Contributions to Knowledge of the Dictyocaulus Infection of the Red Deer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Necropsies and Microscopic Examination
2.2. Anatomopathological Study
2.3. Molecular Procedures
2.3.1. DNA Extraction
2.3.2. End-Point PCR and Primers
2.3.3. Sequencing, Alignment, and Comparison Through Phylogenetic Networks
3. Results
3.1. Prevalence of Infection by Dictyocaulus in Deer at Sampling Location
3.2. Morphological Identification and Anatomopathological Findings in Dictyocaulus Infection
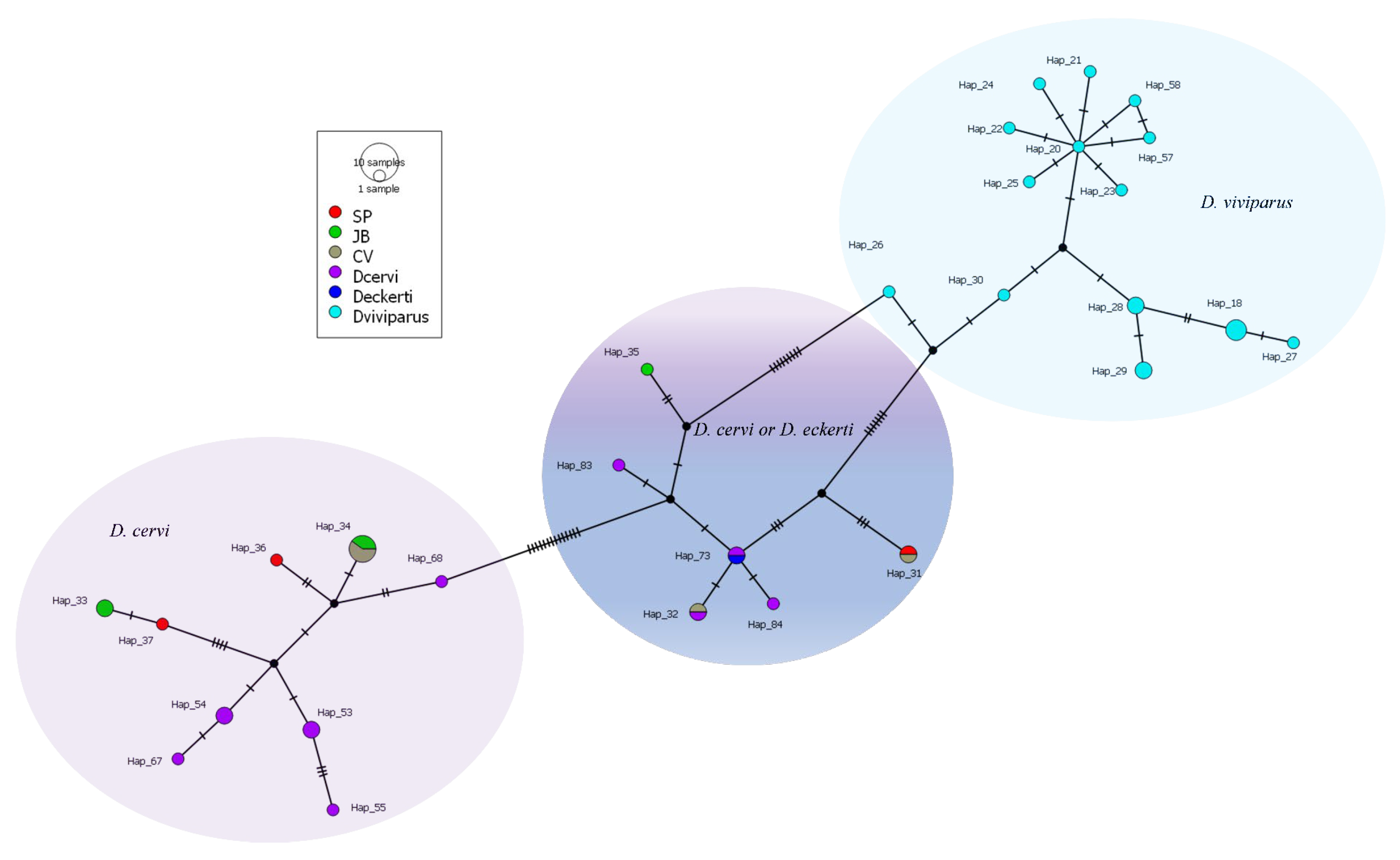
3.3. Barcoding Findings Through Sequencing COI Gene
4. Discussion
4.1. Prevalence of Infection by Dictyocaulus in Deer in Extremadura
4.2. Findings After Anatomopathological Study
4.3. Molecular Assessment of Dictyocaulus spp.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huaman, J.L.; Helbig, K.J.; Carvalho, T.G.; Doyle, M.; Hampton, J.; Forsyth, D.M.; Pople, A.R.; Pacioni, C.A. Review of viral and parasitic infections in wild deer in australia with relevance to livestock and human health. Wildl. Res. 2023, 50, 593–602. [Google Scholar] [CrossRef]
- Shamsi, S.; Brown, K.; Francis, N.; Barton, D.P.; Jenkins, D.J. First findings of Sarcocystis species in game deer and feral pigs in Australia. Int. J. Food Microbiol. 2024, 421, 110780. [Google Scholar] [CrossRef] [PubMed]
- Vainutis, K.S.; Voronova, A.N.; Andreev, M.E.; Pankratov, D.V.; Shchelkanov, M.Y. Morphological and molecular description of Dictyocaulus xanthopygus sp. nov. (Nematoda: Trichostrongyloidea) from the Manchurian wapiti Cervus elaphus xanthopygsus. Syst. Parasitol. 2023, 100, 557–570. [Google Scholar] [CrossRef]
- Dougherty, E.C. A brief survey of the genus Dictyocaulus Railliet and Henry, 1907 (Nematoda: Trichostrangylidae.). Proc. Helminthol. Soc. Wash. 1946, 13, 49–54. [Google Scholar]
- Pyziel, A.M.; Laskowski, Z.; Demiaszkiewicz, A.W.; Höglund, J. Interrelationships of Dictyocaulus spp. in wild ruminants with morphological description of Dictyocaulus cervi N. Sp. (Nematoda: Trichostrongyloidea) from red deer, Cervus elaphus. J. Parasitol. 2017, 103, 506–518. [Google Scholar] [CrossRef]
- Gibbons, L.M.; Höglund, J. Dictyocaulus capreolus N. Sp. (Nematoda: Trichostrongyloidea) from roe deer, Capreolus capreolus and moose, Alces alces in Sweden. J. Helminthol. 2002, 76, 119–124. [Google Scholar] [CrossRef]
