Ssc-miR-130b Enhances Cell Proliferation and Represses Adipogenesis of Primary Cultured Intramuscular Preadipocytes in Pigs
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statements
2.2. Isolation and Culture of PIMPA
2.3. Experimental Groups and Adipogenic Differentiation
2.4. MiR-130b Mimic and Inhibitor Transfection
2.5. Oil Red O (ORO) Staining and Quantification
2.6. Total RNA Extraction and qRT-PCR
2.7. Western Blot
2.8. Real-Time PCR Quantification of miR-130b
2.9. The Effect of miR-130b on PIMPA Proliferation
2.10. The Effect of miR-130b on the Differentiation of PIMPA
2.11. Statistical Analysis
3. Results
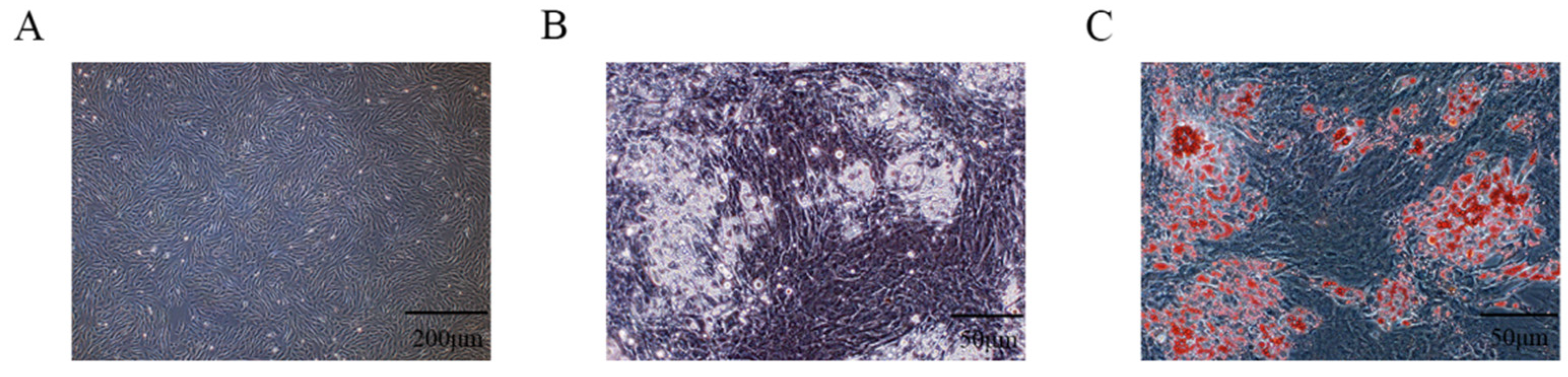
3.1. Isolation and Identification of PIMPA
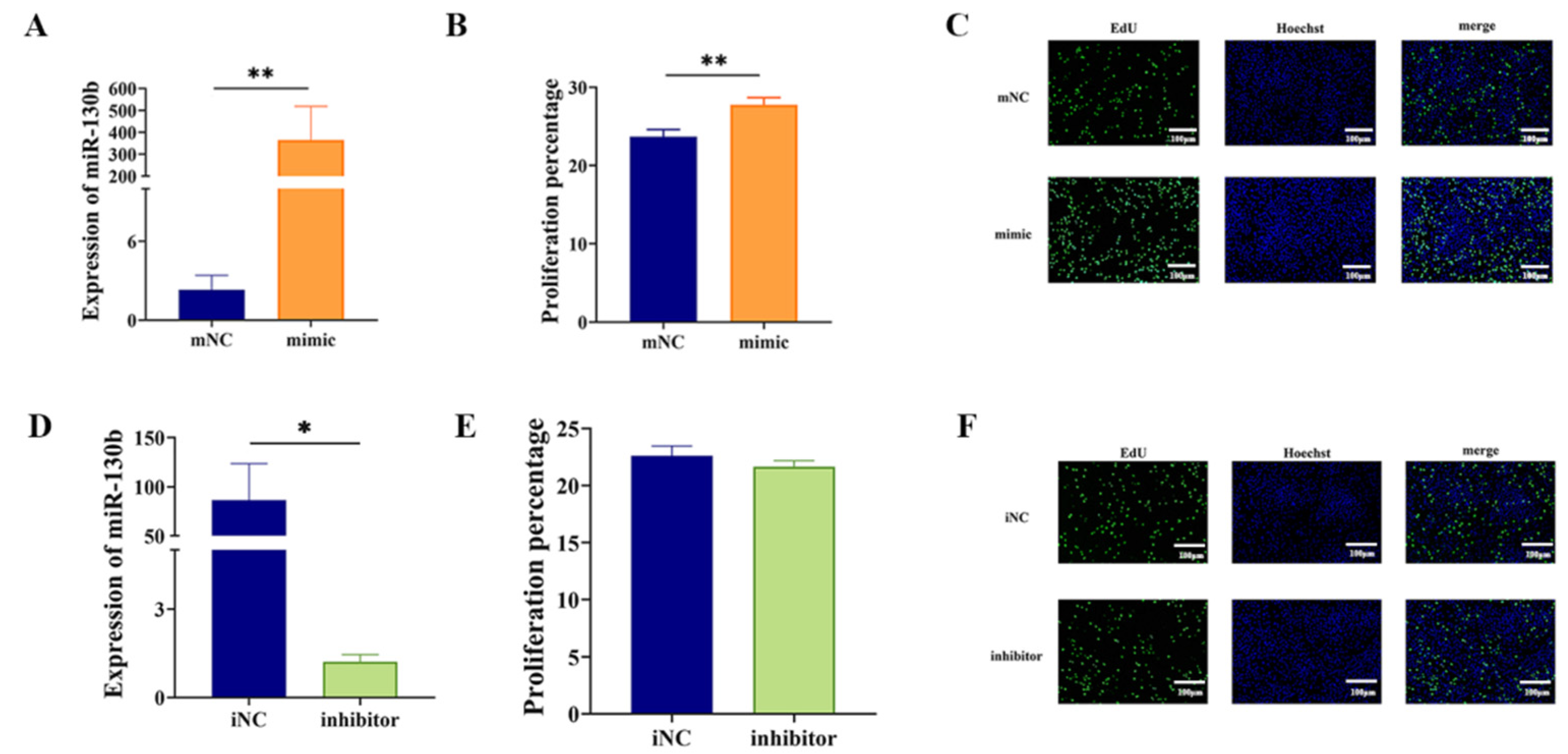
3.2. MiR-130b Overexpression Significantly Promoted the Proliferation of PIMPA
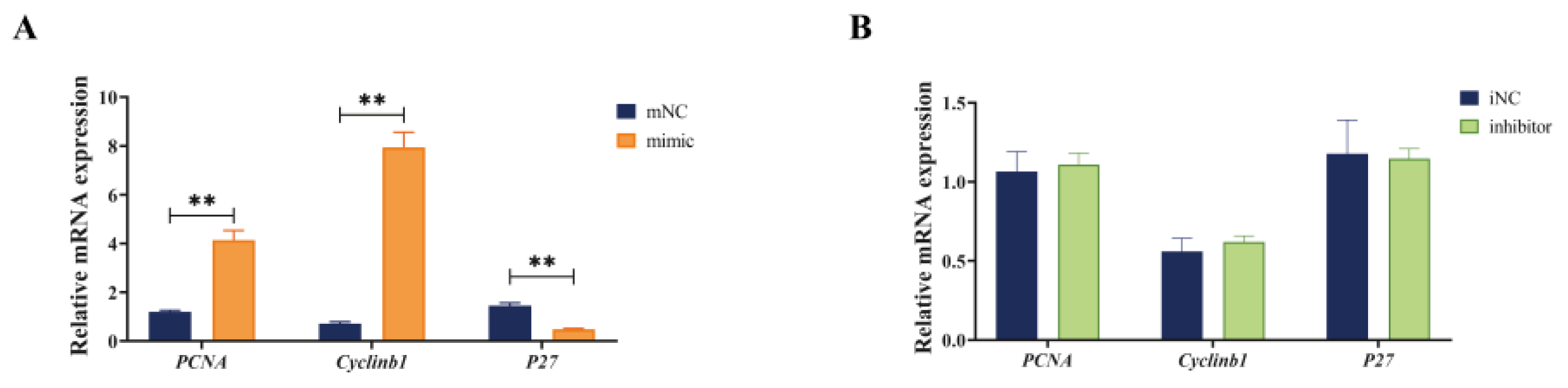
3.3. Overexpression of miR-130b Promoted the Expression of PCNA and cyclinb1 and Inhibited the Expression of P27 in PIMPA
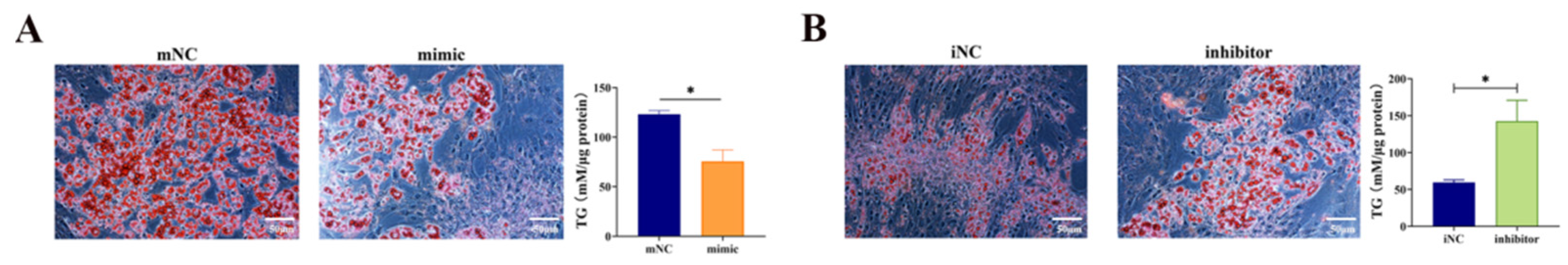
3.4. Overexpression of miR-130b Significantly Inhibited the Adipogenic Differentiation of PIMPA
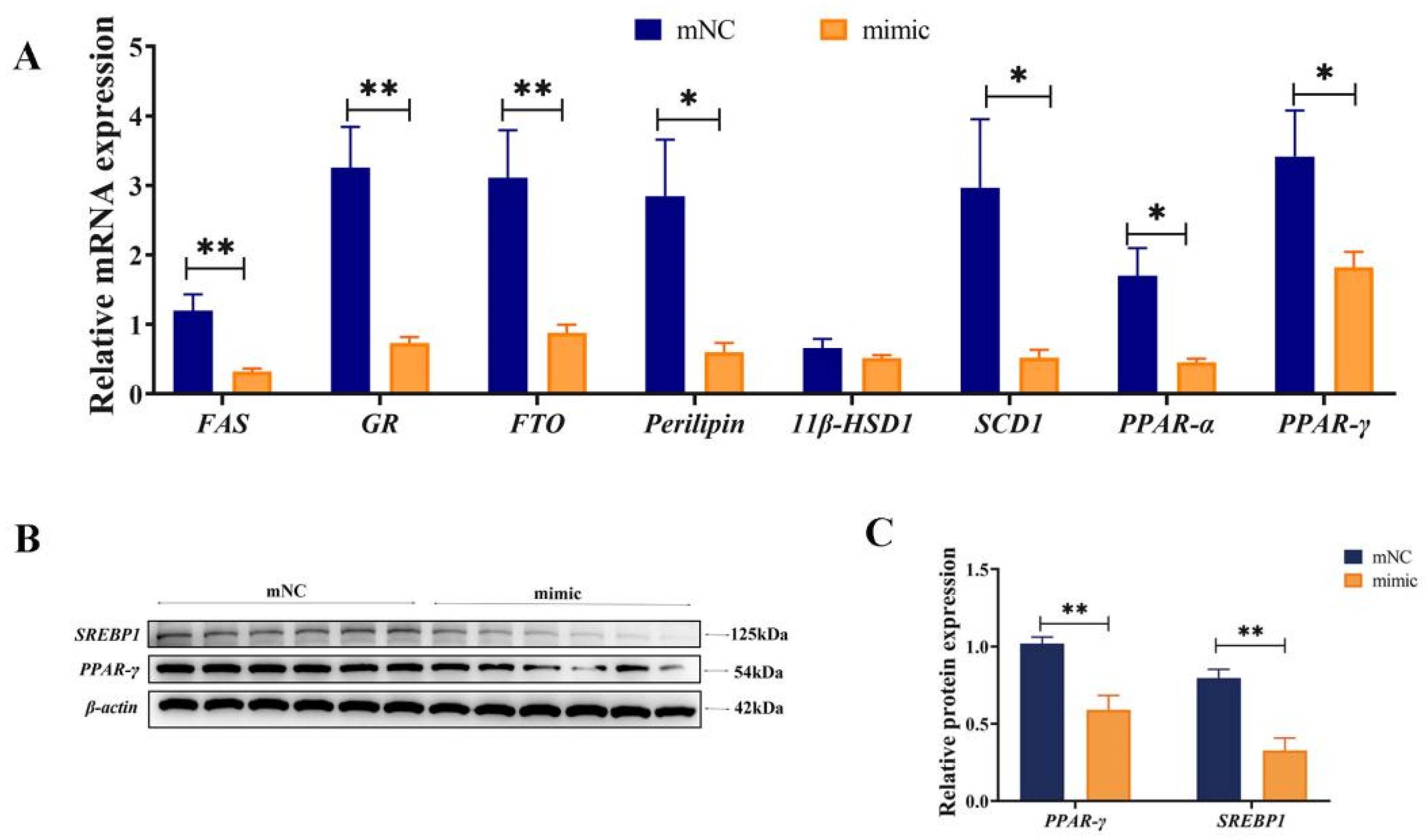
3.5. Overexpression of miR-130b Significantly Inhibited the Expression of Key Genes for Adipogenesis
3.6. miR-130b Inhibition Significantly Promoted the Expression of Key Genes for Adipogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | acetyl-CoA carboxylase |
| DEPC | diethylpyrocarbonate |
| FAS | fatty acid synthesis |
| FTO | fat mass and obesity-associated protein |
| GR | glucocorticoid receptor |
| IMF | intramuscular fat |
| miRNAs | microRNAs |
| ORO | oil red o |
| PCNA | proliferating cell nuclear antigen |
| PIMPA | porcine primary intramuscular preadipocytes |
