1. Introduction
Yellow-feathered broilers, a distinctive breed in China, are highly valued for their distinctive flavor profiles and superior nutritional composition [
1]. This growing consumer preference has driven their expanding market share in China’s meat sector [
2]. However, modern intensive breeding practices aimed at accelerating growth rates—particularly through high-fat diets—impose substantial intestinal stress, compromising barrier integrity in broilers [
3]. Such barrier dysfunction can trigger a cascade of metabolic dysregulation, manifesting as suppressed growth metrics, amplified stress susceptibility, and impaired immunocompetence [
4]. These challenges are exacerbated by the breed’s extended production cycle, necessitating more rigorous gut health management compared to commercial broiler strains [
5].
The gastrointestinal tract serves as the primary interface between external environments and internal homeostasis, with its multilayered barrier system providing a critical defense against luminal pathogens and antigens [
6]. The intestinal barrier system consists of a tightly connected epithelial cell layer, tight junction protein (TJP) expression, mucus secretion, intestinal immune cells, and the resident symbiotic microbiota [
7]. The system is categorized into four distinct types based on their functions: the physical barrier, the chemical barrier, the immune barrier, and the microbial barrier. TJP, specialized structures situated between intestinal epithelial cells, function as a critical regulatory barrier that governs paracellular permeability, maintaining gut barrier integrity [
8].
Emerging evidence shows that synbiotic interventions (combined prebiotics and probiotics) can optimize the intestinal ecosystem by reshaping microbial composition, enhancing α-diversity, and competitively excluding enteropathogens [
9,
10]. Within this paradigm, yeast cultures—a class of multifunctional probiotic preparations—deliver a bioactive matrix comprising fermentation substrates, microbial proteins, yeast metabolites, and cell wall components like β-glucans and mannooligosaccharides [
11]. Notably, yeast-derived components have been shown to increase the ileal villus height-to-crypt depth (VH/CD) ratio in broilers [
12]. Yeast cell walls also enhance intestinal barrier integrity in broilers through the upregulation of TJP gene expression, thereby improving gut health [
13,
14]. Additionally, dietary yeast culture supplementation has been found to modulate the composition and diversity of the cecal microbiota in broilers [
11]. Beyond structural benefits, red yeast enhances gastrointestinal health in poultry by adsorbing mycotoxins, reducing toxin-induced intestinal dysfunction [
11,
15]. Importantly, studies have shown that
Rhodotorula yeast culture (RYC) is rich in metabolites such as carotenoids, which provide significant benefits in reducing immune stress and enhancing intestinal barrier function [
16].
Bacillus subtilis (BS), a prominent member of the animal intestinal microbiota, produces bioactive compounds such as lipopeptides, surfactants, and bacteriocins, which can be used as a viable alternative to traditional feed antibiotics [
17,
18]. Beyond its antimicrobial effects, BS enhances intestinal health in broilers by improving VH/CD, upregulating TJ expression, and reducing pathogenic colonization in the cecum, thereby synergistically improving gut barrier integrity and microbiota homeostasis [
19,
20]. Additionally, BS facilitates nutrient release, modulates digestive enzyme activity, and optimizes microbial community structure, further supporting intestinal function [
21,
22].
Recent studies have demonstrated that combining dietary supplementation with BS and
Saccharomyces cerevisiae can significantly improve growth performance in
Salmonella Typhimurium-challenged broilers, primarily by enhancing intestinal morphology [
23]. Further research suggests that BS achieves its maximal viable biomass when co-administered with yeast, indicating a potential microbial synergy [
24].
Despite these findings, most studies have focused on the isolated application of yeast products or BS, leaving a critical gap in understanding their combined effects—particularly in yellow-feathered broilers. Specifically, systematic studies evaluating the impact of RYC and BS cosupplementation on intestinal barrier function are lacking. This study hypothesizes that the synergistic use of RYC and BS will enhance intestinal barrier function through complementary mechanisms: RYC primarily supports intestinal morphology via nutrient provision, while BS exerts its benefits through cecal microbiota modulation.
2. Materials and Methods
This study was conducted in accordance with the ethical guidelines and protocols of the People’s Republic of China. All experimental procedures were assessed and approved by the Animal Care and Use Committee at Inner Mongolia Agricultural University (Hohhot, China; Approval No. NND2024124).
2.1. Feed Additives
The
Rhodotorula yeast culture (β-glucan ≥ 1.45 mg/g, mannan oligosaccharides ≥ 0.33 mg/g, carotenoids ≥ 1.60 mg/g) was provided by the State Key Laboratory of Animal Nutrition at the Beijing Institute of Animal Husbandry and Veterinary Medicine, Chinese Academy of Agricultural Sciences (Beijing, China). The RYC was produced by fermenting
Rhodotorula mucilaginosa in a liquid broth with soybean meal as the solid substrate. The final product contained yeast cell walls, cellular metabolites, and residual medium components [
25,
26]. The
Bacillus subtilis was provided by Beihai Yiqiang Biotechnology Co., Ltd. (Beihai, China) and had a viable count of 1 × 10
11 CFU/g. Both the RYC and BS were used in powder form for this study.
The additives were incorporated into the feed in a three-stage mixing process. In the initial mixing stage, the probiotics (RYC or BS) were thoroughly blended with 100 g of mixed feed according to the experimental design. During the secondary mixing stage, this 100 g mixture was then combined with 900 g of standard feed, producing 1 kg of supplemented feed. In the final dilution stage, the 1 kg supplemented feed was mixed with 9 kg of standard feed, resulting in 10 kg of intermediate feed. For complete feed production, the 10 kg intermediate feed was blended with 30 kg of standard feed, yielding 40 kg of final feed. To ensure uniform distribution, the feed was mixed thoroughly at each stage to prevent ingredient separation [
27].