- Carreno, R.A.; Nadler, S.A. Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J. Parasitol. 2003, 89, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Carreno, R.A.; Diez-Baños, N.; Hidalgo-Argüello, M.R.; Nadler, S.A. Characterization of Dictyocaulus species (Nematoda: Trichostrongyloidea) from three species of wild ruminants in northwestern Spain. J. Parasitol. 2009, 95, 966–970. [Google Scholar] [CrossRef]
- Gibbons, L.M.; Khalil, L.F. A Revision Of The Genus Dictyocaulus Railliet And Henry, 1907 (Nematoda: Trichostrongyloidea) With The Description of D. Africanus n. sp. from African Artiodactylids. Rev. Zool. Afr. 1988, 102, 151–175. [Google Scholar]
- Höglund, J.; Morrison, D.A.; Divina, B.P.; Wilhelmsson, E.; Mattsson, J.G. Phylogeny of Dictyocaulus (lungworms) from eight species of ruminants based on analyses of Ribosomal RNA Data. Parasitology 2003, 127, 179–187. [Google Scholar] [CrossRef]
- Gasser, R.B.; Jabbar, A.; Mohandas, N.; Höglund, J.; Hall, R.S.; Littlewood, D.T.J.; Jex, A.R. Assessment of the genetic relationship between Dictyocaulus species from Bos taurus and Cervus elaphus using complete mitochondrial genomic datasets. Parasites Vectors 2012, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Pyziel, A.M.; Laskowski, Z.; Klich, D.; Demiaszkiewicz, A.W.; Kaczor, S.; Merta, D.; Kobielski, J.; Nowakowska, J.; Anusz, K.; Höglund, J. Distribution of large lungworms (Nematoda: Dictyocaulidae) in free-roaming populations of red deer Cervus elaphus (L.) with the description of Dictyocaulus skrjabini n. sp. Parasitology 2023, 150, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.B.; Huby-Chilton, F.; Gasser, R.B.; Beveridge, I. The evolutionary origins of nematodes within the order Strongylida are related to predilection sites within hosts. Mol. Phylogenet. Evol. 2006, 40, 118–128. [Google Scholar] [CrossRef]
- Cafiso, A.; Castelli, M.; Tedesco, P.; Poglayen, G.; Buccheri-Pederzoli, C.; Robetto, S.; Orusa, R.; Corlatti, L.; Bazzocchi, C.; Luzzago, C. Molecular characterization of Dictyocaulus nematodes in wild red deer Cervus elaphus in two areas of the Italian alps. Parasitol. Res. 2023, 122, 881–887. [Google Scholar] [CrossRef]