| PPAR-α | peroxisome proliferator activated receptor-α |
| PPAR-γ | peroxisome proliferator-activated receptor-gamma |
| PPIA | peptidylprolyl isomerase A |
| RT-qPCR | reverse transcription-quantitative polymerase chain reaction |
| SCD-1 | stearyl-CoA-desaturase-1 |
| SREBP-1 | sterol regulatory element binding protein-1 |
| TNF | tumor necrosis factor |
| 11β-HSD1 | 11 beta-hydroxysteroid dehydrogenase type 1 |
References
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Tian, X.K.; Li, D.; He, Y.L.; Yang, P.Y.; Cheng, Y.; Zhao, X.; Sun, J.C.; Yang, G.S. Transcriptome, proteome and metabolome analysis provide insights on fat deposition and meat quality in pig. Food Res. Int. 2023, 166, 112550. [Google Scholar] [CrossRef]
- Qimuge, N.; He, Z.Z.; Qin, J.; Sun, Y.M.; Wang, X.; Yu, T.Y.; Dong, W.Z.; Yang, G.S.; Pang, W.J. Overexpression of DNMT3A promotes proliferation and inhibits differentiation of porcine intramuscular preadipocytes by methylating p21 and PPARg promoters. Gene 2019, 696, 54–62. [Google Scholar] [CrossRef]
- Yi, X.D.; He, Z.Z.; Tian, T.T.; Kou, Z.Y.; Pang, W.J. LncIMF2 promotes adipogenesis in porcine intramuscular preadipocyte through sponging MiR-217. Anim. Biotechnol. 2023, 34, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Sun, X.M.; Cai, H.F.; Sun, Y.J.; Plath, M.; Li, C.; Lan, X.; Lei, C.J.; Lan, X.Y.; Lei, C.Z.; et al. Long non-coding RNA ADNCR suppresses adipogenic differentiation by targeting miR-204. Biochim. Biophys. Acta 2016, 1859, 871–882. [Google Scholar] [CrossRef]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernández-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Rodríguez-Barrueco, R.; Latorre, J.; Devis-Jáuregui, L.; Lluch, A.; Bonifaci, N.; Llobet, F.J.; Olivan, M.; Coll-Iglesias, L.; Gassner, K.; Davis, M.L.; et al. A microRNA Cluster Controls Fat Cell Differentiation and Adipose Tissue Expansion By Regulating SNCG. Adv. Sci. 2022, 9, e2104759. [Google Scholar] [CrossRef]