2.2. Animals, Research Protocol, and Nutritional Regimens
A total of 192 one-day-old yellow-feathered broilers with similar initial body weights (40.00 ± 0.64 g) were randomly assigned to four treatment groups. Each group consisted of six replicate pens, with eight chicks per pen (four males and four females) to ensure sex balance. Based on previous research [
28,
29], the optimal inclusion levels of RYC and BS in the broiler diets were determined. The experiment was designed as a 2 × 2 factorial interaction study to evaluate the effects of RYC and BS, alone or in combination, on yellow-feathered broilers. The four experimental groups were as follows: Control group (basal diet only), BS group (basal diet + 5 × 10
9 CFU/kg BS), RYC group (basal diet + 5000 mg/kg RYC), and BS + RYC group (basal diet + both additives: 5 × 10
9 CFU/kg BS + 5000 mg/kg of RYC). The basal diet was formulated according to the Nutrition Requirement for Yellow-Feather Broilers (NY/T 3645-2020) [
30]. The detailed composition and nutritional profile of the basal diet are provided in
Table 1.
2.3. Assessment of Growth Performance and Acquisition of Samples
Feed intake and residual feed were recorded daily throughout the trial. Body weights were measured at two time points: the initial weight was recorded after a 2-h fasting period posthatching, and the final weight was measured at 56 days following a 12-h fast to ensure an empty gastrointestinal tract. Average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (F/G) were calculated per replicate using the following formulas:
At 56 days, six broilers per group (three males and three females, representing average body weight per sex) were selected for sampling. Following the administration of anesthesia, the selected yellow-feathered broilers were euthanized via carotid artery transection. A longitudinal surgical section was made along the ventral median axis to access the coelomic compartment. The duodenum, jejunum, ileum, and cecum were sequentially removed. During sampling, the contents of the distal end of the right cecum were collected uniformly, and 1.5 mL of the contents were rapidly transferred into a 2 mL freezing tube and flash-frozen in liquid nitrogen. A 2 cm segment of intestinal tissue from the midileum was excised, and the luminal digesta were flushed with 4 °C normal saline. The tissue was then placed into a 2 mL cryogenic storage tube and flash-frozen in liquid nitrogen. Duodenal, jejunal, and ileal samples were taken from approximately 1 cm of tissue 3 cm from the pylorus, 5 cm proximal to the yolk sac diverticulum, and 10 cm proximal to the ileocecal junction, respectively, for morphometric analysis. The samples were fixed in a 4% paraformaldehyde solution. All procedures were conducted in accordance with the methods described by Liu et al. [
31].
2.4. The Structural Characteristics of Enteric Tissue
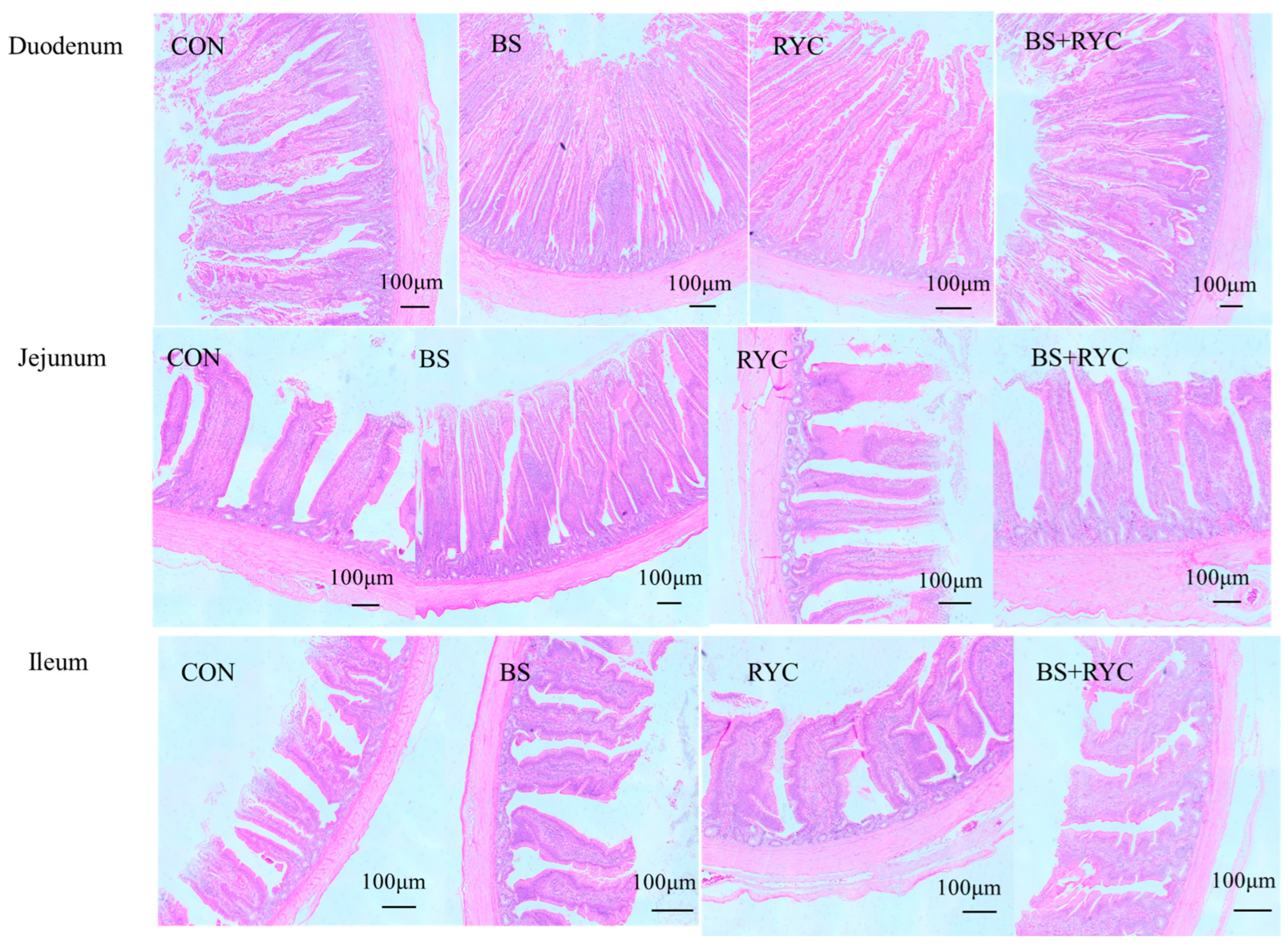
Fixed intestinal tissue samples were processed through progressive ethanol dehydration, paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining. Morphometric measurements, including villus height (VH), crypt depth (CD), and the VH/CD ratio, were performed using a Nikon Eclipse E200 microscope (Tokyo, Japan) and Image View 4 software.
