Efficacy of PCV2 Vaccination Under Natural Conditions: A Longitudinal Study Using PCR and Virus Isolation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Condition
2.2. Serum Samples
2.3. PCV2 and PRRS Gene Detection by qPCR/qRT-PCR
2.4. Antibody Responses Induced by PCV2 and PRRSV
2.5. PCV2 Isolation from Serum Samples
2.6. Immunofluorescent Assays
2.7. Statistical and Correlation Analysis
3. Results
3.1. PCV2 Copy Number in Vaccinated and Non-Vaccinated Pigs’ Serum
3.2. Serological Test for PCV2 Antibody Detection
3.3. Clinical Signs and Mortality
3.4. PCV2 Isolation
3.5. PRRSV Infection-Based Antibody Test and RT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCV2 | Porcine circovirus type 2 |
| PRRSV | Porcine reproduction and respiratory syndrome virus |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Kang, S.J.; Bae, S.M.; Lee, H.J.; Jeong, Y.J.; Lee, M.A.; You, S.H.; Lee, H.; Hyun, B.H.; Lee, N.; Cha, S.H. Porcine circovirus (PCV) genotype 2d-based virus-like particles (VLPs) induced broad cross-neutralizing antibodies against diverse genotypes and protected dual-challenge infection of a PCV2d virus and a type 1 porcine reproductive and respiratory syndrome. Pathogens 2021, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Gourgues, S.; Fraile, L.; Segalés, J.; Hernández-Caravaca, I.; López-Úbeda, R.; García-Vázquez, F.A.; Gomez-Duran, O.; Grosse-Liesner, B. Effect of porcine circovirus 2 (PCV-2) maternally derived antibodies on performance and PCV-2 viremia in vaccinated piglets under field conditions. Porc. Health Manag. 