Study on the Correlation Between Aggressive Behavior and Gut Microbiota and Serum Serotonin (5-HT) in Working Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
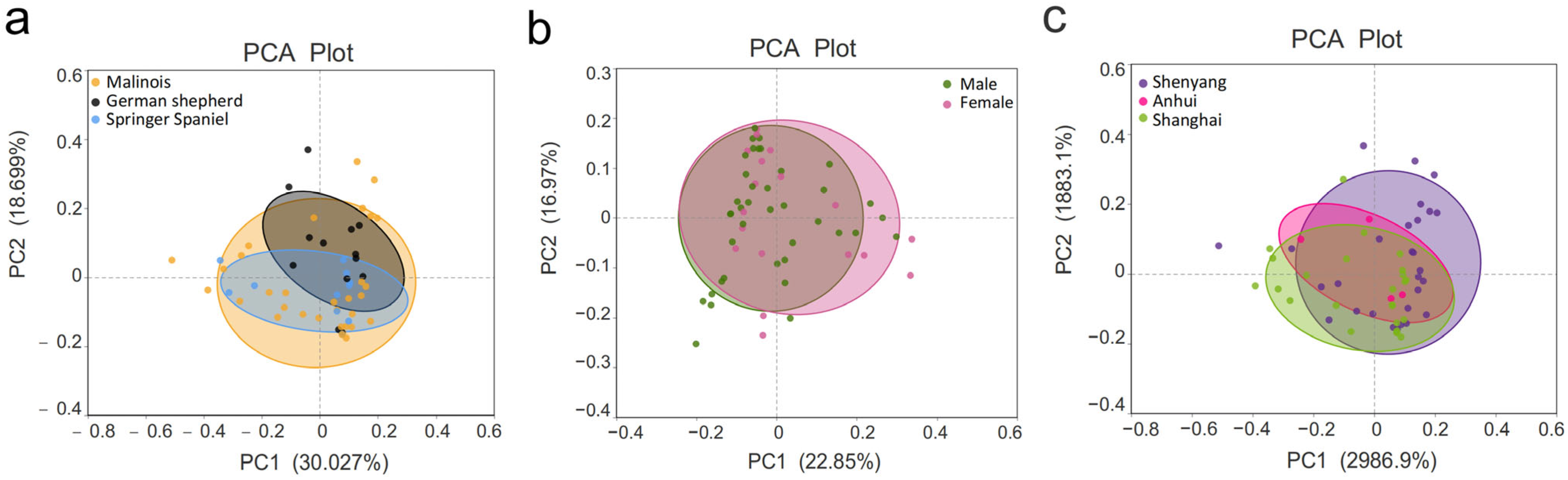
2.1. Animals
2.2. Assessment of Aggressive Behavior
2.3. Sample Collection
2.4. Extraction of Microbial Genomic DNA
2.5. PCR Amplification
2.6. Library Quality Control, Pooling, and High-Throughput Sequencing
2.7. 5-HT Measurement
2.8. Data and Statistical Analysis
3. Results
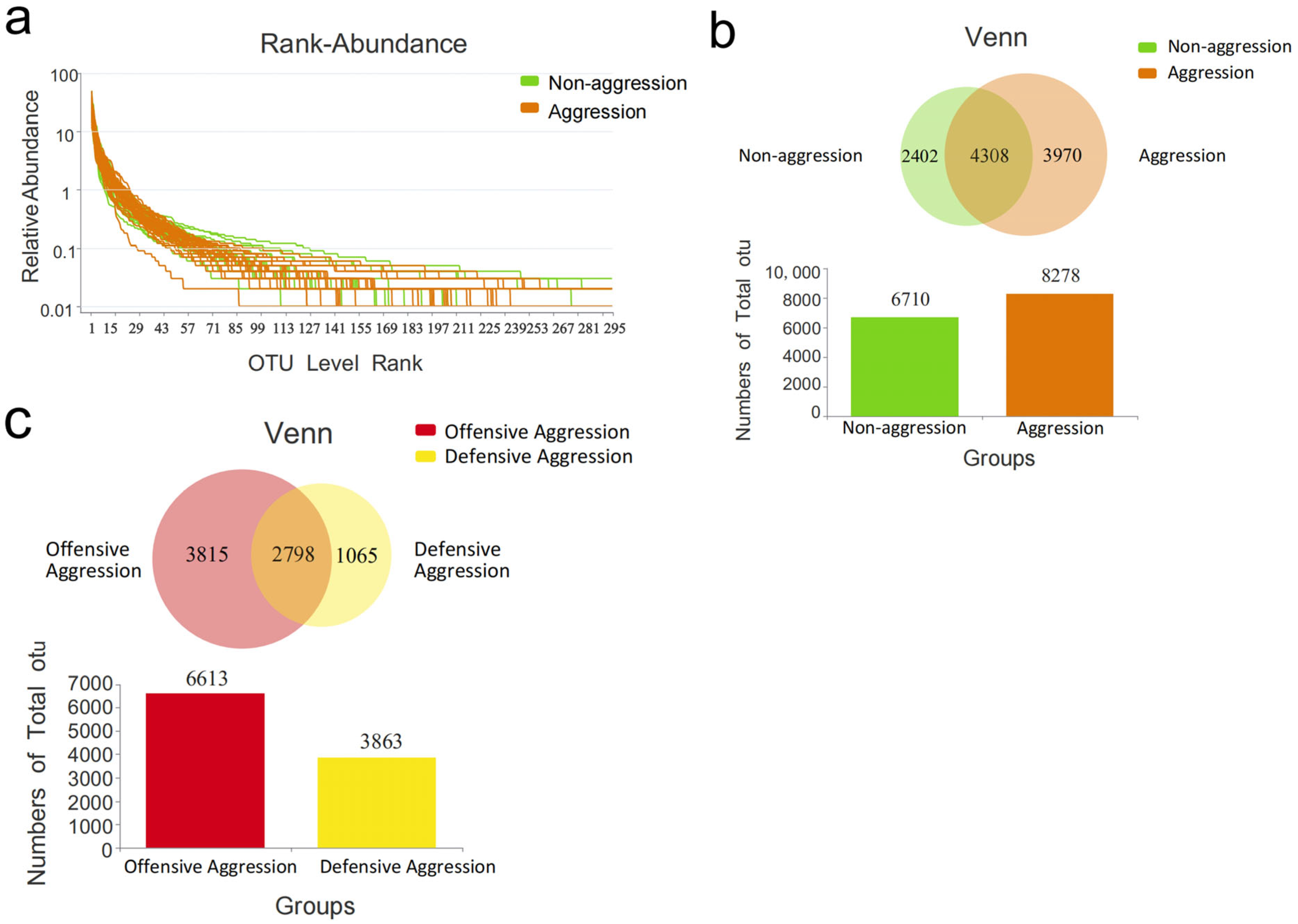
3.1. Sample Sequencing Data
3.2. α-Diversity Analysis
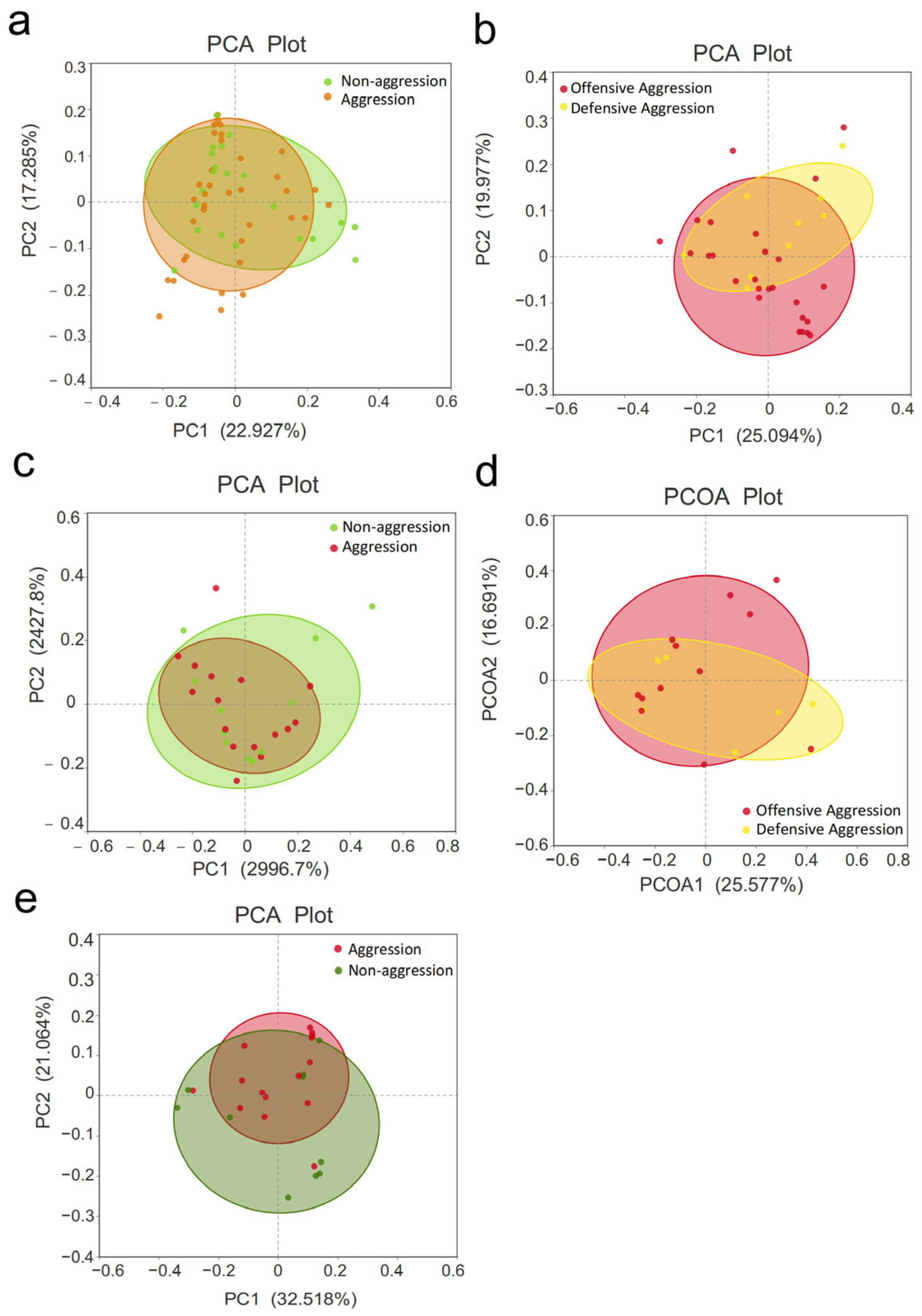
3.3. β-Diversity Analysis
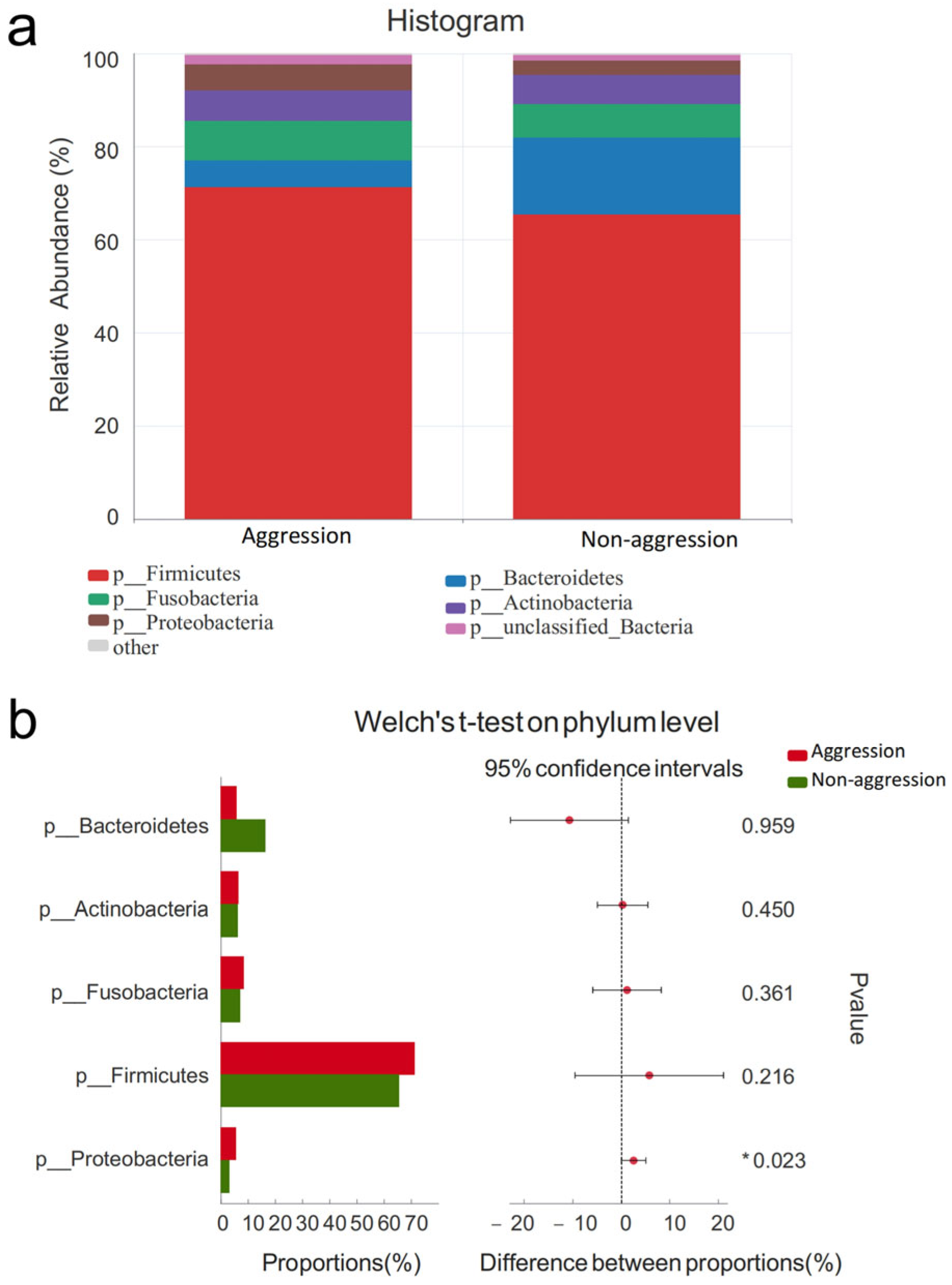
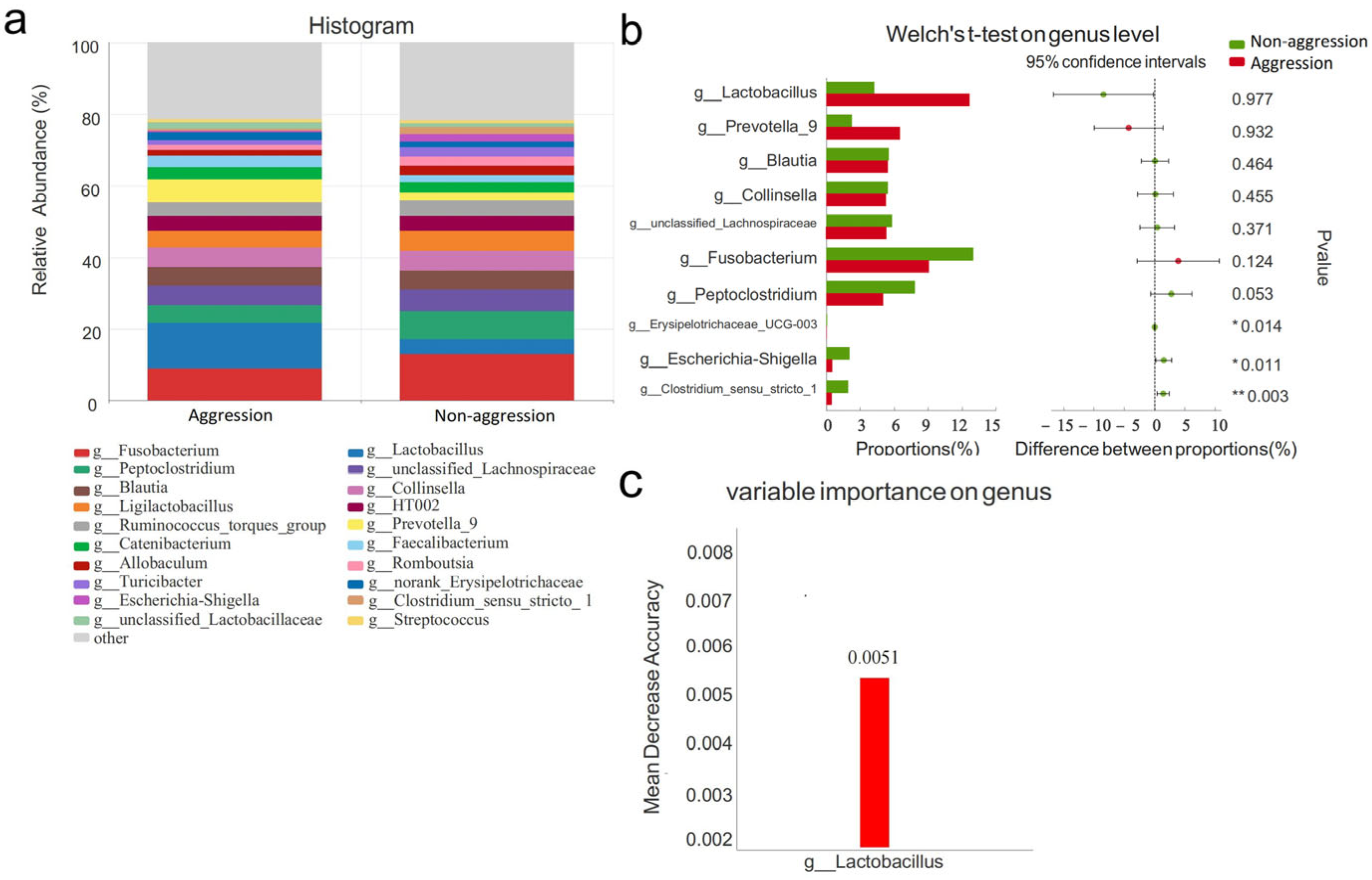
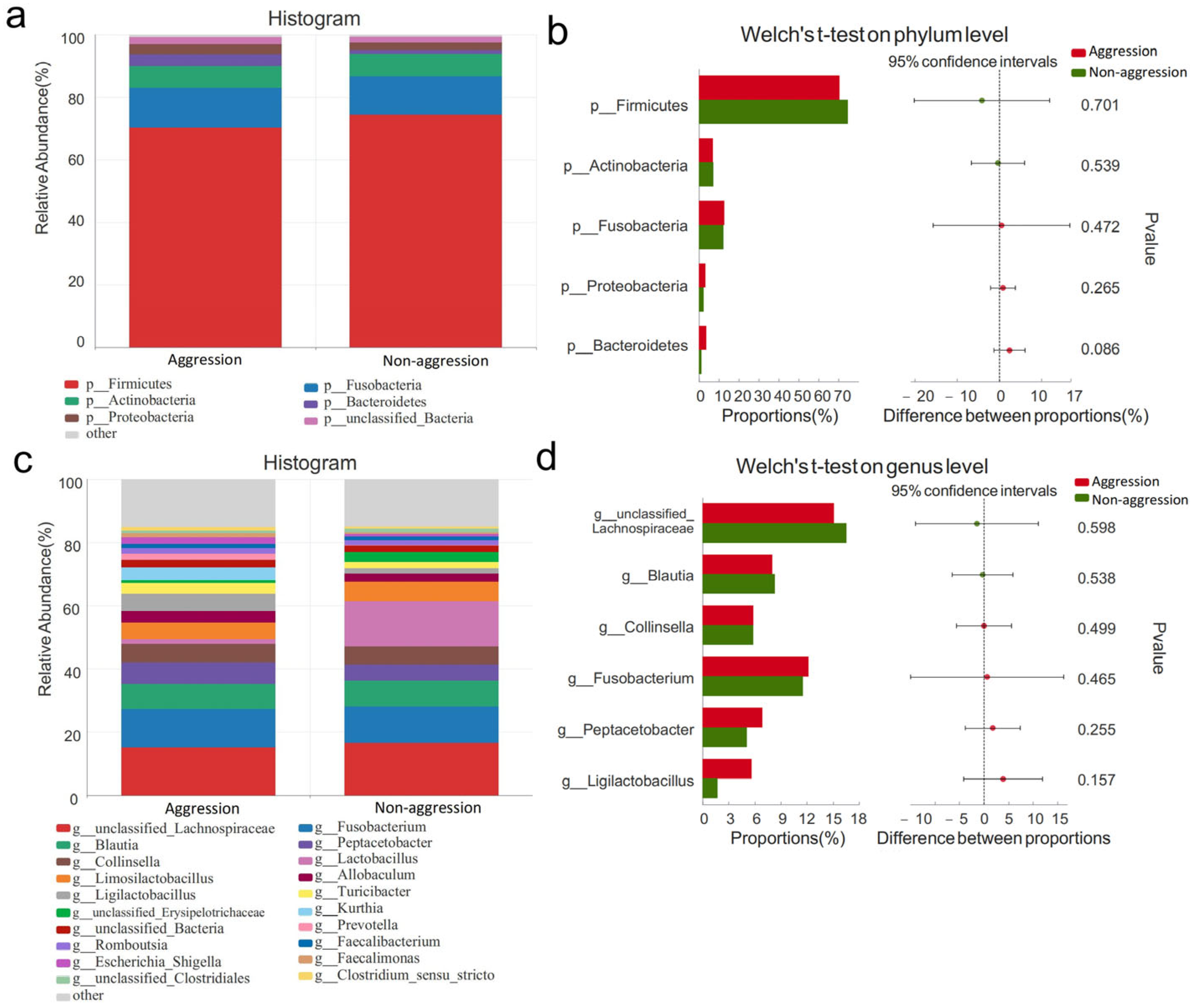
3.4. Composition of Gut Microbiota
3.4.1. Comparison Between Aggressive and Non-Aggressive Groups
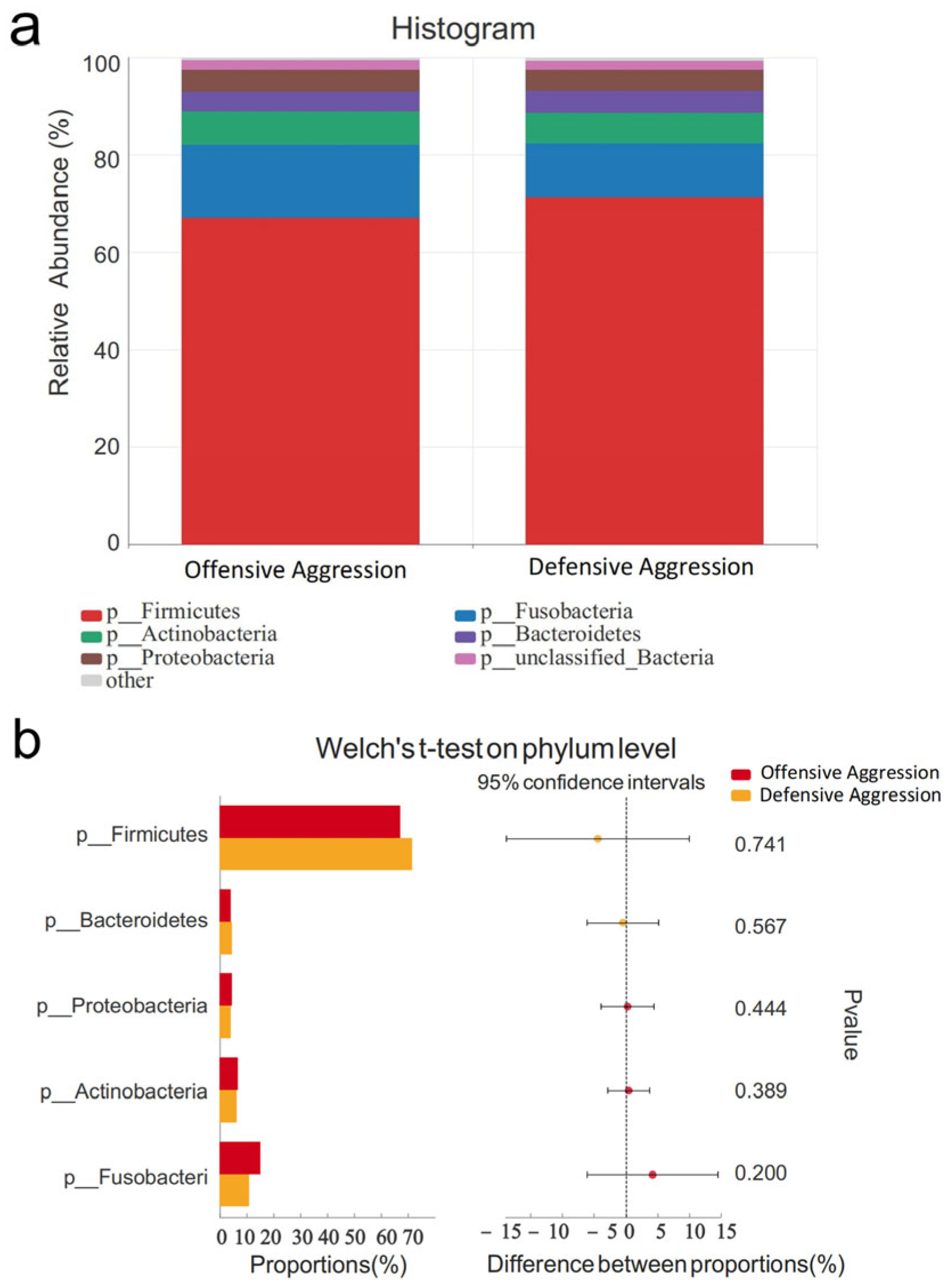
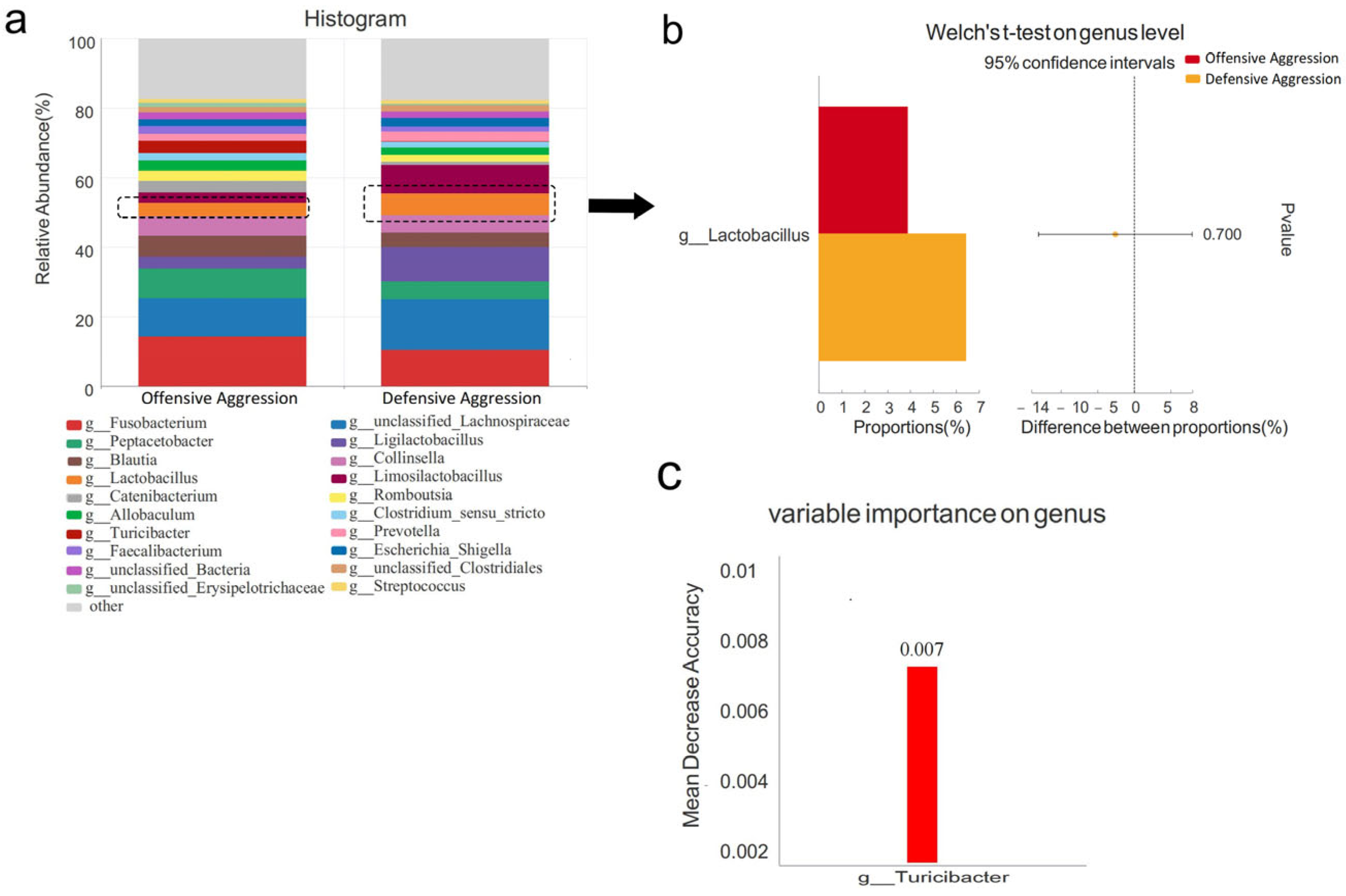
3.4.2. Comparison Between Offensive and Defensive Aggression Groups
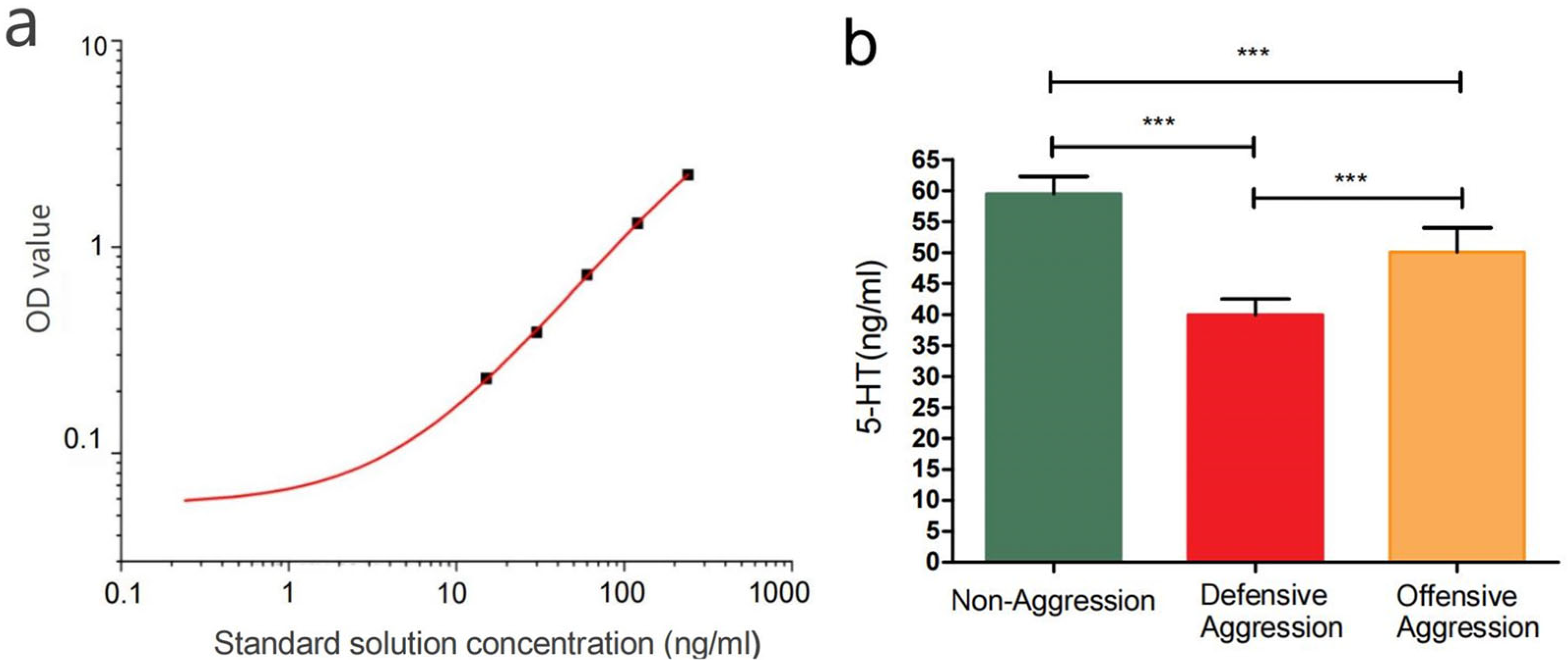
3.5. 5-HT Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frantz, L.A.F.; Mullin, V.E.; Pionnier-Capitan, M.; Lebrasseur, O.; Ollivier, M.; Perri, A.; Linderholm, A.; Mattiangeli, V.; Teasdale, M.D.; Dimopoulos, E.A.; et al. Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 2016, 352, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Mariti, C.; Ricci, E.; Carlone, B.; Moore, J.L.; Sighieri, C.; Gazzano, A. Dog attachment to man: A comparison between pet and working dogs. J. Vet. Behav. 2013, 8, 135–145. [Google Scholar] [CrossRef]
- Lord, K.; Schneider, R.; Coppinger, R. Evolution of Working Dogs. In The Domestic Dog: Its Evolution, Behaviour, and Interactions with People; Cambridge University Press: Cambridge, UK, 2016; pp. 42–66. [Google Scholar]
- VonHoldt, B.M.; DeCandia, A.L.; Heppenheimer, E.; Janowitz-Koch, I.; Shi, R.; Zhou, H.; German, C.A.; Brzeski, K.E.; Cassidy, K.A.; Stahler, D.R.; et al. Heritability of interpack aggression in a wild pedigreed population of North American grey wolves. Mol. Ecol. 2020, 29, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Fatjo, J.; Amat, M.; Mariotti, V.M.; de la Torre, J.L.R.; Manteca, X. Analysis of 1040 cases of canine aggression in a referral practice in Spain. J. Vet. Behav. 2007, 2, 158–165. [Google Scholar] [CrossRef]
