Simple Summary
This study investigated the molecular identification and antimicrobial resistance characteristics of Klebsiella pneumoniae producing extended-spectrum beta-lactamase (ESBL) in captive wild and migratory birds in northeastern Bangladesh. Out of 219 fecal samples, 42.47% tested positive for K. pneumoniae, with a higher prevalence observed in captive birds. The isolates showed significant resistance to ampicillin and streptomycin, while trimethoprim-sulfamethoxazole and levofloxacin were the most effective antibiotics. PCR detected key resistance genes, including blaTEM-1&2 (100%), blaSHV-1 (45.2%), and blaOXA-1,4&30, along with strA, tetA, and sul1. The multiple antibiotic resistance (MAR) index varied from 0.18 to 0.64, with 63.4% of isolates classified as multidrug-resistant (MDR). These findings suggest that wild and migratory birds may serve as reservoirs and disseminators of MDR and ESBL-producing K. pneumoniae, posing a potential threat to public and animal health. Continuous surveillance and a One Health approach are recommended.
Abstract
The emergence and dissemination of antibiotic-resistant Klebsiella pneumoniae, particularly those are extended-spectrum beta-lactamase (ESBL) producers, thought to pose a serious threat to global health. This study aimed to isolate and identify the ESBL-producing K. pneumoniae from captive wild and migratory birds in Bangladesh along with their antimicrobial resistance characteristics. In this investigation, standard bacteriological methods were used to detect K. pneumoniae in 219 fecal samples. The positive isolates were confirmed by PCR and antibiotic susceptibility testing was performed by disk diffusion method. K. pneumoniae was detected in 93 (42.47%, 95% CI: 35.8–49.3) out of 219 fecal samples. The prevalence of K. pneumoniae was higher in captive wild birds (50%; 40/80) compared to migratory birds (38.1%; 53/139). The isolates showed high resistance to ampicillin (69.9%) and streptomycin (64.5%). Conversely, the highest sensitivity was recorded for trimethoprim-sulfamethoxazole (84.95%), followed by levofloxacin (79.57%) and gentamicin (69.89%). Molecular screening revealed that all positive isolates harbored blaTEM-1&2 encoding genes, with 45.2% and 15.1% carried blaSHV-1 and blaOXA-1,4&30, respectively. Additionally, resistance genes strA (30.1%), tetA (9.7%), and sul1 (9.7%) were detected. The Multiple Antibiotic Resistance (MAR) index ranged from 0.18 to 0.64, with 63.4% of isolates classified as MDR. The isolation of MDR and ESBL producing K. pneumoniae from captive wild and migratory birds suggests that these birds may serve as reservoirs for the spread of these bacteria, potentially impacting public health in the study region.
1. Introduction
Wild and migratory birds represent a large proportion of the estimated 10,000 bird species globally [1]. Migratory and wild birds are a potential reservoir and are able to propagate zoonotic pathogens and resistant bacteria [2]. These birds frequently travel across countries and continents, facilitating the spread of infectious agents through fecal contamination in water bodies such as lakes, ponds, wetlands, haor etc. [3,4]. Through migration, the birds may acquire or disseminate antimicrobial resistant (AMR) and multidrug resistant (MDR) bacteria [4,5]. Notably, fecal shedding by birds can introduce pathogens into shared environments, which may subsequently infect humans, domestic animals, or wildlife [6]. This risk is particularly relevant in regions like Bangladesh, where seasonal migratory bird influxes are common and many communities rely on open water sources for daily activities [7,8].
Klebsiella pneumoniae (K. pneumoniae) belongs to the family Enterobacteriaceae. Enterobacteria are bacteria that live in the intestines of mammals and some birds, and they can spread throughout the environment and become ubiquitous if the right conditions exist [9]. Because they are found in intestine of wild and migratory birds, they have great significance in animal and human health. K. pneumoniae is a common Gram-negative bacterium recognized as a primary pathogen, often associated with respiratory diseases in birds [10]. It may be isolated from stool samples and the oropharynx of various species of healthy parrots and passerines [10,11,12]. It frequently acts as a respiratory pathogen, particularly among immunosuppressed and stressed birds [13]. The microbiota of caged birds is likely to change as a result of their management, favoring Gram-negative bacteria colonization [14]. In certain cases, K. pneumoniae can lead to encephalitis, lung infections, and renal failure. Localized infections of the skin, lips, upper respiratory tract, and crops, specifically in psittacine birds, are more prevalent [15].
K. pneumoniae is one of the most common producers of extended-spectrum β-lactamases (ESBLs), along with Escherichia coli. Several virulence factors, including a polysaccharide capsule, muco-viscous exopolysaccharides, lipopolysaccharides (LPS), adhesins, and iron acquisition systems have the capacity to acquire antimicrobial resistance determinants, making K. pneumoniae particularly dangerous [16]. ESBL-producing strains are particularly concerning due to their resistance to β-lactam antibiotics and carbapenems genes, leaving limited treatment options such as imipenem or meropenem [17,18]. The emergence of ESBL- and carbapenemase-producing K. pneumoniae is a critical priority pathogen for global health intervention due to its role in hospital-acquired infections and its limited therapeutic options [19,20,21].
Despite its clinical relevance, the role of wild and migratory birds as reservoirs of ESBL-producing K. pneumoniae in South Asia, particularly Bangladesh, remains poorly understood. Recent surveillance studies from other regions (e.g., China, Europe, and Egypt) have revealed varying prevalence rates of K. pneumoniae in avian populations, ranging from 1.5% to over 50% [4,18,22]. However, molecular data from avian hosts in Bangladesh are lacking. Therefore, the present study aimed to isolate and characterize K. pneumoniae from fecal samples of captive wild and migratory birds in northeastern Bangladesh. Specifically, the study focused on identifying the prevalence of ESBL-producing strains, profiling antimicrobial resistance patterns, and detecting key resistance genes using molecular techniques.