2019, 5, 21. [Google Scholar] [CrossRef]
- Czyżewska-Dors, E.B.; Dors, A.; Pomorska-Mól, M.; Podgórska, K.; Pejsak, Z. Conditions, Efficacy of the porcine circovirus 2 (PCV2) vaccination under field conditions. Vet. Ital. 2018, 54, 219–224. [Google Scholar] [CrossRef]
- Takahagi, Y.; Toki, S.; Nishiyama, Y.; Morimatsu, F.; Murakami, H. Differential effects of porcine circovirus type 2 (PCV2) vaccination on PCV2 genotypes at Japanese pig farms. J. Vet. Med. Sci. 2010, 72, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef]
- Kristensen, C.S.; Baadsgaard, N.P.; Toft, N. A meta-analysis comparing the effect of PCV2 vaccines on average daily weight gain and mortality rate in pigs from weaning to slaughter. Prev. Vet. Med. 2011, 98, 250–258. [Google Scholar] [CrossRef]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Shibata, I.; Okuda, Y.; Yazawa, S.; Ono, M.; Sasaki, T.; Itagaki, M.; Nakajima, N.; Okabe, Y.; Hidejima, I. PCR detection of Porcine circovirus type 2 DNA in whole blood, serum, oropharyngeal swab, nasal swab, and feces from experimentally infected pigs and field cases. J. Vet. Med. Sci. 2003, 65, 405–408. [Google Scholar] [CrossRef]
