Impact of Burdizzo and Surgical Castration on Immune and Oxidative Stress Markers in Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing and Management
2.2. Experimental Procedure
2.3. Blood Collection
2.4. Peripheral Blood Mononuclear Cell Isolation
2.5. Flow Cytometry Analysis
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Malondialdehyde (MDA) Assessment
2.8. Nitric Oxide Assessment
2.9. Total Antioxidant Capacity Assessment
2.10. Statistical Analysis
3. Results
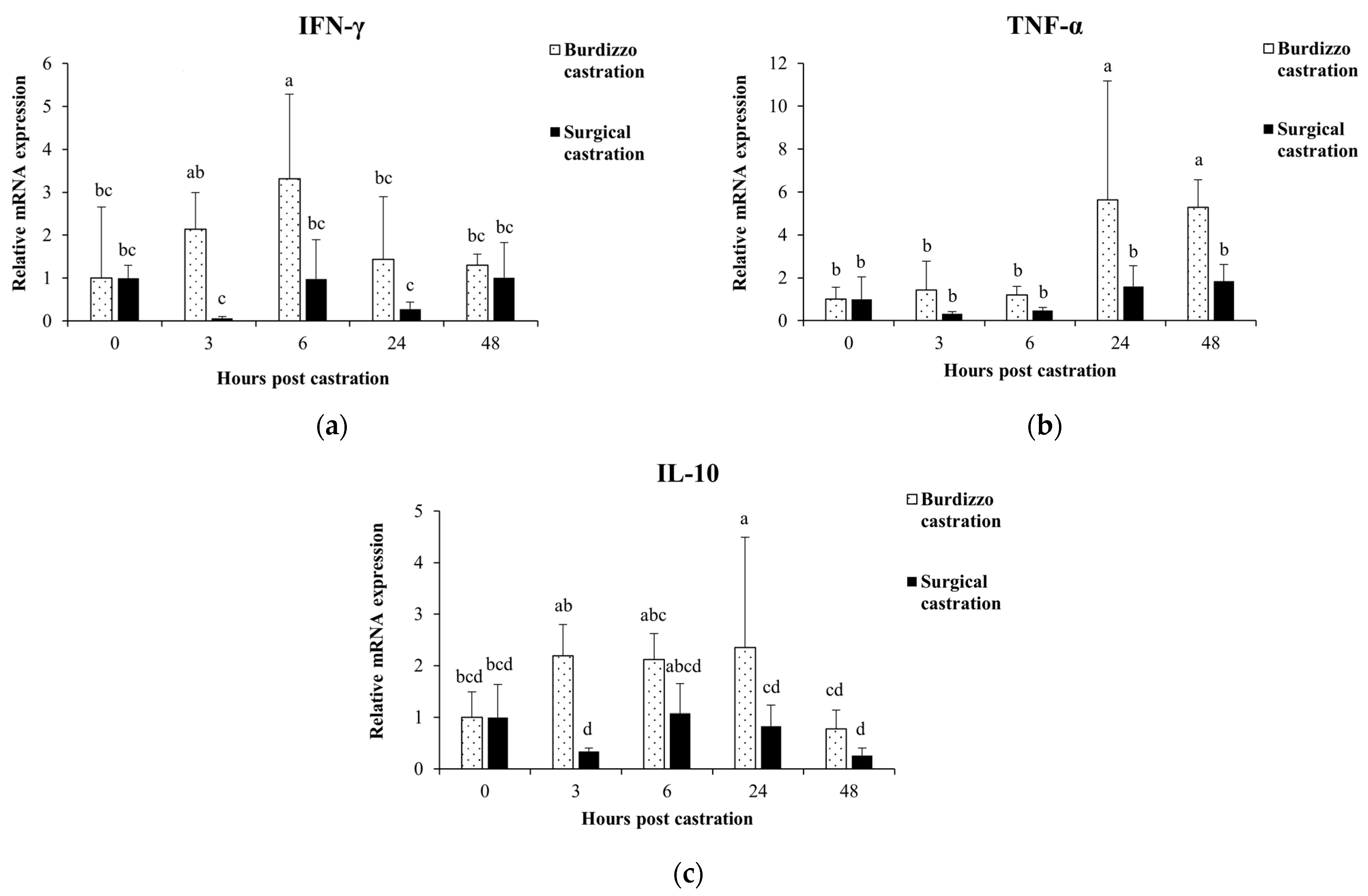
3.1. Effect of Castration on Cytokine Gene Expression in PBMCs
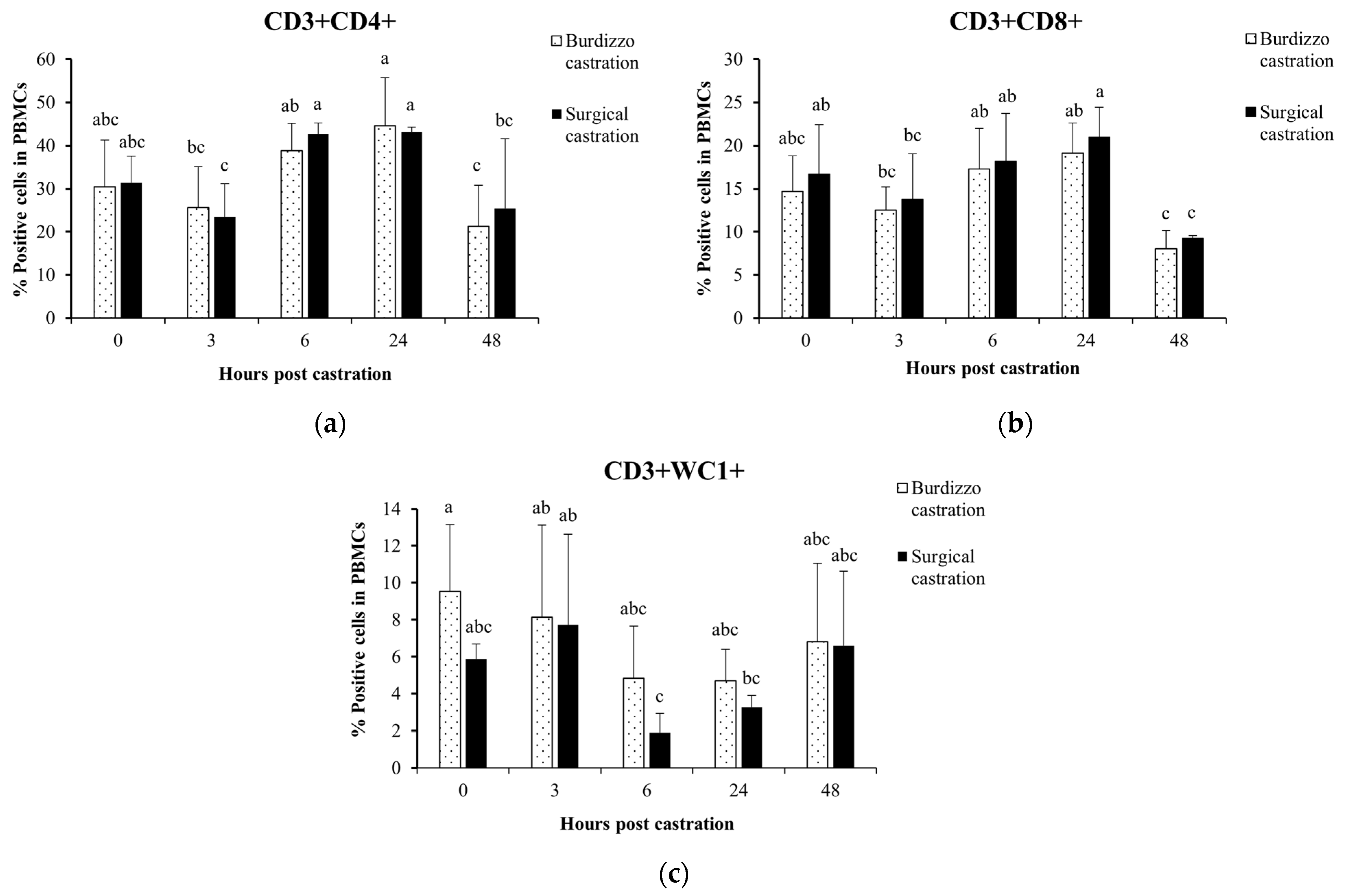
3.2. Effect of Castration on T Lymphocyte Subsets in the PBMCs
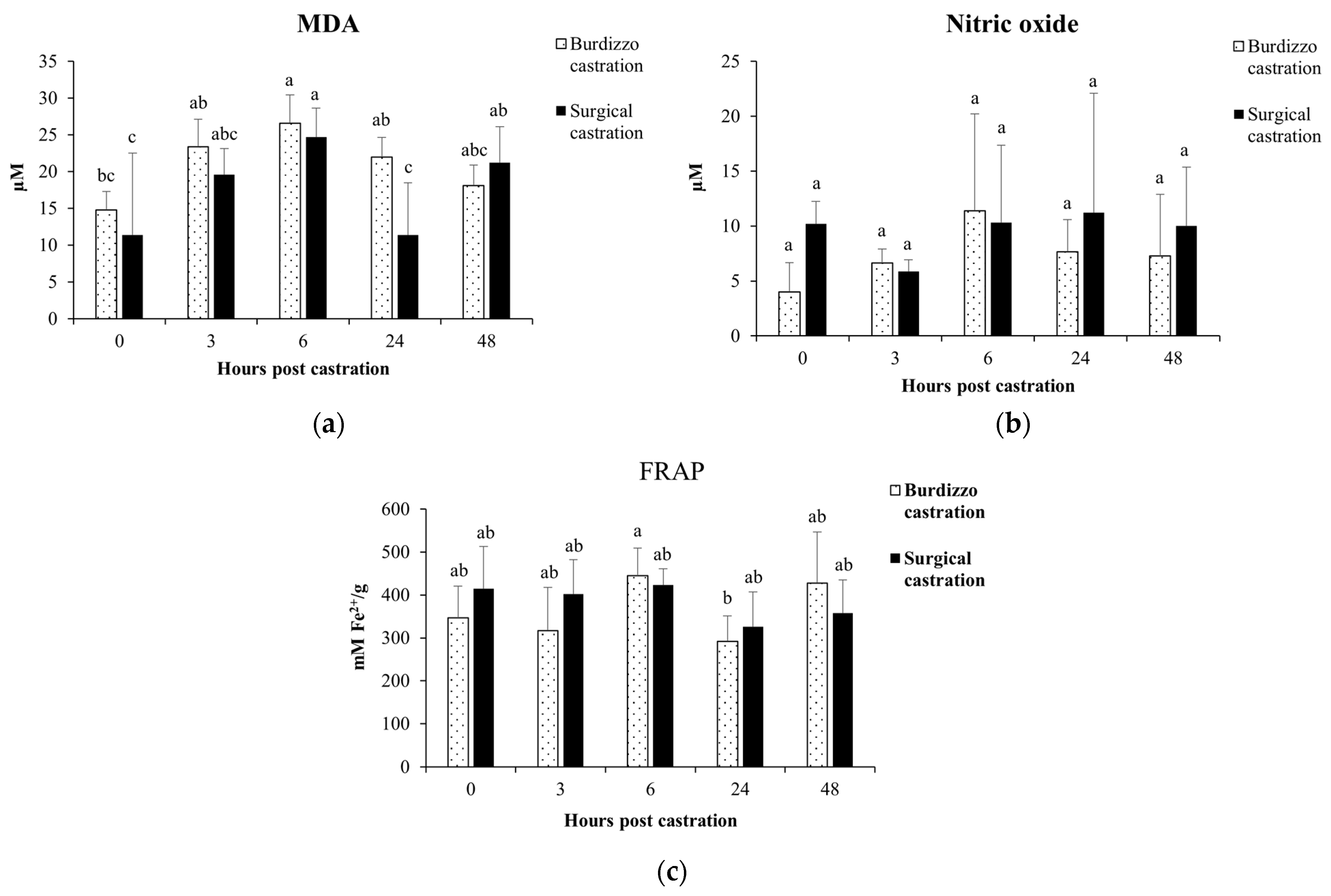
3.3. Effect of Castration on MDA, Total Antioxidant Capacity and NO in Calves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, P.L. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 2021, 15, 100295. [Google Scholar] [CrossRef]
- Herrero, M.; Grace, D.; Njuki, J.; Johnson, N.; Enahoro, D.; Silvestri, S.; Rufino, M.C. The roles of livestock in developing countries. Animal 2012, 7, 318. [Google Scholar] [CrossRef]
- Hilmiati, N.; Ilham, N.; Nulik, J.; Rohaeni, E.S.; deRosari, B.; Basuki, T.; Hau, D.K.; Ngongo, Y.; Lase, J.A.; Fitriawaty, F.; et al. Smallholder cattle development in Indonesia: Learning from the past for an outcome-oriented development model. Int. J. Des. Nat. Ecodynamics 2024, 19, 169–184. [Google Scholar] [CrossRef]
- Smith, S.B.; Gotoh, T.; Greenwood, P.L. Current situation and future prospects for global beef production: Overview of special issue. Asian-Australas. J. Anim. Sci. 2018, 31, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Hubbart, J.A.; Blake, N.; Holásková, I.; Mata Padrino, D.; Walker, M.; Wilson, M. Challenges in sustainable beef cattle production: A subset of needed advancements. Challenges 2023, 14, 14. [Google Scholar] [CrossRef]