- Liu, Y.K.; Wei, Y.L.; Dou, Y.Q.; Li, C.L.; Song, C.L.; Zhang, Z.; Qi, K.L.; Li, X.J.; Qiao, R.M.; Wang, K.J.; et al. Effect of miR-149-5p on intramuscular fat deposition in pigs based on metabolomics and transcriptomics. BMC Genom. 2023, 24, 293. [Google Scholar] [CrossRef]
- Liu, H.C.; Wei, W.; Lin, W.M.; Yu, W.S.; Luo, W.; Niu, Y.F.; Zhang, L.F.; Chen, J. miR-32-5p Regulates Lipid Accumulation in Intramuscular Fat of Erhualian Pigs by Suppressing KLF3. Lipids 2021, 56, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.L.; Liu, H.T.; Zhang, W.; Li, X.J.; Liu, L.Q.; Zhou, M.; Wang, J.R.; Su, S.G.; Ding, X.D.; et al. miR-381-3p Inhibits Intramuscular Fat Deposition through Targeting FABP3 by ceRNA Regulatory Network. Biology 2022, 11, 1497. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, Y.; Wang, X.Y.; Zhang, D.; Zhang, H.H.; Wu, Q.; He, Y.Q.; Wang, J.Y.; Zhang, L.; Xia, H.F.; et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 2013, 56, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Tekcan, E.; Kara, N.; Aydın, H.M.; Abur, Ü.; Abbaszadeh, M. Evaluation of the promoter methylation status of hypoxia factor 3A and interleukin-6 genes and expression levels of mir-130b and mir-146b in childhood obesity. Rev. Assoc. Med. Bras. 2022, 68, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Lee, M.J.; Abdelmohsen, K.; Kim, W.; Kim, M.M.; Srikantan, S.; Martindale, J.L.; Hutchison, E.R.; Kim, H.H.; Marasa, B.S.; et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol. Cell Biol. 2011, 31, 626–638. [Google Scholar] [CrossRef]