2.5. Intestinal Biomarkers
A 0.5 g sample of ileal tissue was placed in a freezing tube, and 4.5 mL of phosphate-buffered saline was added to prepare a 1:9 tissue homogenate. The homogenate was centrifuged at 3500 rpm for 15 min. After centrifugation, the supernatant was collected and stored for further analysis; the remaining steps were carried out strictly according to the instructions. The total protein concentration in the supernatant was measured using a commercial BCA assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). This was done with the Bicinchoninic Acid Assay according to the manufacturer’s guidelines. Each protein concentration measurement was performed in three technical replicates to ensure precision with intra-assay CV < 5%, inter-assay CV < 8%. Assays for chicken tissue immunoglobulins (IgG, JYM0001Ch; IgM, JYM0060Ch; sIgA, JYM0036Ch) and cytokines (TNF-α, JYM0033Ch; IFN-γ, JYM0007Ch; IL-1β, JYM0041Ch; IL-6, JYM0028Ch; IL-10, JYM0040Ch) were performed using ELISA kits (Genome Biotechnology Co., Ltd., Wuhan, China). For ELISA assays, samples were analyzed in duplicate per the manufacturer’s recommendations, with intra-assay CV < 10%, inter-assay CV < 12%.
2.6. Real-Time qPCR Assay
RNA isolation was performed according to the manufacturer’s guidelines (Takara, Dalian, China). Briefly, 50 mg of tissue samples were mechanically homogenized in 1 mL of Trizol lysate using an FA-25D homogenizer (FLUKO, Shanghai, China). mRNA extraction was conducted following the method described by Ji et al. [
32]. RNA concentration and purity were measured using an Implen P330 spectrophotometer (Implen, Munich, Germany).
For cDNA synthesis, the Evo M-MLV RT MIX KIT (Accurate Bio-technology Co., Ltd., Changsha, China) was used. The reaction mixture consisted of 2 μL 500 μg/μL RNA, 2 μL gDNA Clean Reaction Mix Ver.2, and 6 μL enzyme-free water, which was incubated in a PCR instrument (Labcycler, Senso Quest, Germany) for 2 min. Subsequently, 4 μL 5 × Evo M-MLV RT Reaction Mix Ver.2 and 6 μL enzyme-free water were added. The reaction conditions were as follows: 37 °C for 15 min, 85 °C for 5 s, and 4 °C for cooling.
The qPCR assay was conducted using the YBR Green Pro Taq HS qPCR Kit (Rox Plus; Accurate Biotechnology Co., Ltd., Changsha, China). The PCR reaction system included 2 μL of cDNA, 0.5 μL of F-strand primer, 0.5 μL of R-strand primer, 10 μL of 2 × SYBR Green Pro Taq HS Premix, and 7.2 μL of enzyme-free water. RT-qPCR was performed on a LightCycler 480 II instrument (Roche, Basel, Switzerland) with the following program: 95 °C for 30 s (1 cycle), followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s.
β-actin was used as an endogenous normalization control to standardize the experimental data. The relative expression levels of the following target genes were analyzed:
Occludin (
OCLN),
zonula occludin-1 (
TJP1),
junctional adhesion molecule-2 (
JAM2),
mucin-2 (
MUC2),
interleukin-1beta (
IL1B),
interleukin-6 (
IL6),
interleukin-10 (
IL10),
tumor necrosis factor-alpha (
TNFA),
interferon-γ (
IFNG),
myeloid differentiation factor 88 (
MyD88),
Toll-like receptor 4 (
TLR4), and
nuclear transcription factor-κBp50 (
NFKB1). The relative mRNA expression of the target gene was calculated using the 2
-∆∆Ct method [
33]. Primer sequences are provided in
Table 2.