2. Materials and Methods
2.1. Study Area and Species Selection
From January 2022 to July 2022, a cross-sectional investigation was conducted in Haripur, the Hakaluki Haor of Moulvibazar, the Tanguar Haor, and parts of the Tahirpur upazila of Sunamganj District, based on the accessibility and density of birds (Figure 1). Feces from captive wild birds were obtained from Tilagor Eco-Park, Sylhet, and the Bangladesh Bannyaprani Sheba Foundation, Sreemangal, Bangladesh. Migratory bird species were prioritized due to their potential role in the long-distance dissemination of pathogenic bacteria. Captive wild birds were also included to provide a baseline for AMR prevalence in local avian populations. Species were selected based on prior studies indicating their exposure to anthropogenic influences in agricultural areas, wetlands, or rural feeders.
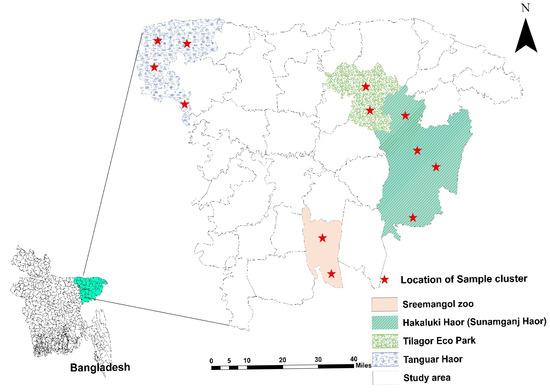
Figure 1.
Study area map represents the location of sample collection. The figure was generated using ArcMap 10.8.
2.2. Sample Collection
In all, 219 fecal samples were gathered from various sites in Sylhet region, Bangladesh. These locations were chosen because they are home to a variety of species of migrating and wild birds in captivity. A sterile swab stick was used to aseptically collect feces samples. The feces were then placed in sterile containers containing buffered peptone water (BPW) (Hi media, India) at 1:10 dilution, and were labeled accordingly. For consistency, all samples were processed within 24 h to ensure data reliability.
2.3. Isolation and Identification of K. pneumoniae
The samples from BPW were cultured on MacConkey Agar (MCA; Oxoid, Southampton, UK) and then on Eosin Methylene Blue Agar (EMBA; Oxoid, UK), and by streaking and incubation at 37 °C for 18–24 h. Culture positive samples were sub-cultured several times to obtain a pure culture. The growth of large, mucoid, pink, or pink to purple colonies on EMB and MacConkey agar plates demonstrated the growth of K. pneumoniae. Gram’s staining method and various biochemical tests were performed to screen the single pure colonies for additional confirmation. These tests included catalase, sugar fermentation, methyl red, Voges–Proskauer, and citrate utilization (Supplementary Materials). Pure cultures were stored in two separate Eppendorf tubes and preserved in Brain Heart Infusion broth (BHIB; Oxoid, UK) with 15% glycerol supplement, and stored at −40 °C until further use.
2.4. Genomic DNA Extraction
The DNA extraction was carried out according to the manufacturer’s instructions (AddBio Inc. Ltd., Dajeon, Republic of Korea). Briefly, 200 μL of cultivated cells were extracted overnight and centrifuged at 13,000× g rpm for 30 s. The supernatant was disposed of and 200 μL of lysis solution was pipetted in and suspended. After adding 20 mg/mL of proteinase K solution, the mixture was incubated at 56 °C to ensure full lysis. The mixture was thoroughly homogenized and incubated at 56 °C for 10 min following centrifugation and the addition of binding solution. Following the addition of 100% ethanol, the lysate was cautiously moved to a spin column tube, centrifuged, and then cleaned out using washing solutions. The spin column was again centrifuged at 13,000× g rpm for 1 min in order to remove any last traces of ethanol. Lastly, 30 μL of elution buffer was added to the spin column and was allowed to sit for 1 min at room temperature. The genomic DNA was eluted by centrifugation and kept at −20 °C for further examination. The quantity and purity of extracted DNA were verified using a NanoDrop spectrophotometer.
2.5. Molecular Detection of K. pneumoniae by PCR
Species-specific primers were used for the amplification of the rpoB gene of K. pneumoniae (F-CAACGGTGTGGTTACTGACG and R-TCTACGAAGTGGCCGTTTTC). Each PCR reaction contained 25 µL reaction volume with 12.5 µL of 2× master mix, 2 µL of primer mix, and 5 µL of a DNA sample. The 25 µL volume was adjusted by adding 5.5 µL of nuclease-free water appropriate for high-performance liquid chromatography. PCR was performed using a thermal cycler (DLAB Scientific Inc., Alhambra, CA, USA) with the following cycling conditions: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min, with a final extension at 72 °C for 10 min. After that, the PCR product was visualized by electrophoresis, revealing a unique band of 108 bp for K. pneumoniae (Figure S1A). A 100 bp ladder (AddBio Inc., Dajeon, Republic of Korea) was included to assist with PCR product size estimation.
2.6. Double-Disk Synergy Test (DDST) for ESBL Detection
The phenotypic detection of ESBL production in K. pneumoniae isolates was performed using the Double-Disk Synergy Test (DDST) [23]. A bacterial suspension equivalent to a 0.5 McFarland standard was prepared from each isolate and evenly inoculated onto Mueller–Hinton agar plates using a sterile cotton swab. An amoxicillin-clavulanic acid disk (30 µg) was placed at the center of the plate, and cefotaxime (30 µg) and ceftriaxone (30 µg) disks were positioned 20 mm apart (center-to-center) on either side of the central disk. Plates were incubated at 37 °C for 24 h.
Following incubation, plates were examined for the presence of a characteristic “keyhole” or synergy zone between the cephalosporin disks and the amoxicillin-clavulanate disk. The appearance of this zone indicated the inhibition of ESBL activity by clavulanate, confirming ESBL production.
2.7. Antimicrobial Susceptibility Testing
The agar diffusion method developed by Kirby–Bauer was employed to assess antibiotic sensitivity. The antibiotics that have been utilized come in disk form. Three categories (sensitive, intermediate, and resistant) were established from the clear zone that developed during this test. Using a tube, K. pneumoniae isolates were extracted from one or two pure colonies and mixed in physiological NaCl. The turbidity of the solution was assessed using the McFarland 0.5 standard. A volume of 0.2 mL was then obtained and gently rubbed across the Mueller–Hinton agar (MHA: Oxoid, UK) medium. Eleven antibiotics (Oxoid, UK) were used for sensitivity testing (Table S1). Findings were ascertained after 18–24 h incubation at 37 °C. Using a manual millimeter scale, after measuring the diameter of the zone of inhibition encircling the discs, the findings were contrasted with the CLSI breakpoints [24].