- Alonso-Villa, E.; Mangas, A.; Bonet, F.; Campuzano, Ó.; Quezada-Feijoo, M.; Ramos, M.; García-Padilla, C.; Franco, D.; Toro, R. The Protective Role of miR-130b-3p Against Palmitate-Induced Lipotoxicity in Cardiomyocytes Through PPARγ Pathway. Int. J. Mol. Sci. 2024, 25, 12161. [Google Scholar] [CrossRef]
- Li, Y.Y.; He, C.S.; Ran, L.; Wang, Y.; Xiong, Y.; Wang, Y.L.; Zhu, J.J.; Lin, Y.Q. miR-130b duplex (miR-130b-3p/miR-130b-5p) negatively regulates goat intramuscular preadipocyte lipid droplets accumulation by inhibiting Krüppel-like factor 3 expression. J. Anim. Sci. 2023, 101, skad184. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wei, D.W.; Cheng, G.; Li, S.J.; Wang, L.; Wang, Y.N.; Wang, X.Y.; Zhang, S.; Wang, H.; Zan, L. Bta-miR-130a/b regulates preadipocyte differentiation by targeting PPARG and CYP2U1 in beef cattle. Mol. Cell. Probes 2018, 42, 10–17. [Google Scholar] [CrossRef]
- Pan, S.F.; Zheng, Y.T.; Zhao, R.Q.; Yang, X.J. MicroRNA-130b and microRNA-374b mediate the effect of maternal dietary protein on offspring lipid metabolism in Meishan pigs. Br. J. Nutr. 2013, 109, 1731–1738. [Google Scholar] [CrossRef]
- Pan, S.F.; Yang, X.J.; Jia, Y.M.; Li, R.S.; Zhao, R.Q. Microvesicle-shuttled miR-130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPAR-g expression. J. Cell Physiol. 2014, 229, 631–639. [Google Scholar] [CrossRef]
- Pan, S.F.; Yang, X.J.; Jia, Y.; Li, Y.M.; Chen, R.R.; Wang, M.; Cai, D.M.; Zhao, R.Q. Intravenous injection of microvesicle-delivery miR-130b alleviates high-fat diet-induced obesity in C57BL/6 mice through translational repression of PPAR-γ. J. Biomed. Sci. 2015, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Xie, Y.H.; Chen, W.; Zhang, Y.; Zeng, Y.Q. miR-34a Regulates Lipid Droplet Deposition in 3T3-L1 and C2C12 Cells by Targeting LEF1. Cells 2022, 12, 167. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yao, X.; Ma, M.; Zhang, H.; Wang, H.; Zhao, L.; Liu, S.N.; Sun, C.; Li, P.; Wu, Y.T.; et al. miR-130b inhibits proliferation and promotes differentiation in myocytes via targeting Sp1. J. Mol. Cell Biol. 2021, 13, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Liu, H.H.; Chen, M.T.; Ren, S.S.; Cheng, P.P.; Zhang, H. miR-301b~miR-130b-PPARγ axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef]
- Chen, W.K.; Zhang, H.J.; Zou, M.X.; Wang, C.; Yan, Y.G.; Zhan, X.L.; Li, X.L.; Wang, W.J. LncRNA HOTAIR influences cell proliferation via miR-130b/PTEN/AKT axis in IDD. Cell Cycle 2022, 21, 323–339. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, F.F.; Ge, J.; Zhu, J.Y.; Shi, X.E.; Li, X.; Yu, T.Y.; Chu, G.Y.; Yang, G.S. miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes. Int. J. Mol. Sci. 2016, 17, 2047. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, A.J.; Abell, A.D.; Bruning, J.B. Targeting PCNA with Peptide Mimetics for Therapeutic Purposes. Chembiochem 2020, 21, 442–450. [Google Scholar] [CrossRef]
- Samulin, J.; Berget, I.; Grindflek, E.; Lien, S.; Sundvold, H. Changes in lipid metabolism associated gene transcripts during porcine adipogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 153, 8–17. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef]
- Czech, M.P.; Tencerova, M.; Pedersen, D.J.; Aouadi, M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 2013, 56, 949–964. [Google Scholar] [CrossRef]