- Kim, D.; Ha, Y.; Oh, Y.; Chae, C. Prevalence of porcine circovirus types 2a and b in pigs with and without post-weaning multi-systemic wasting syndrome. Vet. J. 2011, 188, 115–117. [Google Scholar] [CrossRef]
- Tischer, I.; Peters, D.; Rasch, R.; Pociuli, S. Replication of porcine circovirus: Induction by glucosamine and cell cycle dependence. Arch. Virol. 1987, 96, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Misinzo, G.; Delputte, P.L.; Nauwynck, H.J. Inhibition of Endosome-Lysosome System Acidification Enhances Porcine Circovirus 2 Infection of Porcine Epithelial Cells. J. Virol. 2008, 82, 1128–1135. [Google Scholar] [CrossRef]
- Hosono, S.; Shiokawa, M.; Kobayashi, T.; Fukusho, A.; Aoki, H. Porcine circovirus type 2 induces a strong cytopathic effect in the serum-free culture cell line CPK-NS. J. Virol. Methods 2019, 273, 113706. [Google Scholar] [CrossRef] [PubMed]
- Marks, F.S.; Almeida, L.L.; Driemeier, D.; Canal, C.; Barcellos, D.E.S.N.; Guimarães, J.A.; Reck, J. Porcine circovirus 2 (PCV2) increases the expression of endothelial adhesion/junction molecules. Braz. J. Microbiol. 2016, 47, 870–875. [Google Scholar] [CrossRef]
- Opriessnig, T.; Madson, D.M.; Prickett, J.R.; Kuhar, D.; Lunney, J.K.; Elsener, J.; Halbur, P.G. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet. Microbiol. 