- Palacio, J.; León, M.; García-Belenguer, S. Aspectos epidemiológicos de las mordeduras caninas. Gac. Sanit. 2005, 19, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Moxon, R.; Whiteside, H.; England, G.C.W. Incidence and impact of dog attacks on guide dogs in the UK: An update. Vet. Rec. 2016, 178, 367. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xiaowei, Z.; Chuanlin, W.; Wei, L. Investigation of posttraumatic stress disorder in children after animal-induced injury in China. Pediatrics 2010, 126, e320–e324. [Google Scholar] [CrossRef]
- Salman, M.D.; Hutchison, J.; Ruch-Gallie, R.; Kogan, L.; New, J.C., Jr.; Kass, P.H.; Scarlett, J.M. Behavioral Reasons for Relinquishment of Dogs and Cats to 12 Shelters. J. Appl. Anim. Welf. Sci. 2000, 3, 93–106. [Google Scholar] [CrossRef]
- Francesco Ferrari, P.; Palanza, P.; Parmigiani, S.; de Almeida, R.M.M.; Miczek, K.A. Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. Eur. J. Pharmacol. 2005, 526, 259–273. [Google Scholar] [CrossRef]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacon, G.; Villegas, A.; Palacio, J. Assessment of serotonin in serum, plasma, and platelets of aggressive dogs. J. Vet. Behav. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Reisner, I.R.; Mann, J.J.; Stanley, M.; Huang, Y.-Y.; Houpt, K.A. Comparison of cerebrospinal fluid monoamine metabolite levels in dominant-aggressive and non-aggressive dogs. Brain Res. 1996, 714, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha-Bang, S.; Knudsen, G.M. The Modulatory Role of Serotonin on Human Impulsive Aggression. Biol. Psychiatry 2021, 90, 447–457. [Google Scholar] [CrossRef]
- Çakiroǧlu, D.; Meral, Y.; Sancak, A.A.; Çifti, G. Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet. Rec. 2007, 161, 59–61. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Neuropods. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 739–747. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef]
- Nagpal, J.; Cryan, J.F. Microbiota-brain interactions: Moving toward mechanisms in model organisms. Neuron 2021, 109, 3930–3953. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Garcia-Reyero, N.; Martyniuk, C.J.; Tubbs, C.W.; Bisesi, J.H., Jr. Regulation of endocrine systems by the microbiome: Perspectives from comparative animal models. Gen. Comp. Endocrinol. 2020, 292, 113437. [Google Scholar] [CrossRef]
- Foxx, C.L.; Heinze, J.D.; González, A.; Vargas, F.; Baratta, M.V.; Elsayed, A.I.; Stewart, J.R.; Loupy, K.M.; Arnold, M.R.; Flux, M.C.; et al. Effects of Immunization with the Soil-Derived Bacterium Mycobacterium vaccae on Stress Coping Behaviors and Cognitive Performance in a “Two Hit” Stressor Model. Front. Physiol. 2020, 11, 524833. [Google Scholar] [CrossRef]