- Granneman, J.G.; Moore, H.P.; Granneman, R.L.; Greenberg, A.S.; Obin, M.S.; Zhu, Z.X. Analysis of lipolytic protein trafficking and interactions in adipocytes. J. Biol. Chem. 2007, 282, 5726–5735. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862 Pt B, 1221–1232. [Google Scholar] [CrossRef]
- Brasaemle, D.L.; Rubin, B.; Harten, I.A.; Gruia-Gray, J.; Kimmel, A.R.; Londos, C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 2000, 275, 38486–38493. [Google Scholar] [CrossRef]
- Dalen, K.T.; Schoonjans, K.; Ulven, S.M.; Weedon-Fekjaer, M.S.; Bentzen, T.G.; Koutnikova, H.; Auwerx, J.; Nebb, H.I. Adipose tissue expression of the lipid droplet-associating proteins S3-12 and perilipin is controlled by peroxisome proliferator-activated receptor-gamma. Diabetes 2004, 53, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Luo, Y.L.; Jia, G.; Liu, G.M.; Zhao, H.; Huang, Z.Q. FTO Promotes Adipogenesis through Inhibition of the Wnt/β-catenin Signaling Pathway in Porcine Intramuscular Preadipocytes. Anim. Biotechnol. 2017, 28, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhou, B.; Luo, Y.L.; Huang, Z.Q.; Jia, G.; Liu, G.M.; Zhao, H. Tissue Distribution of Porcine FTO and Its Effect on Porcine Intramuscular Preadipocytes Proliferation and Differentiation. PLoS ONE 2016, 11, e0151056. [Google Scholar] [CrossRef]
- Ovilo, C.; Pérez-Enciso, M.; Barragán, C.; Clop, A.; Rodríquez, C.; Oliver, M.A.; Toro, M.A.; Noruera, J.L. A QTL for intramuscular fat and backfat thickness is located on porcine chromosome 6. Mamm. Genome 2000, 11, 344–346. [Google Scholar] [CrossRef]
- Fan, B.; Du, Z.Q.; Rothschild, M.F. The fat mass and obesity-associated (FTO) gene is associated with intramuscular fat content and growth rate in the pig. Anim. Biotechnol. 2009, 20, 58–70. [Google Scholar] [CrossRef]