- Jenkins, D.; Baker, A.; Porter, M.; Shamsi, S.; Barton, D.P. Wild fallow deer (Dama Dama) as definitive hosts of Fasciola hepatica (liver fluke) in Alpine New South Wales. Aust. Vet. J. 2020, 98, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Doyle, E.; Barwick, J.; Chambers, M.; Kakhn, L. Prevalence and pathology of liver fluke (Fasciola hepatica) in fallow deer (Dama dama). Vet. Parasitol. 2021, 293, 109427. [Google Scholar] [CrossRef]
- Jarrett, W.F.H.; Jennings, F.W.; Mcintyre, W.I.M.; Mulligan, W.; Thomas, B.A.C.; Urquhart, G.M. Immunological studies on Dictyocaulus viviparus infection. Immunology 1959, 2, 252–261. [Google Scholar]
- Jarrett, W.F.H.; Sharp, N.C.C. Vaccination against parasitic disease: Reactions in vaccinated and immune hosts in Dictyocaulus viviparus infection. J. Parasitol. 1963, 49, 177–189. [Google Scholar] [CrossRef]
- Claerebout, E.; Geldhof, P. Helminth vaccines in ruminants: From development to application. Vet. Clin. Food Anim. Pract. 2020, 36, 159–171. [Google Scholar] [CrossRef]
- Molina, V.M.; Arbeláez, J.M.; Prada, J.A.; Blanco, R.D.; Oviedo, C.A. Posible resistencia de Dictyocaulus viviparus al fenbendazol en un bovino. Rev. Fac. Med. Vet. Zootec. 2016, 63, 54–63. [Google Scholar] [CrossRef]
- Campbell, P.; Forbes, A.; Mcintyre, J.; Bartoschek, T.; Devine, K.; O’neill, K.; Laing, R.; Ellis, K. Inefficacy of ivermectin and moxidectin treatments against Dictyocaulus viviparus in dairy calves. Vet. Rec. 2024, 195, E4265. [Google Scholar] [CrossRef]
- Mckenzie, R.A.; Green, P.E.; Thornton, A.M.; Chung, Y.S.; Mackenzie, A.R.; Cybinski, D.H.; St George, T.D. Diseases of deer in southeastern Queensland. Aust. Vet. J. 1985, 62, 424. [Google Scholar] [CrossRef] [PubMed]
- Mylrea, G.E.; Mulley, R.C.; English, A.W. Gastrointestinal helminths in fallow deer (Dama dama) and their response to treatment with anthelminthics. Aust. Vet. J. 1991, 68, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Habela Martínez-Estéllez, M.Á.; Moreno Casero, A.M.; Peña, J.; Montes, G.; Gómez Carmona, J.M.; Hermoso De Mendoza Salcedo, J. Parásitos asociados a tuberculosis en ciervos (Cervus elaphus) de Extremadura. In Proceedings of the XXXI Jornadas Científicas y X Internacionales de la Sociedad Española de Ovinotecnia y Caprinotecnia (Seoc), Zamora, Spain, 20–22 September 2006; pp. 337–339, ISBN 84-934535-8-7. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=8691504 (accessed on 10 June 2025).