2.8. Identification of ß-lactamase Encoding Genes
Reference primers were used to detect ß-lactamase genes (Table 1). All Klebsiella pneumoniae isolates underwent PCR screening to identify the ß-lactamase genes, including blaTEM-1&2, blaSHV-1, and blaOXA-1,4 &30 (Figure S1B–E). Thermal cycle conditions are described in the Supplementary Materials.

Table 1.
Primer sequence and amplicon size used in this study.
2.9. Detection of AMR Genes
The targeted AMR genes tetA and strA were amplified using multiplex PCR using two sets of particular primer pairs (Table 1). Every PCR reaction had a volume of 25 μL and included 5 μL of DNA template from PCR positive isolates, 12.5 μL of 2× master mix (Add Bio Inc., Daejeon, Republic of Korea), 0.5 μL of each primer (10 pmol/L concentration) for forward and reverse, and 5.5 μL of nuclease-free water. The sul1 genes were found using a different uniplex PCR test. To perform the PCR assay, a combination of 25 μL reaction volume was used, which included 5 μL of DNA template obtained from isolates of K. pneumoniae, 12.5 μL of 2× master mix (Add Bio Inc., Dajeon, Republic of Korea), 1 μL each of the forward and reverse primers (10 pmol/L concentration), and 5.5 μL of nuclease-free water. Thermal cycle conditions are described in the Supplementary Materials.
2.10. MAR Index and MDR
Using the formula MAR = (the number of antibiotics to which an isolate was resistant)/(the total number of antibiotics tested), the MAR index was computed in accordance with the parameters as previously established [28]. When an isolate showed resistance to at least three different classes of antibiotics, it was classified as multidrug resistant (MDR). The MAR index ranged from 0 to 1, where values close to 1 indicated strong resistance and values around zero indicated high sensitivity. A significant level of resistance or high-risk bacterial contamination was indicated by an index of 0.20 or above.
2.11. Statistical Analysis
The information was assimilated, categorized, and structured into Excel spreadsheets. The prevalence for different diseases was calculated using following formula [29,30]:
Chi-square (χ2) analysis was done to evaluate the relationships between the different explanatory factors. Fisher’s Exact Test was used when the predicted count in a cell was less than five and it happened in at least 20% of the cells [31]. Spearman correlation coefficient was performed using package R (RStudio 4.3.3). The data analysis was conducted with SPSS version 26. The threshold of significance was defined as p < 0.05. The study area map was created using ArcMap 10.8 and the other plot was created using GraphPad Prism 8.4.
3. Results
3.1. Prevalence and Distribution of K. pneumoniae
The overall prevalence of K. pneumoniae isolated from both migratory and captive wild birds was 42.5% (93/219; 95% CI: 35.8–49.3) (Table 2). The number of positive isolates varied significantly (p < 0.05) across different geographical locations in Sylhet Division. The highest prevalence of K. pneumoniae was recorded in Haripur (63.2%; 12/19). At Tilagor Eco-Park, only 25% (9/36) of the fecal samples from captive wild birds were positive for K. pneumoniae. Similar prevalence rates were observed in the Sunamganj Haor (43.6%) and the Sreemangal Zoo (44.3%) (Table 2). The frequency rate of K. pneumoniae isolated from captive wild birds was also observed (Table 3). Notably, the prevalence of K. pneumoniae was higher in fecal samples from captive wild birds (50%; 40/80) compared to migratory birds (38.1%; 53/139) (Figure 2).

Table 2.
Prevalence of Klebsiella pneumoniae isolated from captive wild and migratory birds among the different locations in Sylhet Division.

Table 3.
Frequency rate of K. pneumoniae isolated from captive wild birds.
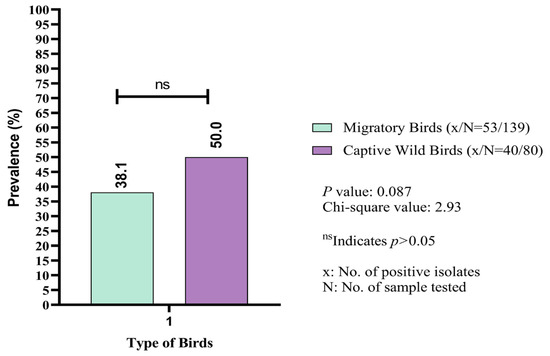
Figure 2.
Comparative assessment of prevalence (%) of Klebsiella pneumoniae between migratory and captive wild birds.
3.2. Antibiogram Profile
Most antimicrobials showed a minimum zone of inhibition (>10 mm) against the isolated K. pneumoniae, except for streptomycin. The overall zone of inhibition ranged between 15–25 mm for most positive isolates (Figure 3A). Streptomycin exhibited no inhibitory activity against 16 isolates. Among the selected antibiotics, the highest resistance was observed with ampicillin (69.89%), followed by streptomycin (64.5%). Conversely, the highest sensitivity was recorded for trimethoprim-sulfamethoxazole (84.95%), followed by levofloxacin (79.57%) and gentamicin (69.89%). Almost all the selected antimicrobials demonstrated sensitivity to some extent against the isolated pathogens (Figure 3B and Table S2).
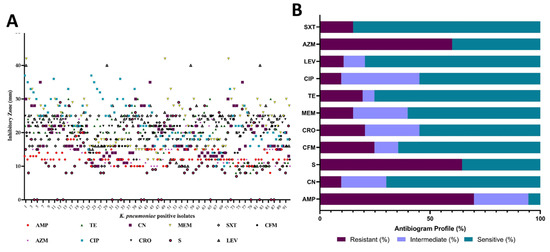
Figure 3.