| Gene Name | GenBank | Primer Sequence (5′-3′) | Annealing Temperature (°C) | PCR Product (bp) | |
|---|---|---|---|---|---|
| Porcine | |||||
| P27 | NM_214316.1 | F | GTCCCTTTCAGTGAGAACCGATAC | 61 | 134 |
| R | TTGCTGCCACATAACGGAATCAT | ||||
| PCNA | NM_001291925.1 | F | GTGATTCCACCACCATGTTC | 57.9 | 145 |
| R | TGAGACGACTCCATGCTCTG | ||||
| Cyclinb1 | NM_001170768.1 | F | AACTGCTCTTGGAGACATCGGT | 61.8 | 196 |
| R | TGGTTCAGGCTCCAGTTCAGG | ||||
| FAS | NM_001099930.1 | F | GTCCTGCTGAAGCCTAACTC | 57.2 | 206 |
| R | TCCTTGGAACCGTCTGTG | ||||
| GR | NM_001008481.1 | F | CCAAACTCTGCCTTGTGTGTTC | 61 | 108 |
| R | TGTGCTGTCCTTCCACTGCT | ||||
| FTO | NM_001112692.1 | F | GGAGAAAGCCAATATCGACACC | 60.1 | 109 |
| R | TCTGCTCTTCCTGTCCACCTC | ||||
| Perilipin | NM_001038638.1 | F | GCCTGACTTTGCTGGATGG | 60.5 | 119 |
| R | CTTGGTGCTGGTGTAGGTCTTCT | ||||
| 11β-HSD1 | NM_214248.3 | F | CCATGCTGAAGCAGAGCAAC | 59.6 | 115 |
| R | AAGAACCCGTCCAGAGCAAA | ||||
| SCD1 | NM_213781.1 | F | CCCAGCCGTCAAAGAGAA | 56.5 | 200 |
| R | CGATGGCGTAACGAAGAAA | ||||
| PPAR-α | NM_001044526.1 | F | GAGCCTGAGGAAACCCTTCT | 58.2 | 128 |
| R | GGTCTCCGCACCAAATGA | ||||
| PPAR-γ | NM_214379.1 | F | GCCCTTCACCACTGTTGATT | 58.5 | 210 |
| R | GAGTTGGAAGGCTCTTCGTG | ||||
| Caspase-3 | NM_214131.1 | F | TGCTGCAAATCTCAGGGAGACCT | 64.6 | 288 |
| R | GTGCCTCGGCAGGCCTGAAT | ||||
| PPIA | NM_214353.1 | F | AGGATTTATGTGCCAGGGTG | 50.9 | 126 |
| R | ATGGACAAGATGCCAGGAC | ||||
| Antibody Name | Species Source | Number | Company |
|---|---|---|---|
| PPAR-γ | Rabbit polyclonal antibody | BS6442 | Bioworld (Shanghai, China) |
| β-actin | Rabbit polyclonal antibody | AP0060 | Bioworld (Shanghai, China) |
| SREBP1 | Rabbit polyclonal antibody | 14088-1-AP | Proteintech (Wuhan, China) |