- Pyziel, A.M.; Laskowski, Z.; Höglund, J. Development of a multiplex PCR for identification of Dictyocaulus lungworms in domestic and wild ruminants. Parasitol. Res. 2015, 114, 3923–3926. [Google Scholar] [CrossRef] [PubMed]
- Halvarsson, P.; Baltrušis, P.; Kjellander, P.; Höglund, J. Parasitic strongyle nemabiome communities in wild ruminants in Sweden. Parasites Vectors 2022, 15, 341. [Google Scholar] [CrossRef]
- Bangoura, B.; Brinegar, B.; Creekmore, T.E. Dictyocaulus cervi-like lungworm infection in a rocky mountain elk (Cervus canadensis nelsoni) from wyoming, USA. J. Wildl. Dis. 2020, 57, 71–81. [Google Scholar] [CrossRef]
- Pato Rivero, F.J. Estudio Epidemiológico de las Infecciones que Afectan al Aparato Respiratorio y Gastrointestinal de los Corzos en Galicia. Ph.D. Thesis, University of Lugo, Lugo, Spain, 2012. Available online: https://investigacion.usc.es/documentos/5d1df67029995204f766c047 (accessed on 10 June 2025).
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Bowles, J.; Blair, D.; Mcmanus, D.P. Genetic variants within the genus echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Assessment of the universality and utility of a set of conserved mitochondrial COI primers in insects. Insect Mol. Biol. 1997, 6, 143–150. [Google Scholar] [CrossRef]
- Wollan, G.T.; Quevedo, E.M. Molecular Methods Used to Identify a New Species of Dictyocaulus (Family Dictyocaulidae) in White-Tailed Deer; Presented at College of Science & Engineering, Department Biology, Campus Winona, Ballroom—Kryzsko Commons, Poster Session (nº 57); Winona State University: Winona, MN, USA, 2024. [Google Scholar]
- Rozas Liras, J.A.; Librado Sanz, P.; Sánchez Del Barrio, J.C.; Messeguer Peypoch, X.; Rozas, R. Dnasp Version 5. DNA Sequence Polymorphism Program (Genètica, Microbiologia I Estadística). 2010. Available online: https://diposit.ub.edu/dspace/handle/2445/53451?locale=es (accessed on 10 June 2025).
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-Joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Lseigh, J.W.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Panadero, R.; Carrillo, E.B.; López, C.; Díez-Baños, N.; Díez-Baños, P.; Morrondo, M.P. Bronchopulmonary helminths of roe deer (Capreolus capreolus) in the northwest of Spain. Vet. Parasitol. 2001, 99, 221–229. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Jansen, J.; Van Nispen Tot Pannerden, H.P.M.; Van Der Burg, W.P.J.; Noorman, N.; Poutsma, J.; Kotter, J.F. Untersuchungen über die helminthen-fauna beim reh (Capreolus capreolus L.) in den niederlanden. Z. Für Jagdwiss. 1990, 36, 104–109. [Google Scholar] [CrossRef]