Zone of inhibition (mm) of 11 antimicrobials against positive isolates of K. pneumoniae (A). AMP: ampicillin, TE: tetracycline, CN: gentamicin, MEM: meropenem, SXT: trimethoprim-sulfamethoxazole, CFM: cefixime, AZM: azithromycin, CIP: ciprofloxacin, CRO: ceftriaxone, S: streptomycin, LEV: levofloxacin. The antibiogram profile showing the percent (%) of sensitive, intermediate, and resistance of the tested antimicrobials (B).
3.3. MAR and MDR
The MAR index for multiple antibiotic resistance values varied from 0.18 (resistant to at least two antibiotics) to 0.64, with an average of 0.33. One isolate was resistant to eight out of 11 antimicrobials, and four isolates were resistant to seven antimicrobial agents, with an average MAR index of 0.54. Overall, 59 isolates (63.4%) out of 93 were resistant to more than three classes of antimicrobials (MDR).
3.4. ß-lactamase Encoding and Antimicrobial Resistance Gene Frequency
All K. pneumoniae positive isolates harbored blaTEM-1&2 broad spectrum ß-lactamase encoding gene, while only 14 isolates (15.1%) carried blaOXA-1,4&30. Additionally, blaSHV-1 was detected in 42 (45.2%) of the 93 positive samples (Figure 4A). This study screened for three common antimicrobial resistance genes in captive wild and migratory birds, finding that 28 (30.1%) isolates were positive for strA and nine (9.7%) isolates were positive for tetA, and sul1 (Figure 4B).
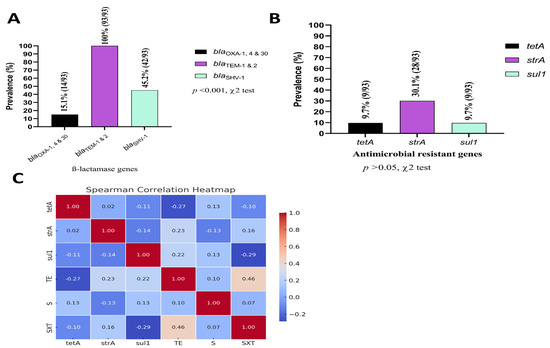
Figure 4.
Frequency (%) of ß-lactamase encoding genes (A); Frequency (%) of selected antimicrobial resistant genes (B); Spearman correlation coefficient (phenotype–genotype correlation) among the most common antibiotics used against K. pneumoniae in this region with their respective genes (C). TE: tetracycline, SXT: trimethoprim-sulfamethoxazole, S: streptomycin.
3.5. Phenotype–Genotype Correlations
The Spearman correlation analysis identified several key findings regarding the relationships between genotypes and antibiotic susceptibility test (AST) phenotypes (Figure 4C). The tetracycline resistant gene (tetA) exhibited a moderate negative correlation with tetracycline (TE) (rs = −0.27), while showing a weak positive correlation with streptomycin (S) (rs = 0.13). strA demonstrated a moderate positive correlation with TE (rs = 0.23), indicating a potential link between the gene and TE resistance, along with a weak positive correlation with trimethoprim-sulfamethoxazole (SXT) (rs = 0.16). Similarly, sul1 presented a weak positive correlation with TE (rs = 0.22) and a negative correlation with SXT (rs = −0.29). Overall, the analysis revealed mostly weak correlations between the genotypes and the AST phenotypes, with a few moderate associations observed for specific gene–antibiotic combinations.
3.6. Association Between Resistance Genes and Phenotypic Antibiotic Resistance
The association between genotypic resistance markers and corresponding phenotypic antibiotic resistance was evaluated using binary logistic regression analysis (Table 4). The analysis showed that the presence of the tetA gene was significantly associated with tetracycline resistance, with isolates harboring this gene being 5.75 times more likely to exhibit resistance (p = 0.016). Similarly, a strong association was observed between the sul1 gene and resistance to trimethoprim-sulfamethoxazole, with 6.25 times higher odds of resistance in gene-positive isolates (p = 0.012). However, no statistically significant association was found between the strA gene and streptomycin resistance (OR = 2.04, p = 0.203), suggesting that other resistance mechanisms may be involved.

Table 4.
Binary logistic regression analysis showing the association between resistance genes and phenotypic antibiotic resistance.
4. Discussion
Recent studies have reported a significant increase in Klebsiella pneumoniae infections, particularly strains exhibiting multidrug resistance (MDR), including resistance to extended-spectrum β-lactamases (ESBLs), carbapenems, and fluoroquinolones. These resistance patterns complicate treatment regimens, often resulting in prolonged hospital stays and increased morbidity [17,18,32]. The global spread of ESBL- and carbapenemase-producing K. pneumoniae has become a pressing public health concern, driven by plasmid-mediated gene transfer and antibiotic misuse in both human and veterinary medicine [33].
In this study, 59 K. pneumoniae isolates were identified as MDR, with a notably high resistance to ampicillin (69.9%). This finding indicates a significant prevalence of MDR strains in captive wild and migratory birds [20,34]. Due to ampicillin resistance, up to 90% of K. pneumoniae cases in hospitals in northern India occur in patients in the intensive care unit [35]. The presence of MDR K. pneumoniae in both wild and captive birds, as well as in poultry environments, underscores the importance of a One Health approach to address antibiotic resistance. This approach considers the interconnectedness of human, animal, and environmental health [18,36].
The prevalence of K. pneumoniae in migratory and wild birds varies significantly across different regions, reflecting diverse ecological and environmental factors. In this investigation, 42.47% (93/219) feces samples from captive wild and migratory birds were discovered to have K. pneumoniae. This prevalence is lower than the 55.47% reported in China and 53% in Kiel, Germany, but notably higher than the 1.5% in Egypt and comparable to the 20% in Catalonia, Spain [18,22,37,38].
In this investigation, sixty K. pneumoniae isolates showed 64.5% (60/93) resistance to aminoglycoside, specifically streptomycin. This resistance may be related to the efficacy of streptomycin treatment for humans and animals in Bangladesh. Combining antibiotics such as streptomycin and penicillin could be a beneficial approach to treat animal infections; however, streptomycin use should be closely monitored [39]. Loss of the KpnO porin and changes in the AcrAb-ToIC and KpnEF efflux pump systems can result in altered cellular permeability, which may lead to K. pneumoniae resistance to aminoglycoside drugs [35].