| Caspase-3 | Rabbit polyclonal antibody | 19677-1-AP | Proteintech (Wuhan, China) |
| Bcl-2 | Rabbit polyclonal antibody | 26593-1-AP | Proteintech (Wuhan, China) |
| Name | Sequences (5′-3′) |
|---|---|
| miR-130b mimic | CAGTGCAATGATGAAAGGGCAT |
| GCCCTTTCATCATTGCACTGTT | |
| mimic NC | TTCTCCGAACGTGTCACGTTT |
| ACGTGACACGTTCGGAGAATT | |
| miR-130b inhibitor | ATGCCCTTTCATCATTGCACTG |
| inhibitor NC | CAGTACTTTTGTGTAGTACAA |
| Universal primer | TAGAGTGAGTGTAGCGAGCA |
| External reference | GTGACCCACGATGTGTATTCGC |
| Poly (T) adapter | TAGAGTGAGTGTAGCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTTTTTVN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, Y.; Wang, L.; Du, M.; Zhang, R.; Lu, Y.; Pan, S. Ssc-miR-130b Enhances Cell Proliferation and Represses Adipogenesis of Primary Cultured Intramuscular Preadipocytes in Pigs. Vet. Sci. 2025, 12, 375. https://doi.org/10.3390/vetsci12040375
Yang Y, Chen Y, Wang L, Du M, Zhang R, Lu Y, Pan S. Ssc-miR-130b Enhances Cell Proliferation and Represses Adipogenesis of Primary Cultured Intramuscular Preadipocytes in Pigs. Veterinary Sciences. 2025; 12(4):375. https://doi.org/10.3390/vetsci12040375
Chicago/Turabian StyleYang, Yunqiu, Yongfang Chen, Lijun Wang, Min Du, Rui Zhang, Yao Lu, and Shifeng Pan. 2025. "Ssc-miR-130b Enhances Cell Proliferation and Represses Adipogenesis of Primary Cultured Intramuscular Preadipocytes in Pigs" Veterinary Sciences 12, no. 4: 375. https://doi.org/10.3390/vetsci12040375
APA StyleYang, Y., Chen, Y., Wang, L., Du, M., Zhang, R., Lu, Y., & Pan, S. (2025). Ssc-miR-130b Enhances Cell Proliferation and Represses Adipogenesis of Primary Cultured Intramuscular Preadipocytes in Pigs. Veterinary Sciences, 12(4), 375. https://doi.org/10.3390/vetsci12040375






