiomyocyte apoptosis, oxidative stress, and improves the inflammatory microenvironment in heart failure. | [186] |
| 2023 | piR-has-27620, piR-has-27124 | Upregulation | Blood samples | Identification of peripheral leukocyte piRNA expression and their enrichment in Rap1, PI3K-Akt, and MAPK pathways as RA biomarkers. | [190] |
| 2021 | rno-piR-017330 | Upregulation | Endothelial cells, rats | Identification of piRNAs expression in endothelial cells under inflammatory conditions suggests that piRNAs may regulate inflammatory processes. | [187] |
| 2024 | hsa_piR_019949 | Downregulation | C28/I2, SW1353 | Inhibition of NEAT1 and NLRP3 expression regulates the NOD-like receptor signaling pathway and modulates OA progression. | [185] |
| 2024 | mmu_piR_037459 | Upregulation | Mice cardiomyocytes, mice | Inhibition of collagenase II expression, promotion of chondrocyte apoptosis and inhibition of proliferation, inhibition of USP7 expression, and regulation of OA progression. | [188] |
| 2024 | piR-112710 | Downregulation | Mice cardiomyocytes, mice | Inhibits the Txnip/NLRP3 signaling pathway, reduces the levels of IL-18, IL-1β, and NLRP3, inhibits cardiomyocyte injury, and regulates inflammation progression. | [19] |
| 2025 | pir-has-216911 | Upregulation | HL7702, Huh7, HepG2, Hep3B, nude mice | Inhibition of the TLR4/NFκB/NLRP3 inflammatory signaling pathway suppressed the inflammatory response. | [183] |
| 2020 | piRNAs | Differential expression | Sudani duck | The composition of piRNAs in brain and lung was characterized, suggesting that they may be associated with lung inflammation. | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Zhang, Z.; Wang, Z.; Dong, X.; Hou, Q. piRNAs as Potential Regulators of Mammary Gland Development and Pathology in Livestock. Vet. Sci. 2025, 12, 594. https://doi.org/10.3390/vetsci12060594
Yu W, Zhang Z, Wang Z, Dong X, Hou Q. piRNAs as Potential Regulators of Mammary Gland Development and Pathology in Livestock. Veterinary Sciences. 2025; 12(6):594. https://doi.org/10.3390/vetsci12060594
Chicago/Turabian StyleYu, Wenjing, Zixuan Zhang, Zhonghua Wang, Xusheng Dong, and Qiuling Hou. 2025. "piRNAs as Potential Regulators of Mammary Gland Development and Pathology in Livestock" Veterinary Sciences 12, no. 6: 594. https://doi.org/10.3390/vetsci12060594
APA StyleYu, W., Zhang, Z., Wang, Z., Dong, X., & Hou, Q. (2025). piRNAs as Potential Regulators of Mammary Gland Development and Pathology in Livestock. Veterinary Sciences, 12(6), 594. https://doi.org/10.3390/vetsci12060594