Overuse and incorrect use of antibiotics may lead to antibiotic resistance. Because of the ability of Klebsiella to produce the ESBL enzyme, it becomes resistant to antibiotics. Bacterial plasmids may be the source of resistance genes. The gene that produces the ESBL enzyme is based on a plasmid called beta-lactamase, which transforms into a point mutation that modifies the structure of the active region of the original gene [40]. Beta-lactamase enzymes have the ability to shield Gram-negative bacteria from beta-lactamase antibiotics. The cell wall is the target of the beta-lactam antibiotic. Similar to cell walls, this class of antibiotics also contains a beta-lactam group that interacts with enzymes during the production of cell walls. The cell wall will not fully develop since the enzyme will no longer be active. Bacterial cells without cell walls and those with incomplete cell walls result in death [41]. Resistant bacteria can be transferred by pets, wild animals, and food-producing animals [42]. The feces of animals can spread resistant bacteria to nearby people who may have been exposed to the bacteria through the air during animal transportation, as well as to workers in farms and slaughterhouses. These workers may also be infected with resistance bacteria that possess the ability to proliferate the bacteria to the environment [43]. Humans, cattle, wild animals, and non-clinical isolates of K. pneumoniae have all been found to produce ESBLs [41,44].
In this investigation, blaTEM encoding genes 93/93 (100%) were present in all K. pneumoniae positive isolates, whereas blaSHV was found in 42 (45.2%) of the 93 positive samples. These results align with those of another study in chickens that found a 100% prevalence of the blaTEM gene, reinforcing the high prevalence of this gene in avian sources [45]. In a study involving K. pneumoniae isolates from different sources, 84.6% of the isolates were found to carry the blaTEM gene, and 73% carried the blaSHV gene [46]. This high prevalence of blaTEM is consistent with the prevalence reported in this investigation. In addition, the findings are consistent with studies on Salmonella spp. from migratory birds, which carried the 94.34% blaTEM gene, while 13.33% carried blaSHV, indicating a similar trend of blaTEM dominance [47]. While blaSHV was less common than blaTEM, its presence in a significant portion of isolates indicates that it is also a relevant contributor to beta-lactam resistance in these samples. The existence of the ESBL-coding gene suggests that human resistance genes have migrated to animals, especially the multidrug-resistant K. pneumoniae, which is dangerous for livestock and public health [48].
The MAR index, which ranged from 0.18 to 0.64 with an average of 0.33, indicates a significant level of resistance, similar to patterns observed in other studies involving K. pneumoniae and other bacteria. This resistance is particularly concerning in environments where antibiotic use is prevalent, such as poultry farms, which can serve as reservoirs for resistant strains that may affect both animals and humans [37]. The presence of MDR isolates, which were resistant to more than three classes of antimicrobials, was observed in 63.4% of the isolates of captive wild and migratory birds, aligning with similar resistance patterns found in various animal and environmental studies [46,47].
Furthermore, this study highlights the prevalence of AMR genes in captive wild and migratory birds, specifically noting the presence of strA, tetA and sul1 genes. The study found that 30.1% of isolates were positive for the strA gene, which confers resistance to streptomycin. This is consistent with findings from other studies isolated from wild birds in Korea [49]. The tetA gene, associated with tetracycline resistance, was found in 9.7% of isolates. This aligns with research that reported a significant presence of tetracycline resistance genes in wild birds in Italy [50]. Other studies have reported similar findings, with sul1 being prevalent in wild bird populations [47,49]. Wild birds are increasingly recognized as reservoirs and vectors for AMR genes. They can acquire resistant bacteria from contaminated environments and spread them over long distances during migration [51]. The presence of AMR genes in wild birds poses a risk to public health, as these genes can be transferred to human pathogens. This is particularly concerning in areas where human and wildlife habitats overlap [18].
While this study’s findings are interesting, it has several limitations. Environmental samples (e.g., water, soil) were not included, which limits our ability to distinguish between true colonization in birds and potential environmental contamination. The study was also restricted to a specific geographic region and a six months sampling period, which may not capture broader temporal or spatial variations. Additionally, molecular characterization was limited to a few resistance genes, potentially overlooking other important resistance determinants or plasmid types contributing to the observed multidrug resistance.
5. Conclusions
This study confirms the presence of K. pneumoniae in 42.47% of fecal samples from captive wild and migratory birds in northeastern Bangladesh, with a substantial proportion (63.4%) of isolates exhibiting multidrug resistance. Notably, ß-lactamase encoding genes such as blaTEM-1&2, blaOXA-1,4&30, and blaSHV-1 were detected, indicating a high potential for environmental dissemination of resistance. These findings underscore the role of wild and migratory birds as reservoirs and potential vectors for MDR and ESBL-producing K. pneumoniae, posing a threat to public and animal health. Continuous surveillance and prudent antibiotic stewardship under the One Health approach are crucial to mitigate this emerging risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci12060556/s1; Figure S1. (A–E): Electrophoresis on 1.5% agarose gel showing specific amplified band of different gene of Klebsiella pneumoniae. amplification by PCR; Lane M: 100 bp Marker DNA; Lane NC: Control (– v e); A: Lane (1-10) reaction specific (+ ve) for Klebsiella pneumoniae (108 bp); B: Lane (1-10) samples for ESBL genes (blaTEM-1 & 2 at 800 bp, blaSHV-1 at 713, blaOXA-1,4 & 30 at 564); C: Lane 1-7 samples for strA gene (893 bp); D: Lane 1-5 for tetA gene (502 bp); E: Lane (1-5) samples for sul1 gene (433 bp); Table S1: List of Antibiotic disks for antimicrobial susceptibility testing; Table S2: Antibiogram profile of K. pneumoniae.