- Shimalov, V.; Shimalov, V. Helminth fauna of cervids in Belorussian Polesie. Parasitol. Res. 2002, 89, 75–76. [Google Scholar] [CrossRef]
- Pyziel, A.M.; Dolka, I.; Werszko, J.; Laskowski, Z.; Steiner-Bogdaszewska, Z.; Wiśniewski, J.; Demiaszkiewicz, A.W.; Anusz, K. Pathological lesions in the lungs of red deer Cervus elaphus (L.) induced by a newly-described Dictyocaulus cervi (Nematoda: Trichostrongyloidea). Vet. Parasitol. 2018, 261, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hugonnet, L.; Cabaret, J. Infection of roe-deer in France by the lung nematode, Dictyocaulus eckerti skrjabin, 1931 (Trichostrongyloidea): Influence of environmental factors and host density. J. Wildl. Dis. 1987, 23, 109–112. [Google Scholar] [CrossRef]
- Dacal, V.; Vázquez, L.; Pato, F.J.; Cienfuegos, S.; Panadero-Fontán, R.; López Sández, C.; Morrondo, P. Cambios en la capacidad pulmonar en corzos (Capreolus capreolus) del noroeste de España infectados por nematodos broncopulmonares. Galemys 2010, 22, 233–242. [Google Scholar] [CrossRef]
- Kuzmina, T.; Kharchenko, V.; Malega, A. Helminth fauna of roe deer (Capreolus capreolus) in ukraine: Biodiversity and parasite community. Vestn. Zool. 2010, 44, 15–22. [Google Scholar] [CrossRef]
- Handeland, K.; Davidson, R.K.; Viljugrein, H.; Mossing, A.; Meisingset, E.L.; Heum, M.; Strand, O.; Isaksen, K. Elaphostrongylus and Dictyocaulus infections in Norwegian wild reindeer and red deer populations in relation to summer pasture altitude and climate. Int. J. Parasitol. 2019, 10, 188–195. [Google Scholar] [CrossRef]
- Stoican, E.; Olteanu, G. Beitriige zum studium der helminthofauna des rehes (C. capreolus) in remaining. Probl. Der Parazitol. 1959, 7, 38–46. [Google Scholar]
- Panayotova-Pencheva, M. Morphometric data on Dictyocaulus eckerti (Nematoda: Trichostrongyloidea) in materials from Bulgaria. Comptes Rendus l’Academie Bulg. Sci. 2012, 65, 1225–1232. [Google Scholar]
- Llada, I.M.; Gianechini, L.S.; Lloberas, M.M.; Morrell, E.L.; Odriozola, E.R.; Cantón, G.J. Dictiocaulosis en vacas de cría en la provincia de Buenos Aires, Argentina: Descripción de dos brotes. Analecta Vet. 2020, 40, 25. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, A.; Hussain, R.; Anjum, M.S. Prevalence and pathology of Dictyocaulus viviparus infection in cattle and buffaloes. Vet. Record 2011, 169, 494. [Google Scholar] [CrossRef]