2.7. Ileal Microbiota Analysis
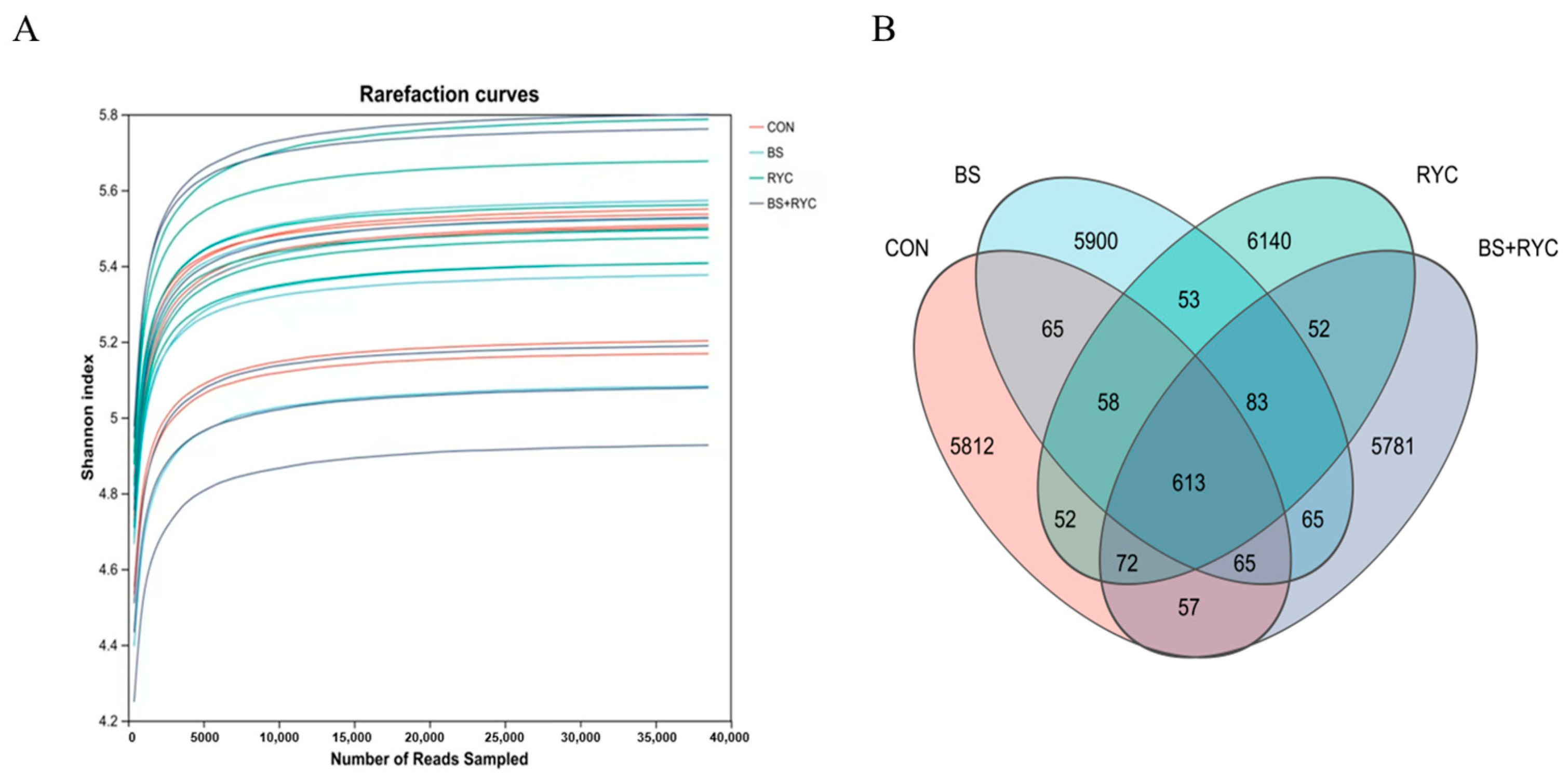
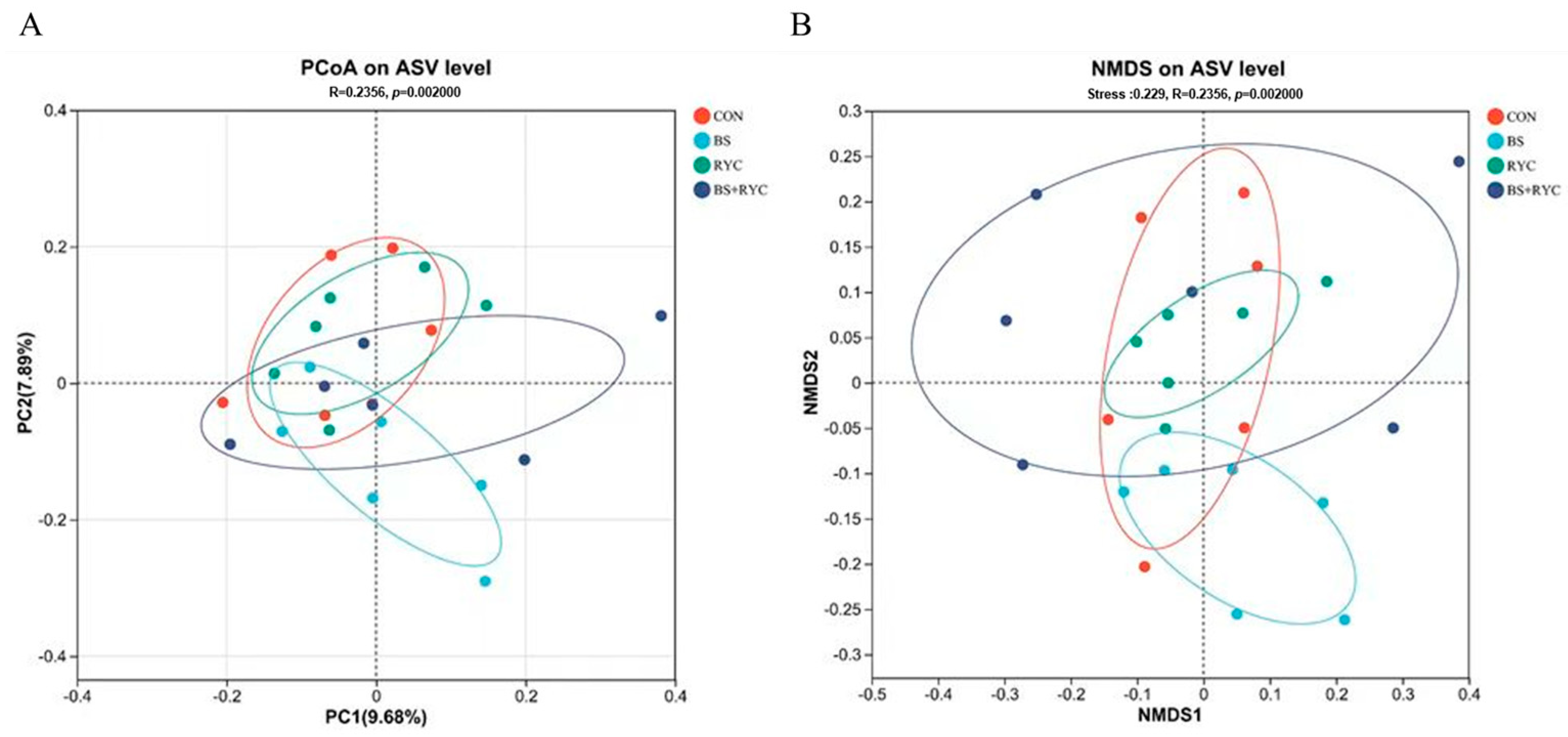
Samples were transported on dry ice to Shanghai Meiji Biomedicals Co. (Shanghai, China) to ensure sample integrity. Total DNA was extracted from the cecal contents of 56-day-old yellow-feathered broilers using the Fast DNA™ Spin Kit (MP Biomedicals, Santa Ana, CA, USA). The V3–V4 variable region of the bacterial 16S ribosomal RNA was amplified using the following primer sequences: forward primer 341F (5′-ACTCCTACGGGAGGCAGCAG-3′) and reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). After quantitative purification, the amplified sequences were sequenced on the Illumina Nextseq 2000 PE300 platform. Raw sequences were processed using QIIME software (version 1.9.1) and clustered into amplicon sequence variants (ASV) [
34]. All raw sequencing datasets have been deposited in the NCBI Sequence Read Archive under BioProject PRJNA1199219.
2.8. Statistical Analysis
All data were analyzed using two-way ANOVA to evaluate the main effects of RYC and BS, as well as their interaction effects, with the replicates serving as the experimental unit. Mean comparisons were performed using Duncan’s multiple range test. Statistical computations were conducted using SPSS version 21 (IBM Corp., Armonk, NY, USA) [
35]. For cecal microbial data, analyses were performed on the online Majorbio cloud platform (
www.majorbio.com, accessed on 10 December 2024). These analyses included alpha diversity analysis, beta diversity analysis, bacterial abundance analysis, and linear discriminant analysis effect size (LEfSe). The data analysis process involved three main steps: filtering raw data, applying the UPARSE algorithm for clustering, and annotating species. A
p < 0.05 was considered statistically significant, and a
p-value between 0.05 and 0.10 was considered indicative of a trend.
4. Discussion
Throughout the feeding trial in this study, broilers across all experimental groups were observed to exhibit comparable feed intake levels and similar daily weight gain trends. These observations suggest that the primary effects of RYC and BS supplementation are not driven by enhanced nutrient utilization for growth but rather by their regulatory roles in