Author Contributions
M.M.I.: Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing. M.B.U.: Formal analysis, Methodology, Validation, Writing—original draft, Writing—critical proofs, manuscript revisions and editing. H.H.: Data curation, Formal analysis, Software, Writing—original draft, Writing—review & editing. M.R.: Data curation, Formal analysis, Writing—original draft, Writing—review & editing. R.B.: Conceptualization, Data curation, Formal analysis, Software, Writing—original draft, Writing—review & editing. P.K.G.: Data curation, Formal analysis, Writing—original draft, Writing—review & editing. M.M.R.: Formal analysis, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing. H.-S.C.: Formal analysis, Methodology, Writing—original draft, Writing—review & editing. M.M.H.: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft, Writing—critical proofs, manuscript revisions and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Technology (Project ID: SRG-231300), People’s Republic of Bangladesh.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of Sylhet Agricultural University, Sylhet, Bangladesh (protocol #AUP2023039) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated and analyzed in this study are included in the main manuscript.
Acknowledgments
The authors like to thank Sylhet Agricultural University Research System (SAURES) authority for their support to smoothly run this research. We also thank to the authorities of Bangladesh Bannyaprani Sheba Foundation (Zoo), Sreemangal and the Tilagor Eco-Park in Sylhet.
Conflicts of Interest
The authors declare no conflict of interest existed while the research was conducted.
References
- Orubu, E.S.F.; Zaman, M.H.; Rahman, T.; Wirtz, V.J. Veterinary antimicrobial resistance containment in Bangladesh: Evaluating the national action plan and scoping the evidence on implementation. J. Glob. Antimicrob. Resist. 2020, 21, 105–115. [Google Scholar] [CrossRef]
- Elsohaby, I.; Samy, A.; Elmoslemany, A.; Alorabi, M.; Alkafafy, M.; Aldoweriej, A.; Al-Marri, T.; Elbehiry, A.; Fayez, M. Migratory Wild Birds as a Potential Disseminator of Antimicrobial-Resistant Bacteria around Al-Asfar Lake, Eastern Saudi Arabia. Antibiotics 2021, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, X.; Chen, S.; Liu, Y.; Peng, D.; Wang, Z.; Li, R. Molecular epidemiology and population genomics of tet(X4), blaNDM or mcr-1 positive Escherichia coli from migratory birds in southeast coast of China. Ecotoxicol. Environ. Saf. 2022, 244, 114032. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.H.; Lu, X.; Woksepp, H.; Sattar, A.; Humak, F.; Ali, J.; Li, R.; Bonnedahl, J.; Mohsin, M. Detection and genomic characterization of Klebsiella pneumoniae and Escherichia coli harboring tet(X4) in black kites (Milvus migrans) in Pakistan. Sci. Rep. 2024, 14, 9054. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lv, C.; Chen, J.; Sun, Y.; Tang, T.; Zhang, Y.; Yang, Y.; Wang, G.; Xu, Q.; Zhang, X.; et al. The global distribution and diversity of wild-bird-associated pathogens: An integrated data analysis and modeling study. Med. 2025, 6, 100553. [Google Scholar] [CrossRef]
- Shah, A.; Alam, S.; Kabir, M.; Fazal, S.; Khurshid, A.; Iqbal, A.; Khan, M.M.; Khan, W.; Qayyum, A.; Hussain, M.; et al. Migratory birds as the vehicle of transmission of multi drug resistant extended spectrum β lactamase producing Escherichia fergusonii, an emerging zoonotic pathogen. Saudi J. Biol. Sci. 2022, 29, 3167–3176. [Google Scholar] [CrossRef]
- Islam, A.; Amin, E.; Munro, S.; Hossain, M.E.; Islam, S.; Hassan, M.M.; Al Mamun, A.; Samad, M.A.; Shirin, T.; Rahman, M.Z.; et al. Potential risk zones and climatic factors influencing the occurrence and persistence of avian influenza viruses in the environment of live bird markets in Bangladesh. One Health 2013, 17, 100644. [Google Scholar] [CrossRef]
- Shoaib, M.; Tang, M.; Aqib, A.I.; Zhang, X.; Wu, Z.; Wen, Y.; Hou, X.; Xu, J.; Hao, R.; Wang, S.; et al. Dairy farm waste: A potential reservoir of diverse antibiotic resistance and virulence genes in aminoglycoside- and beta-lactam-resistant Escherichia coli in Gansu Province, China. Environ. Res. 2024, 263, 120190. [Google Scholar] [CrossRef]
- Kathi, S. Enterobacter spp. Virulence Factors and Biofilm Components: Synthesis, Structure, Function, and Inhibitors. In ESKAPE Pathogens; Springer: Singapore, 2024; pp. 349–365. [Google Scholar] [CrossRef]
- Yehia, N.; Salem, H.M.; Mahmmod, Y.; Said, D.; Samir, M.; Mawgod, S.A.; Sorour, H.K.; AbdelRahman, M.A.; Selim, S.; Saad, A.M.; et al. Common viral and bacterial avian respiratory infections: An updated review. Poult. Sci. 2023, 102, 102553. [Google Scholar] [CrossRef]
- Knobl, P.; Viveiros, J.F.; Franco, L.S.; Davies, Y.M.; Cunha, M.P.V.; Menão, M.C.; Sato, M.I.Z.; de Moura Gomes, V.T.; Moreno, A.M.; Hidasi, H.W.; et al. Identificação de klebsiella spp. nas fezes de psitacídeos cativos. Atas Saúde Ambient. ASA 2017, 5, 189–193. [Google Scholar]
- Lepuschitz, S.; Hauser, K.; Schriebl, A.; Schlagenhaufen, C.; Stöger, A.; Chakeri, A.; Vötsch, K.; Pekard-Amenitsch, S.; Springer, B.; Allerberger, F.; et al. Fecal Klebsiella pneumoniae Carriage Is Intermittent and of High Clonal Diversity. Front. Microbiol. 2020, 11, 581081. [Google Scholar] [CrossRef] [PubMed]