- Ren, C.C.; Sylvia, K.E.; Munley, K.M.; Deyoe, J.E.; Henderson, S.G.; Vu, M.P.; Demas, G.E. Photoperiod modulates the gut microbiome and aggressive behavior in Siberian hamsters. J. Exp. Biol. 2020, 223 Pt 3, jeb212548. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Johnson, T.A.; Zhang, H.; Cheng, H.-W. The Microbiota-Gut-Brain Axis: Gut Microbiota Modulates Conspecific Aggression in Diversely Selected Laying Hens. Microorganisms 2022, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Craddock, H.A.; Godneva, A.; Rothschild, D.; Motro, Y.; Grinstein, D.; Lotem-Michaeli, Y.; Narkiss, T.; Segal, E.; Moran-Gilad, J. Phenotypic correlates of the working dog microbiome. NPJ Biofilms Microbiomes 2022, 8, 66. [Google Scholar] [CrossRef]
- Mondo, E.; Barone, M.; Soverini, M.; D’Amico, F.; Cocchi, M.; Petrulli, C.; Mattioli, M.; Marliani, G.; Candela, M.; Accorsi, P. Gut microbiome structure and adrenocortical activity in dogs with aggressive and phobic behavioral disorders. Heliyon 2020, 6, e3311. [Google Scholar] [CrossRef]
- Kirchoff, N.S.; Udell, M.A.R.; Sharpton, T.J. The gut microbiome correlates with conspecific aggression in a small population of rescued dogs (Canis familiaris). PeerJ 2019, 7, e6103. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.S.V.; Faresjö, Å.; Theodorsson, E.; Jensen, P. Hair cortisol varies with season and lifestyle and relates to human interactions in German shepherd dogs. Sci. Rep. 2016, 6, 19631. [Google Scholar] [CrossRef]
- Rooney, N.J.; Clark, C.C.A.; Casey, R.A. Minimizing fear and anxiety in working dogs: A review. J. Vet. Behav. 2016, 16, 53–64. [Google Scholar] [CrossRef]
- Pellowe, S.; Zhang, A.; Bignell, D.; Peña-Castillo, L.; Walsh, C.J. Gut microbiome composition is related to anxiety and aggression score in companion dogs. Res. Sq. [Preprint]. 2023. [Google Scholar] [CrossRef]
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczyńska, M.; Górecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef]
- Gazit, I.; Goldblatt, A.; Grinstein, D.; Terkel, J. Dogs can detect the individual odors in a mixture of explosives. Appl. Anim. Behav. Sci. 2021, 235, 105212. [Google Scholar] [CrossRef]
- Oesterhelweg, L.; Kröber, S.; Rottmann, K.; Willhöft, J.; Braun, C.; Thies, N.; Püschel, K.; Silkenath, J.; Gehl, A. Cadaver dogs—A study on detection of contaminated carpet squares. Forensic Sci. Int. 2008, 174, 35–39. [Google Scholar]
- Novák, K.; Helena, C.; Václav, B.; Ivona, S.; Marek, K. Factors affecting locomotor activity of search and rescue dogs: The importance of terrain, vegetation and dog certification. Appl. Anim. Behav. Sci. 2022, 253, 105674. [Google Scholar] [CrossRef]