intestinal function. Specifically, RYC and BS were found to enhance intestinal barrier integrity and stimulate immune activity, which may incur physiological costs such as elevated immune resource allocation and increased intestinal mucus production. Consequently, these metabolic demands are likely to divert energy away from direct body protein deposition [
12]. The improvements in intestinal health observed in yellow-feathered broilers following RYC and BS primarily involved enhanced villus morphology, upregulation of the
MUC2 gene, and optimized cecal microbiota composition. These findings are consistent with and corroborated by previous studies [
22,
27,
36].
The structural integrity of intestinal tissue and intercellular junction complexes is fundamental to maintaining gut barrier homeostasis. In broilers, the development of the small intestinal mucosa directly influences digestion and absorption efficiency, thereby impacting overall health and performance [
37]. The VH/CD ratio, a key indicator of intestinal development and absorptive capacity [
38], was significantly increased in yellow-feathered broilers receiving combined RYC and BS supplementation. Findings from this research further verify the interactive effects of co-administration of RYC and BS. Notably, cosupplementation markedly elevated the jejunal VH/CD ratio, indicating synergism. Supporting our results, Xie et al. [
39] demonstrated that fermented feed containing BS and yeast significantly improved the VH/CD ratio in Xuefeng black-bone chickens. The underlying mechanism may involve bacterial proteins and small peptides derived from RYC, which facilitate BS colonization in the jejunum while simultaneously stimulating villus growth and crypt cell proliferation [
14,
31]. Notably, dietary supplementation with BS alone did not significantly enhance intestinal VH and CD in the present study, consistent with the findings of Aliakbarpour et al. [
40], who observed no significant improvement in jejunal villus architecture in 42-day-old broilers. This lack of effect may be attributed to age-dependent microbiota maturation, wherein established gut microbial communities competitively exclude exogenous BS colonization, thereby diminishing its promotive effects on intestinal villi [
41].