- Roberts, S.L.; Powell, J.G.; Hughes, H.D.; Richeson, J.T. Effect of castration method and analgesia on inflammation, behavior, growth performance, and carcass traits in feedlot cattle. J. Anim. Sci. 2018, 96, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Wielgosz-Groth, Z.; Sobczuk-Szul, M.; Nogalski, Z.; Purwin, C.; Pogorzelska-Przybyłek, P.; Winarski, R. The effect of gender and feeding system on the growth rate and blood parameters of Polish HolsteinFriesian x Limousin calves. Pak. Vet. J. 2015, 35, 33–37. [Google Scholar]
- Hess, R.A.; Park, C.J.; Soto, S.; Reinacher, L.; Oh, J.E.; Bunnell, M.; Ko, C.J. Male animal sterilization: History, current practices, and potential methods for replacing castration. Front. Vet. Sci. 2024, 11, 1409386. [Google Scholar] [CrossRef]
- Marquette, G.A.; McGee, M.; Fisher, A.D.; Stanger, K.; Earley, B. Effect of age of suckler beef calves on stress indicators and growth performance in response to Burdizzo castration. J. Appl. Anim. Res. 2021, 49, 221–233. [Google Scholar] [CrossRef]
- Marquette, G.A.; Ronan, S.; Earley, B. Review: Castration—Animal welfare considerations. J. Appl. Anim. Res. 2023, 51, 703–718. [Google Scholar] [CrossRef]
- Adcock, S.J.J. Early life painful procedures: Long-term consequences and implications for farm animal welfare. Front. Anim. Sci. 2021, 2, 759522. [Google Scholar] [CrossRef]
- Fisher, A.D.; Crowe, M.A.; Alonso de la Varga, M.E.; Enright, W.J. Effect of castration method and the provision of local anaesthesia on plasma cortisol, scrotal circumference, growth, and feed intake of bull calves. J. Anim. Sci. 1996, 74, 2336–2343. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Qulu, L.-A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 2024, 39, 1613–1636. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Pérez-Garnelo, S.S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The effect of stress on reproduction and reproductive technologies in beef cattle: A review. Animals 2020, 10, 2096. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.Y.; Earley, B.; Crowe, M.A. Effects of banding or Burdizzo castration of bulls at 2 ages on plasma cortisol, immune, and inflammatory responses. Animal 2009, 3, 1169–1177. [Google Scholar]
- Marti, S.; Meléndez, D.M.; Pajor, E.A.; Moya, D.; Heuston, C.E.M.; Gellatly, D.; Janzen, E.D.; Schwartzkopf-Genswein, K.S. Effect of band and knife castration of beef calves on welfare indicators of pain at three relevant industry ages: II. Chronic pain. J. Anim. Sci. 2017, 95, 4367–4380. [Google Scholar] [CrossRef] [PubMed]
- Ertuğrul, T.; Ceylan, A.; Bayraktaroğlu, A.G.; Özen, A. Effects of castration on IFN-γ and TNF-α expression in the adrenal gland of Angora goat. J. Istanb. Vet. Sci. 2021, 5, 66–71. [Google Scholar] [CrossRef]
- Yoo, S.; Baik, M.; Haque, M.N.; Kim, M. Effect of knife castration on leukocyte cytokine expression and indicators of stress, pain, and inflammation in Korean cattle bull calves. Anim. Biosci. 2023, 36, 521–528. [Google Scholar] [CrossRef]
- Warnock, T.M.; Thrift, T.A.; Irsik, M.; Hersom, M.J.; Yelich, J.V.; Maddock, T.D.; Lamb, G.C.; Arthington, J.D. Effect of castration technique on beef calf performance, feed efficiency, and inflammatory response. J. Anim. Sci. 2012, 90, 2345–2352. [Google Scholar] [CrossRef]
- Niu, X.; Ding, Y.; Chen, S.; Gooneratne, R.; Ju, X. Effect of immune stress on growth performance and immune functions of livestock: Mechanisms and prevention. Animals 2022, 12, 909. [Google Scholar] [CrossRef]