- Gao, C.Q.; Wang, S.N.; Wang, M.M.; Li, J.J.; Qiao, J.J.; Huang, J.J.; Zhang, X.X.; Xiang, Y.Q.; Xu, Q.; Wang, J.L.; et al. Sensitivity of Sniffer Dogs for a Diagnosis of Parkinson’s Disease: A Diagnostic Accuracy Study. Mov. Disord. 2022, 37, 1807–1816. [Google Scholar] [CrossRef]
- Hare, E.; Kelsey, K.M.; Serpell, J.A.; Otto, C.M. Behavior Differences Between Search-and-Rescue and Pet Dogs. Front. Vet. Sci. 2018, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Haverbeke, A.; De Smet, A.; Depiereux, E.; Giffroy, J.-M.; Diederich, C. Assessing undesired aggression in military working dogs. Appl. Anim. Behav. Sci. 2009, 117, 55–62. [Google Scholar] [CrossRef]
- Goddard, M.E.; Beilharz, R.G. A multivariate analysis of the genetics of fearfulness in potential guide dogs. Behav. Genet. 1985, 15, 69–89. [Google Scholar] [CrossRef]
- Hsu, Y.; Serpell, J.A. Development and validation of a questionnaire for measuring behavior and temperament traits in pet dogs. J. Am. Vet. Med. Assoc. 2003, 223, 1293–1300. [Google Scholar] [CrossRef]
- Zapata, I.; Lilly, M.L.; Herron, M.E.; Serpell, J.A.; Alvarez, C.E. Genetic testing of dogs predicts problem behaviors in clinical and nonclinical samples. BMC Genom. 2022, 23, 102. [Google Scholar] [CrossRef]
- Vilson, Å.; Ramadan, Z.; Li, Q.; Hedhammar, Å.; Reynolds, A.; Spears, J.; Labuda, J.; Pelker, R.; Björkstén, B.; Dicksved, J.; et al. Disentangling factors that shape the gut microbiota in German Shepherd dogs. PLoS ONE 2018, 13, e193507. [Google Scholar]
- Omatsu, T.; Omura, M.; Katayama, Y.; Kimura, T.; Okumura, M.; Okumura, A.; Murata, Y.; Mizutani, T. Molecular diversity of the faecal microbiota of Toy Poodles in Japan. J. Vet. Med. Sci. 2018, 80, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e201279. [Google Scholar] [CrossRef]
- Sylvia, K.E.; Jewell, C.P.; Rendon, N.M.; John, E.A.S.; Demas, G.E. Sex-specific modulation of the gut microbiome and behavior in Siberian hamsters. Brain Behav. Immun. 2017, 60, 51–62. [Google Scholar] [CrossRef]
- Pasquaretta, C.; Gómez-Moracho, T.; Heeb, P.; Lihoreau, M. Exploring Interactions between the Gut Microbiota and Social Behavior through Nutrition. Genes 2018, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- Raulo, A.; Allen, B.E.; Troitsky, T.; Husby, A.; A Firth, J.; Coulson, T.; Knowles, S.C.L. Social networks strongly predict the gut microbiota of wild mice. ISME J. 2021, 15, 2601–2613. [Google Scholar] [CrossRef]
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology 2022, 11, 976. [Google Scholar] [CrossRef]