TJPs regulate the function of the intestinal epithelial barrier and ensure its integrity by controlling permeability [
42]. Interestingly, our results indicate that singular RYC administration downregulates ileal
OCLN mRNA expression, demonstrating antagonism when coadministered with BS. In contrast, Kyoung et al. [
14] found that the yeast cell wall components had no significant effect on ileal
OCLN gene expression in broilers. These divergent outcomes may be attributed to multiple factors, including distinct yeast strains employed, disparities in supplementation levels, and differences in the breed and age of the broilers utilized. We speculate that RYC likely targets anti-inflammatory mechanisms as a primary mode of action, reallocating cellular resources to strengthen gut anti-inflammatory competence rather than fortifying the intestinal barrier via increased TJP mRNA expression. The mechanism underlying this effect warrants further investigation. Studies by Bilal et al. [
43] revealed that dietary supplementation with
Bacillus pumilus and
Bacillus subtilis markedly elevated ileal
JAM2 and
TJP1 mRNA levels in broilers at 42 days of age. Consistent with prior findings, BS administration in this study yielded concordant
JAM2 and
TJP1 mRNA expression trends in ileal tissues.
MUC2, the primary structural component of intestinal mucus, plays a pivotal role in gut barrier defense by physically preventing pathogen adhesion and toxin penetration [
44]. Findings from this study reveal that both RYC and BS significantly upregulate ileal
MUC2 mRNA expression yet exhibit no synergistic effect. This enhancement likely involves BS promoting goblet cell proliferation [
40], while RYC provides the essential nutrients for epithelial cell metabolism and mucus production [
45]. By strengthening the intestinal epithelial barrier, these mechanisms collectively enhance the integrity of the mucosal microstructure.
Immunoglobulin levels serve as critical biomarkers for evaluating humoral immune competence in poultry. The present study demonstrated that RYC supplementation significantly increased IgM concentrations in ileum tissue, consistent with Wang et al. [
46], who report that yeast-derived products enhance jejunal immunoglobulins, and Kyoung et al. [
14], who found that yeast cell walls increase the number of goblet cells in the duodenum of broilers. The underlying mechanism may involve β-glucans in RYC activating TLR2-mediated signaling in intestinal epithelial and antigen-presenting cells, subsequently promoting B lymphocyte differentiation into antibody-producing cells [
47]. Findings from this study also reveal that dietary BS administration markedly elevated IgG levels in the ileum of yellow-feathered broilers, consistent with Dong et al.’s [
48] report on serum IgG concentration in broilers fed diets containing
Bacillus subtilis strain BYS2. Surprisingly, the combined supplementation of RYC and BS demonstrated an antagonistic effect on IgM in the ileal tissues of yellow-feathered broilers. We postulate that this observed antagonism may be attributable to organism-level dynamic immunoglobulin homeostasis. This phenomenon may reflect (i) the earlier emergence of IgM in primary immune responses, followed by (ii) IgG class switching during secondary responses [
49]. Such sequential activation patterns could optimize humoral immunity across different stages of pathogen challenge, with IgM providing rapid first-line defense while IgG mediates sustained protection.
Cytokines play a crucial role in cell-mediated immunity, and their expression patterns reflect immune regulation. IFN-γ is a key Th1 cytokine, and its downregulation in our study suggests that RYC may help maintain immune homeostasis by promoting T regulatory cell differentiation. This aligns with Zhou et al. [
50], who found that yeast cell wall polysaccharides suppress
IFNG and
IL1B upregulation in the ileum during
E. coli challenge. The β-glucans in RYC likely contributes to this effect by enhancing
IL10 secretion, which inhibits IFN-γ production—consistent with Wang et al. [
51], who observed similar immunomodulation with yeast hydrolysates in broilers. In addition, RYC contains yeast cell wall components (β-glucans and mannan oligosaccharides), which can reduce intestinal inflammation by adsorbing harmful bacteria [
15]. Meanwhile, BS supplementation significantly increased ileal IL-1β levels, indicating its immune-stimulating properties, possibly due to transient inflammatory responses triggered by microbial colonization [
18]. Khan et al. [
52] reported similar findings, with BS elevating
IL1B expression in the spleen and kidney. The combination of RYC and BS appears to establish a balanced regulatory model. While BS maintains baseline IL-1β (indicating immune activation), RYC counteracts excessive inflammation by scavenging reactive oxygen species via carotenoids [
45,
53].
Interestingly, while RYC alone reduced IL-1β protein levels, the BS + RYC group showed elevated
IL1B mRNA expression. This discrepancy may reflect post-transcriptional regulation or compensatory feedback mechanisms. RYC could suppress IL-1β protein synthesis via NF-κB inhibition, whereas BS colonization transiently stimulates IL1B transcription in immune cells, consistent with Rajput et al. [
54], who found that Saccharomyces boulardii and BS B10 upregulated IL1B in avian dendritic cells. Overall, BS appears to dominantly regulate IL1B mRNA levels in broilers, while RYC modulates downstream inflammatory responses. An interactive effect was observed for RYC and BS regarding IL-6 at the transcriptional level, while IL-6 cytokine levels in the ileum of yellow-feathered broilers remained unaffected. The underlying mechanisms merit future in-depth exploration.