- Durand, D.; Collin, A.; Merlot, E.; Baéza, E.; Guilloteau, L.A.; Le Floc’h, N.; Thomas, A.; Fontagné-Dicharry, S. Implication of redox imbalance in animal health and performance at critical periods, insights from different farm species. Animal 2022, 16, 100543. [Google Scholar] [CrossRef] [PubMed]
- Mogheiseh, A.; Koohi, F.; Nazifi, S.; Tabrizi, A.S.; Salavati, S. Oxidative-antioxidative status and hepatic and renal factors following melatonin administration in castrated and intact dogs. Basic Clin. Androl. 2019, 29, 14. [Google Scholar] [CrossRef]
- Akbari, B.; Najafi, F.; Bahmaei, M.; Mahmoodi, N.M.; Sherman, J.H. Modeling and optimization of malondialdehyde (MDA) absorbance behavior through response surface methodology (RSM) and artificial intelligence network (AIN): An endeavor to estimate lipid peroxidation by determination of MDA. J. Chemom. 2023, 37, e3468. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.N.; Adzim, M.K.R.; Simbak, N.B.; Atif, A.B. Sample size calculation for animal studies using degree of freedom (E): An easy and statistically defined approach for metabolomics and genetic research. Curr. Trends Biomed. Eng. Biosci. 2017, 10, 555785. [Google Scholar]
- Srinontong, P.; Aengwanich, W.; Somphon, S.; Khonwai, S.; Nitsinsakul, T.; Wu, Z.; Chalalai, T.; Saraphol, B.; Srisanyong, W. Comparison of lipopolysaccharide-mediated peripheral blood mononuclear cell activation between Brahman and Brahman × Thai native crossbreed cattle. Vet. World 2024, 17, 804–810. [Google Scholar] [CrossRef]
- Srinontong, P.; Wandee, J.; Aengwanich, W. The effect of gallic acid on malondialdehyde, hydrogen peroxide and nitric oxide that influence viability of broiler blood cells at high ambient temperatures. Br. Poult. Sci. 2023, 64, 512–517. [Google Scholar] [CrossRef]
- Needham, T.; Lambrechts, H.; Hoffman, L.C. Castration of male livestock and the potential of immunocastration to improve animal welfare and production traits: Invited Review. S. Afr. J. Anim. Sci. 2017, 47, 731–742. [Google Scholar] [CrossRef]