- Homer, B.; Judd, J.; Dehcheshmeh, M.M.; Ebrahimie, E.; Trott, D.J. Gut Microbiota and Behavioural Issues in Production, Performance, and Companion Animals: A Systematic Review. Animals 2023, 13, 1458. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2019, 6, 498. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dhaka, P.; Vijay, D.; Vergis, J.; Mohan, V.; Kumar, A.; Kurkure, N.V.; Barbuddhe, S.B.; Malik, S.; Rawool, D.B. Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative Escherichia coli. Int. J. Antimicrob. Agents 2016, 48, 265–270. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Cheng, L.; Wu, Y.; Wu, T.; Wen, J.; Huang, D.; Liao, Z.; Tan, C.; Luo, Y.; et al. Gut microbiota dysbiosis characterized by abnormal elevation of Lactobacillus in patients with immune-mediated necrotizing myopathy. Front. Cell. Infect. Microbiol. 2023, 13, 1243512. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC1–KC5. [Google Scholar] [CrossRef]
- Lockwood, R. Ethology, ecology and epidemiology of canine aggression. In The Domestic Dog: Its Evolution, Behavior, and Interactions with People; Cambridge University Press: Cambridge, UK, 2016; pp. 160–181. [Google Scholar]
- Coria-Avila, G.; Manzo, J.; Beltran-Parrazal, L.; Garcia, L.I. Agresión. In Neurofisiología de la Conducta; Universidad Veracruzana: Xalapa, Mexico, 2015; pp. 317–348. [Google Scholar]
- Hammond, A.; Rowland, T.; Mills, D.S.; Pilot, M. Comparison of behavioural tendencies between “dangerous dogs” and other domestic dog breeds—Evolutionary context and practical implications. Evol. Appl. 2022, 15, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Hemsworth, P.H.; Coleman, G.J. Fear of novel and startling stimuli in domestic dogs. Appl. Anim. Behav. Sci. 2003, 82, 45–64. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Takahashi, A.; Quadros, I.M.; de Almeida, R.M.M.; Miczek, K.A. Brain serotonin receptors and transporters: Initiation vs. termination of escalated aggression. Psychopharmacology 2011, 213, 183–212. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol. Behav. 2012, 105, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rosado, B.; García-Belenguer, S.; León, M.; Chacón, G.; Villegas, A.; Palacio, J. Blood concentrations of serotonin, cortisol and dehydroepiandrosterone in aggressive dogs. Appl. Anim. Behav. Sci. 2010, 123, 124–130. [Google Scholar] [CrossRef]
- Alberghina, D.; Rizzo, M.; Piccione, G.; Giannetto, C.; Panzera, M. An exploratory study about the association between serum serotonin concentrations and canine-human social interactions in shelter dogs (Canis familiaris). J. Vet. Behav. 2017, 18, 96–101. [Google Scholar] [CrossRef]