The TLR4/NF-κB pathway is crucial for the regulation of the intestinal immune system [
55]. Research conducted by Rajput et al. [
56] demonstrates that
Saccharomyces boulardii and
Bacillus subtilis strain B10 modulate broiler gut immune responses through upregulation of TLR, MyD88, and NF-κB mRNA expression. This activation induces NF-κB nuclear translocation via the MyD88-dependent pathway, resulting in the upregulation of
NFKB1 mRNA expression in the ileum tissue, which subsequently leads to the upregulation of
TNFA and
IL1B mRNA expression [
52]. These results align with the previous findings reported by Yang et al. [
57]. In contrast, RYC treatment specifically upregulated
IL10 mRNA expression, suggesting its potential role in mitigating intestinal inflammation through enhanced anti-inflammatory responses and mucosal protection mechanisms [
23]. While BS activated the NF-κB signaling pathway and enhanced inflammatory factors IL-1β, the β-glucan component of RYC simultaneously enhanced IL-10 expression through T cell-mediated pathways. Studies conducted by Bai et al. [
58] demonstrate that yeast culture supplementation in the diet of pseudobagrus ussuriensis mitigates intestinal inflammation through regulation of the TLR2-MyD88-NF-κB signaling cascade. Together, these complementary actions establish a sophisticated immune balance regulatory network. This mechanism creates a balance between immune system activation and inflammation control, which helps prevent excessive immune damage while strengthening the intestinal mucosa’s defense [
59].
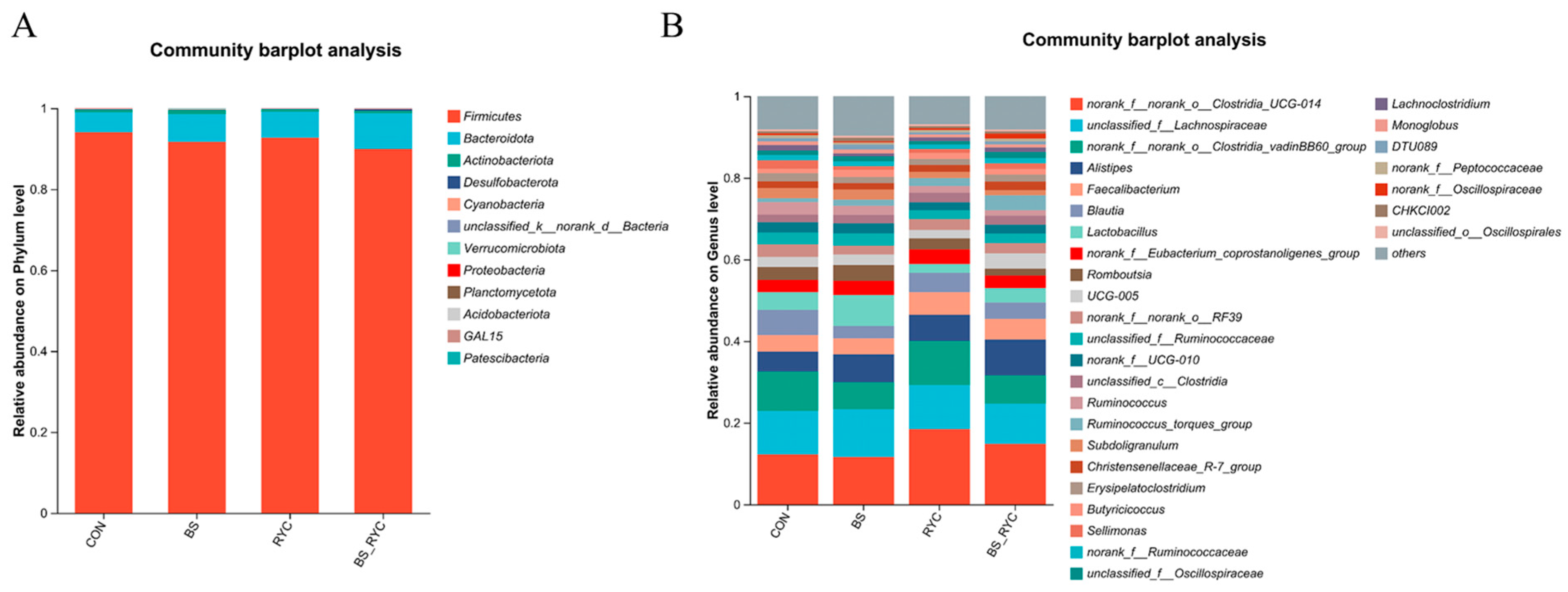
The intestinal microbiota plays a pivotal role in modulating local immune responses and overall immune function in poultry [
60]. In the present study,
Firmicutes emerged as the dominant phylum in the cecal microbiota of yellow-feathered broilers, aligning with findings from Guo et al. [
61]. Dietary supplementation with BS probiotics significantly increased the abundance of
Bacillus spp. in the intestinal microbiota. This observation is consistent with previous research by Liu et al. [
31], demonstrating that
Bacillus-based probiotics in poultry feed yield similar microbial shifts. BS spores exhibit remarkable resilience, surviving gastric acid and bile salt stress before proliferating extensively in the posterior intestinal tract under favorable conditions [
29].