- Abou-Khalil, N.S.; Ali, M.F.; Ali, M.M.; Ibrahim, A. Surgical castration versus chemical castration in donkeys: Response of stress, lipid profile and redox potential biomarkers. BMC Vet. Res. 2020, 16, 310. [Google Scholar] [CrossRef]
- Pang, W.; Earley, B.; Sweeney, T.; Gath, V.; Crowe, M.A. Temporal patterns of inflammatory gene expression in local tissues after banding or burdizzo castration in cattle. BMC Vet. Res. 2009, 5, 36. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.L.; Damani-Yokota, P.; Yirsaw, A.; Loonie, K.; Teixeira, A.F. Special features of γδ T cells in ruminants. Mol. Immunol. 2021, 134, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Druziuk, R.B.; Denefil, O.V. Changes in interleukin content in male rats with castration and stress in the development of adrenaline heart damage. Med. Clin. Chem. 2022, 3, 55–61. [Google Scholar]
- Menges, P.; Kessler, W.; Kloecker, C.; Feuerherd, M.; Gaubert, S.; Diedrich, S.; van der Linde, J.; Hegenbart, A.; Busemann, A.; Traeger, T.; et al. Surgical trauma and postoperative immune dysfunction. Eur. Surg. Res. 2012, 48, 180–186. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total antioxidant capacity: Biochemical aspects and clinical significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Farschtschi, S.; Matte, M.; Pfaffl, M.W. Advantages and challenges of differential immune cell count determination in blood and milk for monitoring the health and well-being of dairy cows. Vet. Sci. 2022, 9, 255. [Google Scholar] [CrossRef]
- Pang, W.Y.; Earley, B.; Murray, M. Banding or Burdizzo castration and carprofen administration on peripheral leukocyte inflammatory cytokine transcripts. Res. Vet. Sci. 2009, 90, 127–132. [Google Scholar] [CrossRef]