2008, 131, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Balasch, M.; Segalés, J.; García, L.; Plana-Durán, J.; Rosell, C.; Ellerbrok, H.; Mankertz, A.; Domingo, M. Experimental Inoculation of Conventional Pigs with Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus 2. J. Virol. 2002, 76, 3232–3239. [Google Scholar] [CrossRef]
- Yim-Im, W.; Huang, H.; Zheng, Y.; Li, G.; Rawal, G.; Gauger, P.; Krueger, K.; Main, R.; Zhang, J. Characterization of PRRSV in clinical samples and the corresponding cell culture isolates. Transbound. Emerg. Dis. 2022, 69, e3045–e3059. [Google Scholar] [CrossRef]
- Drolet, R.; Larochelle, R.; Morin, M.; Delisle, B.; Magar, R. Detection rates of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and swine influenza virus in porcine proliferative and necrotizing pneumonia. Vet. Pathol. 2003, 40, 143–1448. [Google Scholar] [CrossRef]
- Gagnon, C.A.; Del Castillo, J.R.E.; Music, N.; Fontaine, G.; Harel, J.; Tremblay, D. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J. Vet. Diagn. Investig. 2008, 20, 545–558. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Pérez-Martín, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet. Microbiol. 2015, 177, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Brunborg, I.M.; Moldal, T.; Jonassen, C.M. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods. 2004, 122, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.G.; Beach, N.M.; Huang, Y.W.; Halbur, P.G.; Meng, X.J.; Opriessnig, T. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV). Vaccine 2010, 28, 5960–5966. [Google Scholar] [CrossRef]