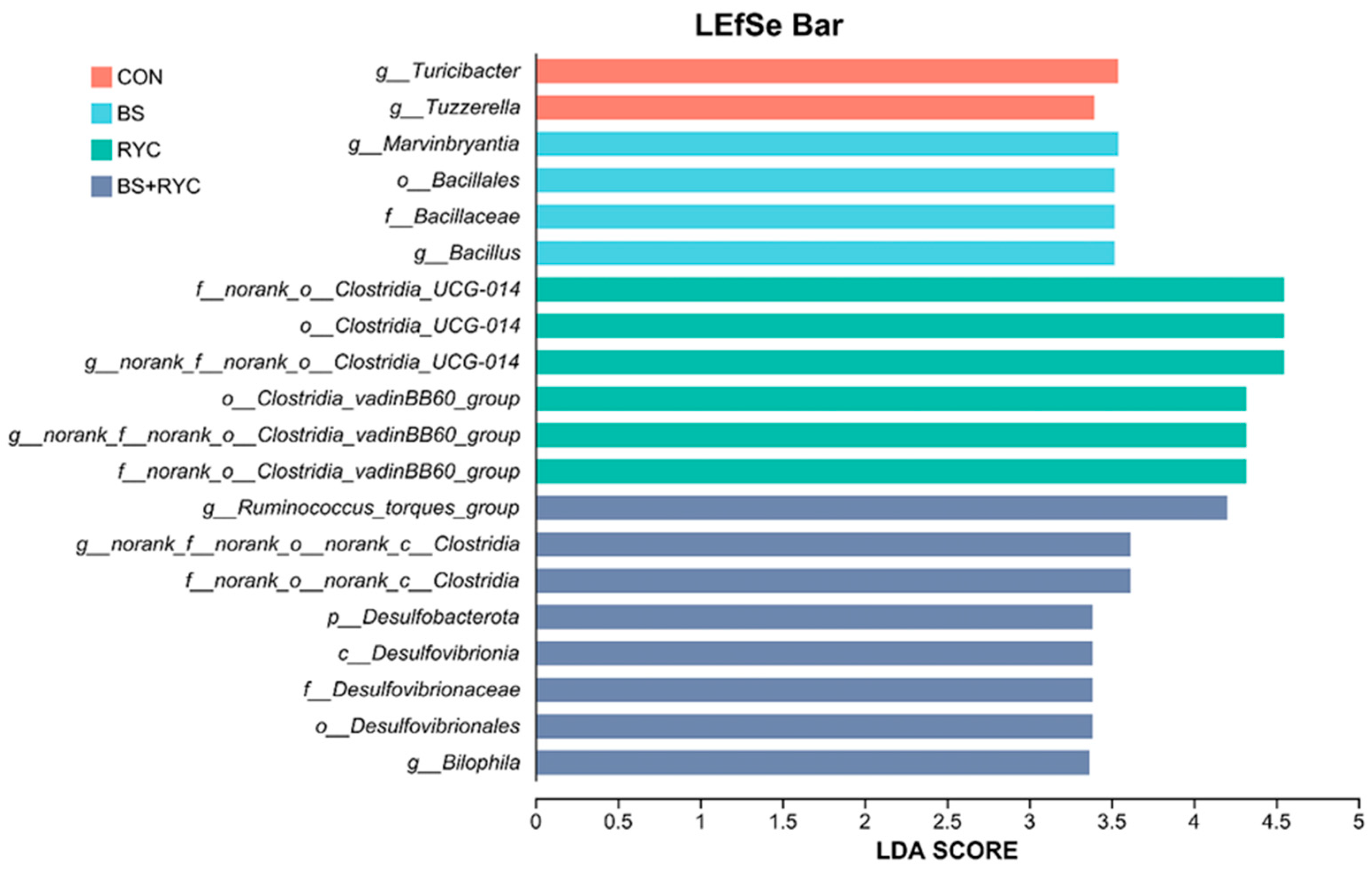
RYC supplementation influenced the cecal microbial community structure, particularly enriching anaerobic
Clostridia species such as
norank_f__norank_o__Clostridia_UCG-014 and
g_norank_f__norank_o__Clostridia_vadinBB60_group [
62]. The mannan oligosaccharides in RYC served as a selective carbon source for these
Clostridia, promoting their colonization and metabolic activity [
63]. These bacteria are critical for fiber degradation and butyrate synthesis, with elevated butyrate levels enhancing intestinal barrier integrity [
64,
65]. RYC jointly optimized the cecal bacterial community composition of yellow-feathered broilers by providing exclusive carbon sources, combining BS-suppressed pathogenic or competing bacteria, and the construction of an anaerobic microenvironment [
25,
66].
The BS-supplemented group exhibited significant enrichment of taxa classified under the
Bacillus order, consistent with findings by Liu et al. [
31], who reported that BS HC6 supplementation in white-feathered broilers enhanced cecal microbiota pathways linked to energy metabolism. In contrast, RYC supplementation prominently enriched the
Clostridia UCG-014 and
Clostridia vadinBB60 branches, both core members of the
Clostridia class. Notably, the presence of unclassified microbial groups suggests that RYC may stimulate the proliferation of metabolically active but poorly characterized microbiota [
36,
67]. The specific interaction effect of RYC and BS co-addition showed significant enrichment of
p_Desulfobacterota, g_Ruminococcus_torques_group, and
g_Bilophila. The different experimental groups formed a characteristic flora structure. The BS group developed a
Bacillus-dominated colony structure, while the BS + RYC group developed a
Desulfovibrio-dominated colony structure. The CON group demonstrated distinct functional differentiation characteristics of the
Firmicutes phylum, whereas the RYC group demonstrated those of the genus
Clostridia. These structural differences likely modulate intestinal barrier function through multiple metabolic pathways, including short-chain fatty acid production, bile acid transformation, and sulfur cycling [
68].