- Brown, K.; Jenkins, D.J.; Alexander, W.G.; Smith, I.; Francis, N.; Shamsi, S.; Barton, D.P. The First finding of Dictyocaulus cervi and Dictyocaulus skrjabini (Nematoda) in feral fallow deer (Dama dama) in Australia. Int. J. Parasitol. 2024, 24, 100953. [Google Scholar] [CrossRef]
- Pyziel, A.M.; Laskowski, Z.; Dolka, I.; Kołodziej-Sobocińska, M.; Nowakowska, J.; Klich, D.; Bielecki, W.; Żygowska, M.; Moazzami, M.; Anusz, K.; et al. Large lungworms (Nematoda: Dictyocaulidae) recovered from the European bison may represent a new nematode subspecies. Int. J. Parasitol. 2020, 13, 213–220. [Google Scholar] [CrossRef]
- Danks, H.A.; Sobotyk, C.; Saleh, M.N.; Kulpa, M.; Luksovsky, J.L.; Kones, L.C.; Verocai, G.G. Opening a can of lungworms: Molecular characterization of Dictyocaulus (Nematoda: Dictyocaulidae) infecting North American bison (Bison bison). Int. J. Parasitol. 2022, 18, 128–134. [Google Scholar] [CrossRef]
- Molento, M.B.; Depner, R.A.; Mello, M.H.A. Suppressive treatment of abamectin against Dictyocaulus viviparus and the occurrence of resistance in first-grazing-season calves. Vet. Parasitol. 2006, 141, 373–376. [Google Scholar] [CrossRef]
- Blanc-Mathieu, R.; Perfus-Barbeoch, L.; Aury, J.-M.; Rocha, M.D.; Gouzy, J.; Sallet, E.; Martin-Jimenez, C.; Bailly-Bechet, M.; Castagnone-Sereno, P.; Flot, J.-F.; et al. Hybridization and polyploidy enable genomic plasticity without sex in the most devastating plant-parasitic nematodes. PLoS Genet. 2017, 13, E1006777. [Google Scholar] [CrossRef]
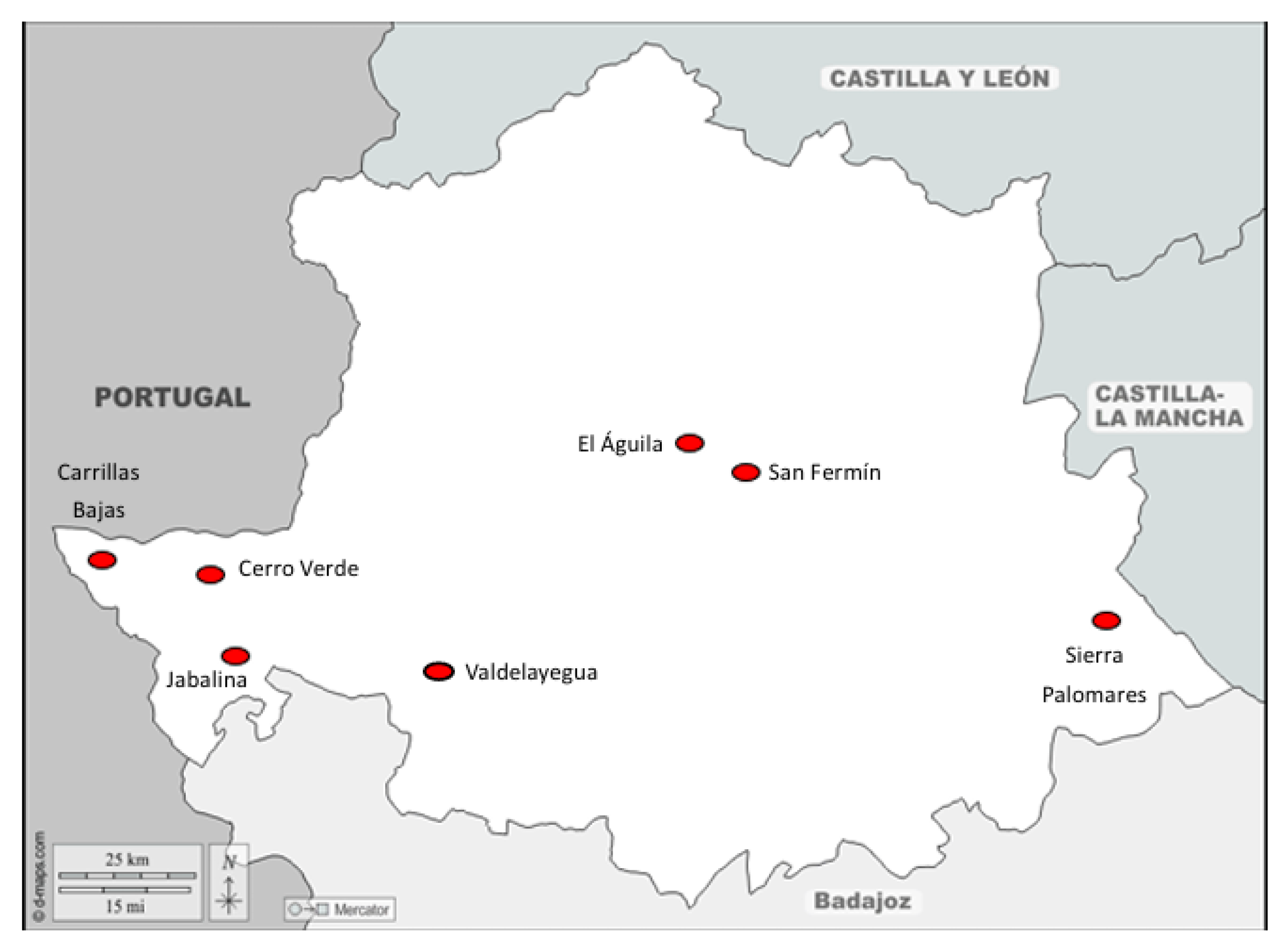


| Name | Location | Date |
|---|---|---|
| Cuadrillas Bajas | Cedillo | 21/09/23 |
| San Fermín | Torrejón el Rubio | 03/12/23 |
| Sierra Palomares | Alía | 27/01/24 |
| Jabalina | Salorino | 02/02/24 |
| Valdelayegua | Aliseda | 09/02/24 |
| Cerro Verde | Carbajo | 11/02/24 |
| El Águila | Serradilla | 17/02/24 |
| Lungs | Location | Macroscopic | Microscopic | Adult Number |
|---|---|---|---|---|
| Deer 1 | Cuadrillas Bajas | + | + | 4 |
| Deer 2 | Cuadrillas Bajas | - | + | nf |
| Deer 3 | Cuadrillas Bajas | - | - | nf |
| Deer 4 | Cuadrillas Bajas | - | - | nf |
| Deer 5 | Cuadrillas Bajas | - | - | nf |
| Deer 6 | San Fermín | - | - | nf |