- El Fertas-Aissani, R.; Messai, Y.; Alouache, S.; Bakour, R. Virulence Profiles and Antibiotic Susceptibility Patterns of Klebsiella Pneumoniae Strains Isolated from Different Clinical Specimens. Pathologie Biologie 2013, 61, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Jun, S.-R.; Kwon, Y.M.; Kiess, A.S.; Adhikari, P. Effects of Housing Types on Cecal Microbiota of Two Different Strains of Laying Hens During the Late Production Phase. Front. Vet. Sci. 2020, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Davies, Y.M.; Cunha, M.P.V.; Dropa, M.; Lincopan, N.; Gomes, V.T.M.; Moreno, L.Z.; Sato, M.I.Z.; Moreno, A.M.; Knöbl, T. Pandemic Clones of CTX-M-15 Producing Klebsiella pneumoniae ST15, ST147, and ST307 in Companion Parrots. Microorganisms 2022, 10, 1412. [Google Scholar] [CrossRef]
- Broberg, C.A.; Palacios, M.; Miller, V.L. Klebsiella: A long way to go towards understanding this enigmatic jetsetter. F1000Prime Rep. 2014, 6, 64. [Google Scholar] [CrossRef]
- Bobbadi, S.; Chinnam, B.K.; Nelapati, S.; Tumati, S.R.; Kandhan, S.; Gottapu, C.; Boddu, S.V. Occurrence and genetic diversity of ESBL producing Klebsiella species isolated from livestock and livestock products. J. Food Saf. 2020, 40, e12738. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Ji, F.; Wang, M.; Wu, B.; Qin, J.; Dong, G.; Zhao, R.; Wang, C. Genomic Characteristics and Molecular Epidemiology of Multidrug-Resistant Klebsiella pneumoniae Strains Carried by Wild Birds. Microbiol. Spectr. 2023, 11, e02691-22. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef]
- McDougall, F.K.; Wyres, K.L.; Judd, L.M.; Boardman, W.S.; Holt, K.E.; Power, M.L. Novel strains of Klebsiella africana and Klebsiella pneumoniae in fruit bats (Pteropus poliocephalus). Res. Microbiol. 2021, 172, 103879. [Google Scholar] [CrossRef]
- Martin, M.J.; Stribling, W.; Ong, A.C.; Maybank, R.; Kwak, Y.I.; Rosado-Mendez, J.A.; Preston, L.N.; Lane, K.F.; Julius, M.; Jones, A.R.; et al. A panel of diverse Klebsiella pneumoniae clinical isolates for research and development. bioRxiv 2022, 9, 000967. [Google Scholar] [CrossRef]
- Raza, S.; Mohsin, M.; Madni, W.A.; Sarwar, F.; Saqib, M.; Aslam, B. First Report of bla CTX-M-15 -Type ESBL-Producing Klebsiella pneumoniae in Wild Migratory Birds in Pakistan. EcoHealth 2017, 14, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Liza, N.A.; Hossain, H.; Rahman Chowdhury, M.S.; Al Naser, J.; Lasker, R.M.; Rahman, A.; Haque, M.A.; Al Mamun, M.; Hossain, M.M.; Rahman, M.M. Molecular epidemiology and antimicrobial resistance of Extended-Spectrum β-Lactamase (ESBL)-producing Klebsiella pneumoniae in retail cattle meat. Vet. Med. Int. 2024, 2024, 3952504. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020; CLSI Supplement M100. [Google Scholar]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Siddiky, N.A.; Sarker, M.S.; Khan, M.S.R.; Begum, R.; Kabir, M.E.; Karim, M.R.; Rahman, M.T.; Mahmud, A.; Samad, M.A. Virulence and Antimicrobial Resistance Profiles of Salmonella Enterica Serovars Isolated from Chicken at Wet Markets in Dhaka, Bangladesh. Microorganisms 2021, 9, 952. [Google Scholar] [CrossRef]
- Lanz, R.; Kuhnert, P.; Boerlin, P. Antimicrobial Resistance and Resistance Gene Determinants in Clinical Escherichia Coli from Different Animal Species in Switzerland. Vet Microbiol 2003, 91, 73–84. [Google Scholar] [CrossRef]
- Al Naser, J.; Hossain, H.; Chowdhury, S.R.; Liza, N.A.; Lasker, R.M.; Rahman, A.; Haque, A.; Hossain, M.; Rahman, M. Exploring of spectrum beta lactamase producing multidrug-resistant Salmonella enterica serovars in goat meat markets of Bangladesh. Vet. Anim. Sci. 2024, 25, 100367. [Google Scholar] [CrossRef]
- Al Emon, A.; Hossain, H.; Chowdhury, M.S.R.; Rahman, M.A.; Tanni, F.Y.; Asha, M.N.; Akter, H.; Hossain, M.M.; Islam, M.R.; Rahman, M.M. Prevalence, antimicrobial susceptibility profiles and resistant gene identification of bovine subclinical mastitis pathogens in Bangladesh. Heliyon 2024, 10, e34567. [Google Scholar] [CrossRef]
- Asha, M.N.; Chowdhury, S.R.; Hossain, H.; Rahman, A.; Al Emon, A.; Tanni, F.Y.; Islam, R.; Hossain, M.; Rahman, M. Antibacterial potential of lactic acid bacteria isolated from raw cow milk in Sylhet district, Bangladesh: A molecular approach. Vet. Med. Sci. 2024, 10, e1463. [Google Scholar] [CrossRef]
- Rahman, M.; Hossain, H.; Chowdhury, S.R.; Hossain, M.; Saleh, A.; Binsuwaidan, R.; Noreddin, A.; Helmy, Y.A.; El Zowalaty, M.E. Molecular Characterization of Multidrug-Resistant and Extended-Spectrum β-Lactamases-Producing Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Raw Meat in Retail Markets. Antibiotics 2024, 13, 586. [Google Scholar] [CrossRef]
- Moradigaravand, D.; Senok, A.; Al-Dabal, L.; Khansaheb, H.H.; Habous, M.; Alsuwaidi, H.; Alsheikh-Ali, A. Unveiling the dynamics of antimicrobial utilization and resistance in a large hospital network over five years: Insights from health record data analysis. PLoS Digit. Health 2023, 2, e0000424. [Google Scholar] [CrossRef]