- Allan, G.M.; McNeilly, F.; Ellis, J.; Krakowka, S.; Meehan, B.; McNair, I.; Walker, I.; Kennedy, S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 2000, 145, 2421–2429. [Google Scholar] [CrossRef]
- Larochelle, R.; Magar, R.; D’Allaire, S. Comparative serologic and virologic study of commercial swine herds with and without postweaning multisystemic wasting syndrome. Can. J. Vet. Res. 2003, 67, 114–1120. [Google Scholar] [PubMed Central]
- Luo, X.; Chen, X.; Qiao, S.; Li, R.; Xie, S.; Zhou, X.; Deng, R.; Zhou, E.; Zhang, G. Porcine Reproductive and Respiratory Syndrome Virus Enhances Self-Replication via AP–1–Dependent Induction of SOCS1. J. Immunol. 2020, 204, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yoo, D. Modulation of innate immune signaling by nonstructural protein 1 (nsp1) in the family Arteriviridae. Virus Res. 2014, 194, 100–109. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
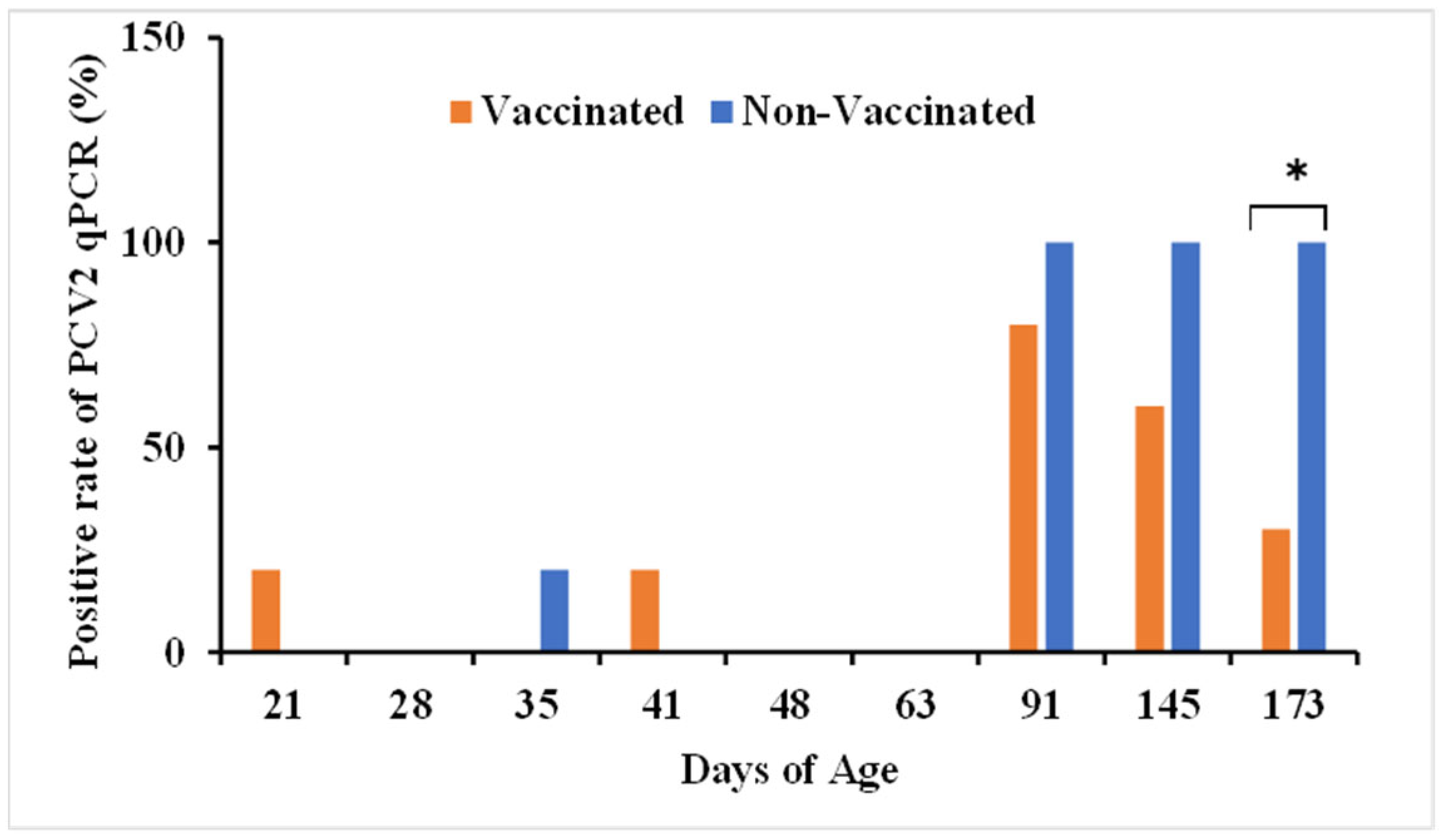
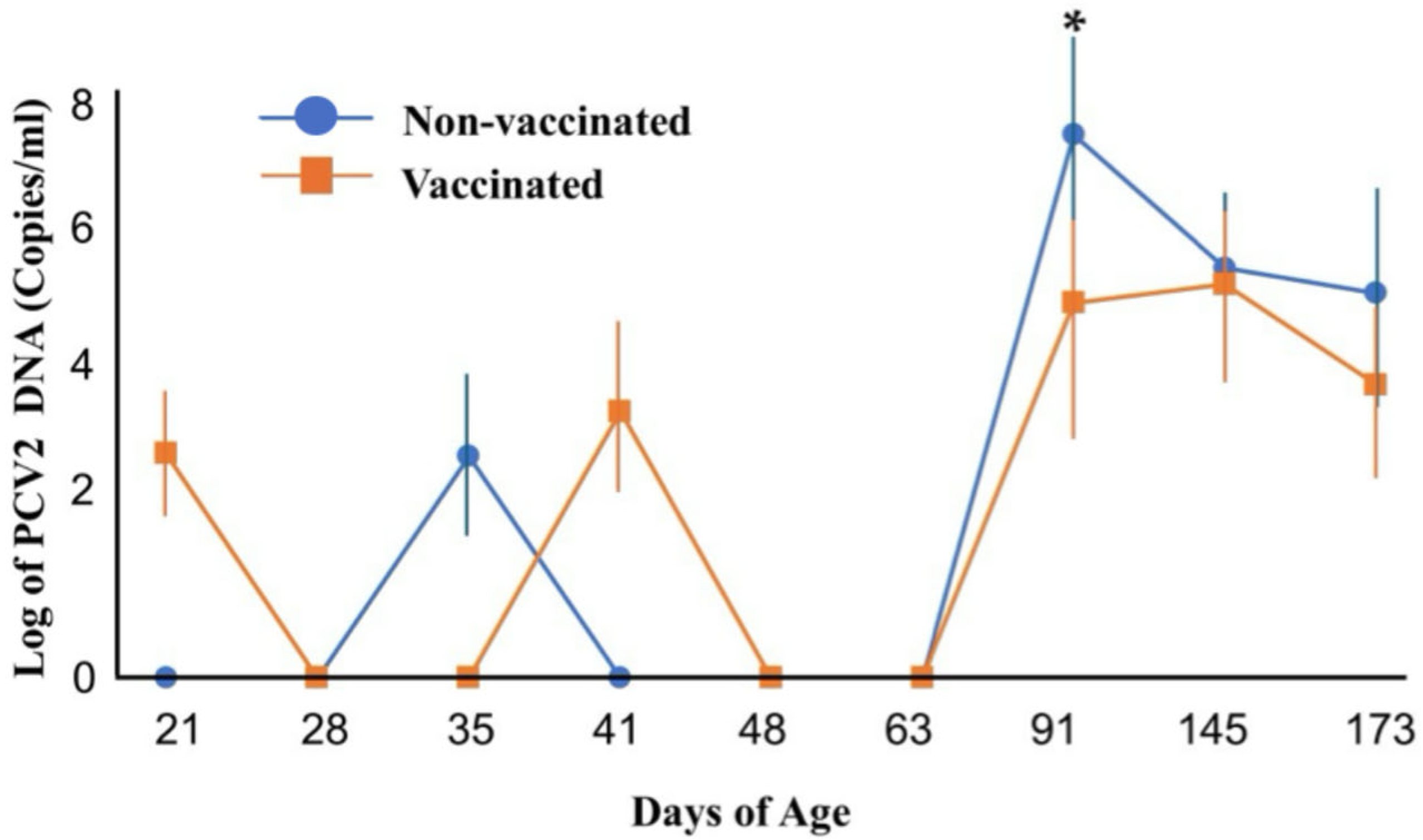
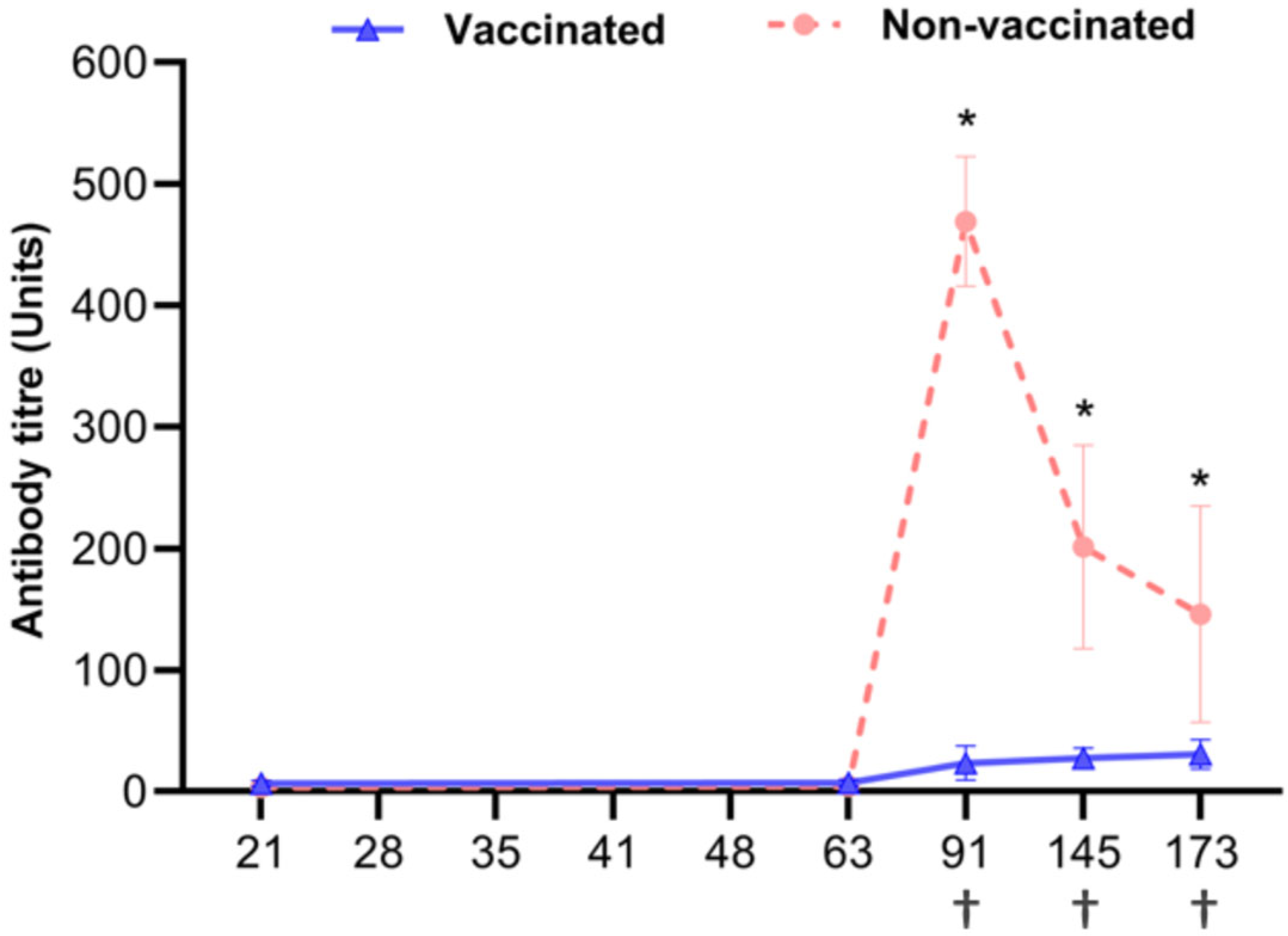

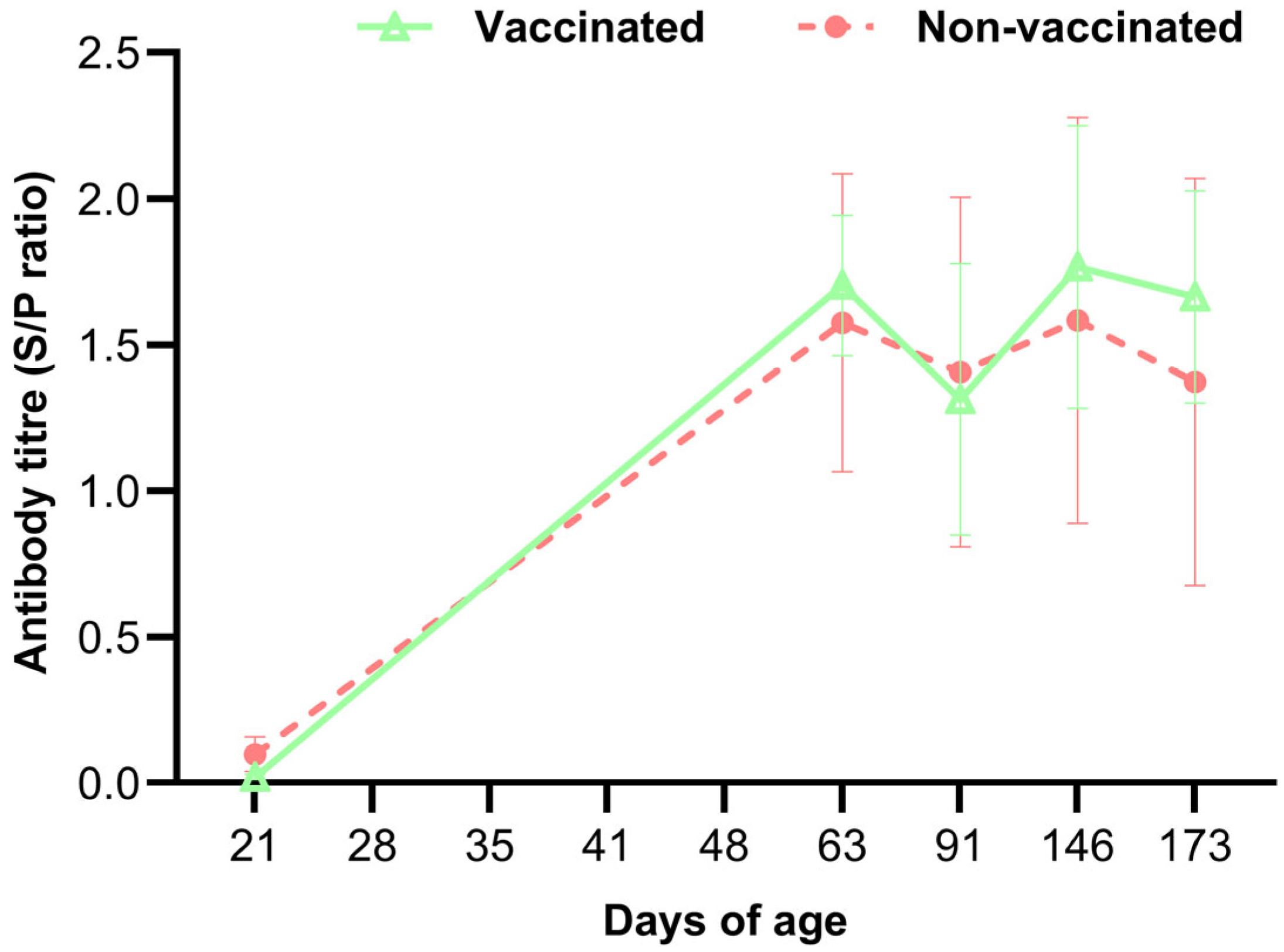
| ID | Serum Copies/mL | Supernatants Copies/mL | IFA +/− |
|---|---|---|---|
| C281 | 5.00 × 103 | <LOD | ND |
| C284 | 5.01 × 102 | 9.00 × 101 | − |
| C292 | 3.10 × 103 | <LOD | ND |
| N276 | 1.78 × 104 | 3.52 × 102 | + |