- van den Berg, L.; Kwant, L.; Hestand, M.S.; van Oost, B.A.; Leegwater, P.A.J. Structure and variation of three canine genes involved in serotonin binding and transport: The serotonin receptor 1A gene (htr1A), serotonin receptor 2A gene (htr2A), and serotonin transporter gene (slc6A4). J. Hered. 2005, 96, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Corchs, F.; Nutt, D.J.; Hince, D.A.; Davies, S.J.; Bernik, M.; Hood, S.D. Evidence for serotonin function as a neurochemical difference between fear and anxiety disorders in humans? J. Psychopharmacol. 2015, 29, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, D.S.; Pinard, G.; Zuroff, D.C.; Annable, L.; Young, S.N. The effect of tryptophan on social interaction in everyday life: A placebo-controlled study. Neuropsychopharmacology 2001, 25, 277–289. [Google Scholar] [CrossRef]
- Den Boer, J.A.; Bosker, F.J.; Slaap, B.R. Serotonergic drugs in the treatment of depressive and anxiety disorders. Hum. Psychopharmacol. 2000, 15, 315–336. [Google Scholar] [CrossRef]
- Ibáñez, M.; Anzola, B. Use of fluoxetine, diazepam, and behavior modification as therapy for treatment of anxiety-related disorders in dogs. J. Vet. Behav. 2009, 4, 223–229. [Google Scholar] [CrossRef]
| Sample-Id | Sex (Male/Female) | Age (Years) | Birth | Breed | Dog Aggression (√/×) | Offensive Aggression/Defensive Aggression (OA/DA) | Region | Diet |
|---|---|---|---|---|---|---|---|---|
| A1 | M | 4.4 | 1 November 2020 | Malinois | √ | DA | Shenyang | Pedigree Innovet |
| A2 | M | 4.4 | 1 November 2020 | Malinois | √ | DA | ||
| A3 | M | 4.4 | 1 November 2020 | Malinois | √ | OA | ||
| A4 | M | 4.2 | 20 December 2020 | German shepherd | √ | OA | ||
| A5 | M | 4.2 | 20 December 2020 | German shepherd | √ | OA | ||
| A6 | M | 3.8 | 13 June 2021 | Malinois | √ | OA | ||
| A7 | M | 3.8 | 13 June 2021 | Malinois | √ | OA | ||
| A8 | M | 3.8 | 13 June 2021 | Malinois | √ | OA | ||
| A9 | F | 3.6 | 23 August 2021 | German shepherd | × | \ | ||
| A10 | F | 3.6 | 23 August 2021 | German shepherd | √ | DA | ||
| A11 | F | 3.1 | 16 February 2022 | Malinois | × | \ | ||
| A12 | M | 4.0 | 27 March 2021 | Malinois | √ | OA | ||
| A13 | M | 3.8 | 9 June 2021 | Malinois | √ | OA | ||
| A14 | M | 4.2 | 14 January 2021 | Malinois | × | \ | ||
| A15 | F | 4.4 | 1 November 2020 | Malinois | √ | DA | ||
| A16 | M | 4.4 | 1 November 2020 | Malinois | √ | DA | ||
| A17 | M | 4.4 | 1 November 2020 | Malinois | √ | OA | ||
| A18 | M | 4.4 | 1 November 2020 | Malinois | √ | OA | ||
| A19 | M | 4.2 | 20 December 2020 | German shepherd | √ | DA | ||
| A20 | M | 4.2 | 20 December 2020 | German shepherd | √ | OA | ||
| A21 | F | 3.5 | 27 September 2021 | German shepherd | √ | OA | ||