- Shariati, A.; Noei, M.; Askarinia, M.; Khoshbayan, A.; Farahani, A.; Chegini, Z. Inhibitory effect of natural compounds on quorum sensing system in Pseudomonas aeruginosa: A helpful promise for managing biofilm community. Front. Pharmacol. 2024, 15, 1350391. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.N.; Tabasum, S.; Ashfaq, Y.; Mukhtar, A.; Haider, M.A.; Fatima, M.; Gang, S.; Tufail, A. Assessment of Antibiotic Resistance Profiles of Pathogenic Bacteria Isolates from Migratory Birds in the River Ravi Stopover Site. Pak. Biomed. J. 2024, 7, 21–26. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, A.; Thakur, N.; Kumar, V.; Chauhan, A.; Bhardwaj, N.; Sr, A.T. Changing trend in the antibiotic resistance pattern of Klebsiella pneumonia isolated from endotracheal aspirate samples of ICU patients of a tertiary care hospital in North India. Cureus 2023, 15, e36317. [Google Scholar] [CrossRef] [PubMed]
- Veloo, Y.; Thahir, S.S.A.; Rajendiran, S.; Hock, L.K.; Ahmad, N.; Muthu, V.; Shaharudin, R. Multidrug-Resistant Gram-Negative Bacteria and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from the Poultry Farm Environment. Microbiol. Spectr. 2022, 10, e0269421. [Google Scholar] [CrossRef]
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High prevalence and diversity of extended-spectrum β-lactamase and emergence of OXA-48 producing Enterobacterales in wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef]
- Liao, X.; Yang, R.-S.; Xia, J.; Chen, L.; Zhang, R.; Fang, L.-X.; Lei, F.; Song, G.; Jia, L.; Han, L.; et al. High colonization rate of a novel carbapenem-resistant Klebsiella lineage among migratory birds at Qinghai Lake, China. J. Antimicrob. Chemother. 2019, 74, 2895–2903. [Google Scholar] [CrossRef]
- Kerantzas, C.A.; Jacobs, W.R., Jr. Origins of combination therapy for tuberculosis: Lessons for future antimicrobial development and application. mBio 2017, 8, e01586-16. [Google Scholar] [CrossRef]
- Umadevi, S.; Kandhakumari, G.; Joseph, N.M.; Kumar, S.; Easow, J.M.; Stephen, S.; Singh, U.K. Prevalence and antimicrobial susceptibility pattern of ESBL producing Gram negative bacilli. J. Clin. Diagn. Res. 2011, 5, 236–239. [Google Scholar]
- Hordijk, J.; Schoormans, A.; Kwakernaak, M.; Duim, B.; Broens, E.; Dierikx, C.; Mevius, D.; Wagenaar, J.A. High prevalence of fecal carriage of extended spectrum β-lactamase/AmpC-producing Enterobacteriaceae in cats and dogs. Front. Microbiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Ansharieta, R.; Ramandinianto, S.C.; Effendi, M.H.; Plumeriastuti, H. Molecular identification of blaCTX-M and blaTEM genes encoding extended-spectrum β-lactamase (ESBL) producing Escherichia coli isolated from raw cow’s milk in East Java, Indonesia. Biodiversitas 2021, 22, 1600–1605. [Google Scholar] [CrossRef]
- Yanestria, S.M.; Dameanti, F.N.A.E.P.; Musayannah, B.G.; Pratama, J.W.A.; Witaningrum, A.M.; Effendi, M.H.; Ugbo, E.N. Antibiotic resistance pattern of extended-spectrum β-lactamase (ESBL) producing Escherichia coli isolated from broiler farm environment in Pasuruan district, Indonesia. Biodiversitas 2022, 23, 4460–4465. [Google Scholar] [CrossRef]
- Effendi, M.H.; Tyasningsih, W.; Yurianti, Y.A.; Rahmahani, J.; Harijani, N.; Plumeriastuti, H. Presence of multidrug resistance (MDR) and extended-spectrum beta-lactamase (ESBL) of Escherichia coli isolated from cloacal swab of broilers in several wet markets in Surabaya, Indonesia. Biodiversitas 2021, 22, 304–310. [Google Scholar] [CrossRef]
- Safika, S.; Nilasari, Z.; Pasaribu, F.H. Detection of antibiotic resistance coding gene in Klebsiella pneumoniae bacteria isolated from broiler chickens in West Java, Indonesia. J. Appl. Pharm. Sci. 2022, 12, 190–198. [Google Scholar] [CrossRef]
- Chaudhary, S.; Rai, T.; Arora, A.; Chandra, M. Multi Drug Resistant (MDR) Klebsiella pnuemoniae Isolated from Different Sources. Agric. Sci. Dig. 2024. [Google Scholar] [CrossRef]
- Begum, R.; Asha, N.A.; Dipu, D.C.C.; Roy, M.; Rahman, A.; Chowdhury, S.R.; Hossain, H.; Islam, R.; Uddin, B.; Rahman, M.; et al. Virulence and Antimicrobial Resistance Patterns of Salmonella spp. Recovered from Migratory and Captive Wild Birds. Vet. Med. Sci. 2024, 10, e70102. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Annals of Clinical Microbiology and Antimicrobials 2020, 19, 1. [Google Scholar] [CrossRef]
- Lee, H.-J.; Woo, Y.-K.; Choi, B.-K.; Jeong, O.-M.; Kim, J.-H.; Kim, D.-W.; Jeong, J.-Y.; Kwon, Y.-K.; Kang, M.-S. High prevalence of a gene cluster conferring resistance to streptomycin, sulfonamide, and tetracycline in Escherichia coli isolated from indigenous wild birds. J. Gen. Appl. Microbiol. 2021, 67, 81–84. [Google Scholar] [CrossRef]
- Di Francesco, A.; Salvatore, D.; Bertelloni, F.; Ebani, V.V. Tetracycline Resistance Genes in Wild Birds from a Wildlife Recovery Centre in Central Italy. Animals 2022, 13, 76. [Google Scholar] [CrossRef]
- Wang, D.; Ji, X.; Jiang, B.; Yuan, Y.; Liang, B.; Sun, S.; Zhu, L.; Liu, J.; Guo, X.; Yin, Y.; et al. Prevalence of Antibiotic Resistance and Virulence Genes in Escherichia coli Carried by Migratory Birds on the Inner Mongolia Plateau of Northern China from 2018 to 2023. Microorganisms 2024, 12, 1076. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).