| N277 | 1.94 × 104 | 1.74 × 102 | + |
| N280 | 1.05 × 104 | 9.87 × 101 | − |
| N282 | 2.73 × 103 | <LOD | ND |
| N283 | 1.22 × 103 | <LOD | ND |
| N284 | 9.15 × 102 | <LOD | ND |
| N290 | 4.65 × 105 | 8.94 × 101 | + |
| N291 | 1.17 × 105 | 1.83 × 102 | + |
| N294 | 1.42 × 104 | 9.73 × 101 | + |
| N297 | 3.25 × 103 | <LOD | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazimpaka, E.; Ringo, R.S.; Hirooka, T.; Okabayashi, T. Efficacy of PCV2 Vaccination Under Natural Conditions: A Longitudinal Study Using PCR and Virus Isolation. Vet. Sci. 2025, 12, 575. https://doi.org/10.3390/vetsci12060575
Mazimpaka E, Ringo RS, Hirooka T, Okabayashi T. Efficacy of PCV2 Vaccination Under Natural Conditions: A Longitudinal Study Using PCR and Virus Isolation. Veterinary Sciences. 2025; 12(6):575. https://doi.org/10.3390/vetsci12060575
Chicago/Turabian StyleMazimpaka, Eugene, Rissar Siringo Ringo, Tasuku Hirooka, and Tamaki Okabayashi. 2025. "Efficacy of PCV2 Vaccination Under Natural Conditions: A Longitudinal Study Using PCR and Virus Isolation" Veterinary Sciences 12, no. 6: 575. https://doi.org/10.3390/vetsci12060575
APA StyleMazimpaka, E., Ringo, R. S., Hirooka, T., & Okabayashi, T. (2025). Efficacy of PCV2 Vaccination Under Natural Conditions: A Longitudinal Study Using PCR and Virus Isolation. Veterinary Sciences, 12(6), 575. https://doi.org/10.3390/vetsci12060575