| A22 | F | 3.8 | 9 June 2021 | Malinois | × | \ | ||
| A23 | F | 3.8 | 9 June 2021 | Malinois | × | \ | ||
| A24 | M | 4.2 | 14 January 2021 | Malinois | × | \ | ||
| A25 | F | 3.5 | 27 September 2021 | German shepherd | × | \ | ||
| A26 | M | 4.2 | 14 January 2021 | Malinois | × | \ | ||
| A27 | F | 4.2 | 14 January 2021 | Malinois | × | \ | ||
| A28 | F | 3.1 | 16 February 2022 | Malinois | × | \ | ||
| B4 | M | 5.5 | 1 October 2019 | Malinois | × | \ | Anhui | Pets Guard |
| B1 | M | 3.9 | 1 May 2021 | Malinois | √ | DA | ||
| B2 | M | 3.9 | 1 May 2021 | Malinois | √ | OA | ||
| B3 | M | 6.6 | 1 September 2018 | Malinois | √ | OA | ||
| C1 | M | 9.4 | 11 November 2015 | Malinois | √ | OA | Shanghai | Pro Plan |
| C2 | M | 4.9 | 10 May 2020 | German shepherd | √ | OA | ||
| C3 | F | 12.8 | 11 June 2012 | German shepherd | √ | OA | ||
| C4 | M | 9.1 | 17 February 2016 | Springer Spaniel | √ | OA | ||
| C5 | F | 2.2 | 7 January 2023 | Malinois | √ | OA | ||
| C6 | M | 5.0 | 24 March 2020 | Malinois | √ | DA | ||
| C7 | M | 3.9 | 20 April 2021 | Malinois | √ | DA | ||
| C8 | M | 5.4 | 27 October 2019 | German shepherd | √ | OA | ||
| C9 | M | 7.6 | 1 September 2017 | Malinois | √ | OA | ||
| C10 | M | 9.6 | 22 August 2015 | Springer Spaniel | √ | OA | ||
| C11 | M | 9.6 | 22 August 2015 | Springer Spaniel | √ | OA | ||
| C13 | F | 4.8 | 5 June 2020 | German shepherd | √ | OA | ||
| C14 | F | 6.3 | 13 December 2018 | Malinois | √ | OA | ||
| C15 | M | 6.3 | 2 December 2018 | German shepherd | √ | OA | ||
| C16 | M | 7.1 | 28 January 2018 | Springer Spaniel | × | \ | ||
| C17 | F | 2.2 | 7 January 2023 | Malinois | × | \ | ||
| C18 | M | 7.1 | 28 January 2018 | Springer Spaniel | × | \ | ||
| C19 | F | 2.2 | 7 January 2023 | Malinois | × | \ | ||
| C20 | M | 5.5 | 18 September 2019 | Springer Spaniel | × | \ | ||
| C21 | F | 6.3 | 12 December 2018 | Springer Spaniel | × | \ | ||
| C22 | F | 2.7 | 27 June 2022 | Springer Spaniel | × | \ | ||
| C23 | M | 2.7 | 27 June 2022 | Springer Spaniel | × | \ | ||
| C24 | M | 2.7 | 27 June 2022 | Springer Spaniel | × | \ | ||
| C25 | M | 7.1 | 28 January 2018 | Springer Spaniel | × | \ |
| Content (%) | Crude Protein | Crude Fat | Crude Fiber | Crude Ash | Calcium | Total Phosphorus | Lysine | |
|---|---|---|---|---|---|---|---|---|
| Dog Food | ||||||||
| Pro Plan | ≥31% | ≥20% | ≤3% | ≤10% | ≥1% | ≥0.8% | ≥1.2% | |
| Pedigree Innovet | ≥24% | ≥14.5% | ≤5% | ≤10% | \ | ≥0.6% | \ | |
| Pets Guard | ≥32% | ≥18% | ≤4% | ≤10% | ≥1.2% | ≥1.0% | ≥1.2% | |