- Petherick, J.C.; Small, A.H.; Mayer, D.G.; Colditz, I.G.; Ferguson, D.M.; Stafford, K.J. A comparison of welfare outcomes for weaner and mature Bos indicus bulls surgically or tension band castrated with or without analgesia: 2. Responses related to stress, health and productivity. Appl. Anim. Behav. Sci. 2014, 157, 23–34. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IFNG | 5′- TTGAATGGCAGCTCTGAGAAAC | 5′- TCTCTTCCGCTTTCTGAGGTTAGA |
| IL-10 | 5′- AAGGTGAAGAGAGTCTTCAGTGAGC | 5′- TGCATCTTCGTTGTCATGTAGG |
| TNFA | 5′- TGACGGGCTTTACCTCATCT | 5′- TGATGGCAGACAGGATGTTG |
| GAPDH | 5′- GGC GTG AAC CAC GAG AAG TAT AA | 5′- CCC TCC ACG ATG CCA AAG T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalalai, T.; Srinontong, P.; Aengwanich, W.; Srisila, K.; Promkrathok, S.; Sununta, M.; Saraphol, B.; Wu, Z. Impact of Burdizzo and Surgical Castration on Immune and Oxidative Stress Markers in Cattle. Vet. Sci. 2025, 12, 537. https://doi.org/10.3390/vetsci12060537
Chalalai T, Srinontong P, Aengwanich W, Srisila K, Promkrathok S, Sununta M, Saraphol B, Wu Z. Impact of Burdizzo and Surgical Castration on Immune and Oxidative Stress Markers in Cattle. Veterinary Sciences. 2025; 12(6):537. https://doi.org/10.3390/vetsci12060537
Chicago/Turabian StyleChalalai, Thanyakorn, Piyarat Srinontong, Worapol Aengwanich, Kanticha Srisila, Sudarat Promkrathok, Mookdawan Sununta, Bhuripit Saraphol, and Zhiliang Wu. 2025. "Impact of Burdizzo and Surgical Castration on Immune and Oxidative Stress Markers in Cattle" Veterinary Sciences 12, no. 6: 537. https://doi.org/10.3390/vetsci12060537
APA StyleChalalai, T., Srinontong, P., Aengwanich, W., Srisila, K., Promkrathok, S., Sununta, M., Saraphol, B., & Wu, Z. (2025). Impact of Burdizzo and Surgical Castration on Immune and Oxidative Stress Markers in Cattle. Veterinary Sciences, 12(6), 537. https://doi.org/10.3390/vetsci12060537