| Deer 7 | San Fermín | - | - | nf |
| Deer 8 | San Fermín | - | - | nf |
| Deer 9 | San Fermín | - | + | nf |
| Deer 10 | San Fermín | - | - | nf |
| Deer 11 | San Fermín | - | - | nf |
| Deer 12 | Sierra Paloma | - | - | nf |
| Deer 13 | Sierra Paloma | - | - | nf |
| Deer 14 | Sierra Paloma | - | - | nf |
| Deer 15 | Sierra Paloma | - | + | nf |
| Deer 16 | Sierra Paloma | + | + | 8 |
| Deer 17 | Jabalina | + | + | 17 |
| Deer 18 | Jabalina | + | + | 22 |
| Deer 19 | Jabalina | - | - | nf |
| Deer 20 | Jabalina | - | - | nf |
| Deer 21 | Jabalina | - | - | nf |
| Deer 22 | Valdelayegua | - | - | nf |
| Deer 23 | Valdelayegua | - | - | nf |
| Deer 24 | Valdelayegua | - | - | nf |
| Deer 25 | Valdelayegua | - | - | nf |
| Deer 26 | Valdelayegua | - | - | nf |
| Deer 27 | Cerro Verde | - | - | nf |
| Deer 28 | Cerro Verde | - | - | nf |
| Deer 29 | Cerro Verde | - | - | nf |
| Deer 30 | Cerro Verde | - | - | nf |
| Deer 31 | Cerro Verde | + | + | 13 |
| Deer 32 | El Águila | - | - | nf |
| Deer 33 | El Águila | - | - | nf |
| Deer 34 | El Águila | - | - | nf |
| Deer 35 | El Águila | - | - | nf |
| Deer 36 | El Águila | - | - | nf |
| TOTAL | 5 | 8 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Velo, M.; Espinosa-Sánchez, A.; Ripa, A.; Hurtado-Preciado, M.A.; Martínez-Estéllez, M.A.H.; Fernández-García, J.L.; Bazo-Pérez, C. Contributions to Knowledge of the Dictyocaulus Infection of the Red Deer. Vet. Sci. 2025, 12, 595. https://doi.org/10.3390/vetsci12060595
González-Velo M, Espinosa-Sánchez A, Ripa A, Hurtado-Preciado MA, Martínez-Estéllez MAH, Fernández-García JL, Bazo-Pérez C. Contributions to Knowledge of the Dictyocaulus Infection of the Red Deer. Veterinary Sciences. 2025; 12(6):595. https://doi.org/10.3390/vetsci12060595
Chicago/Turabian StyleGonzález-Velo, M., A. Espinosa-Sánchez, A. Ripa, M. A. Hurtado-Preciado, M. A. Habela Martínez-Estéllez, J. L. Fernández-García, and C. Bazo-Pérez. 2025. "Contributions to Knowledge of the Dictyocaulus Infection of the Red Deer" Veterinary Sciences 12, no. 6: 595. https://doi.org/10.3390/vetsci12060595
APA StyleGonzález-Velo, M., Espinosa-Sánchez, A., Ripa, A., Hurtado-Preciado, M. A., Martínez-Estéllez, M. A. H., Fernández-García, J. L., & Bazo-Pérez, C. (2025). Contributions to Knowledge of the Dictyocaulus Infection of the Red Deer. Veterinary Sciences, 12(6), 595. https://doi.org/10.3390/vetsci12060595