| A. Aggression Toward Familiar People A1. Does your dog exhibit aggressive behavior when verbally corrected or punished (e.g., scolded, yelled at) by you or your colleagues? A2. Does your dog exhibit aggressive behavior when being bathed or groomed by your colleagues? A3. Does your dog exhibit aggressive behavior when stared at directly by a colleague? A4. Does your dog exhibit aggressive behavior when a colleague steps over it? |
| B. Aggression Toward Strangers B1. Does your dog exhibit aggressive behavior when an unfamiliar adult approaches it directly while it is being walked or exercised on a leash? B2. Does your dog exhibit aggressive behavior when an unfamiliar child approaches it directly while it is being walked or exercised on a leash? B3. Does your dog exhibit aggressive behavior when strangers approach you or your colleagues outside the team? B4. Does your dog exhibit aggressive behavior when strangers approach its kennel? B5. Does your dog exhibit aggressive behavior when strangers attempt to touch or pet it? B6. Does your dog exhibit aggressive behavior when encountering joggers, cyclists, rollerbladers, or skateboarders during a walk? |
| C. Aggression Toward Familiar Dogs C1. Does your dog exhibit aggressive behavior when another familiar dog is in its kennel? C2. Does your dog exhibit aggressive behavior when another familiar dog approaches its favorite resting/sleeping spot? C3. Does your dog exhibit aggressive behavior when approached by another familiar dog while eating? C4. Does your dog exhibit aggressive behavior when another familiar dog approaches it while it is playing with or chewing its favorite toy, bone, or object? |
| D. Aggression Toward Unfamiliar Dogs D1. Does your dog exhibit aggressive behavior when an unfamiliar male dog approaches it while it is being walked or trained on a leash? D2. Does your dog exhibit aggressive behavior when an unfamiliar female dog approaches it while it is being walked or trained on a leash? D3. Does your dog exhibit aggressive behavior when an unfamiliar dog enters its territory? D4. Does your dog exhibit aggressive behavior when barked at, growled at, or lunged at by other unfamiliar dogs? |
| E. Food or Object-Related Aggression E1. Does your dog exhibit aggressive behavior when a colleague takes away its toy, bone, or other objects? E2. Does your dog exhibit aggressive behavior when approached by a colleague while eating? E3. Does your dog exhibit aggressive behavior when a colleague removes its food? E4. Does your dog exhibit aggressive behavior when you or a colleague retrieves food or objects it has stolen? |
| F. Territory-Related Aggression F1. Does your dog exhibit aggressive behavior toward strangers approaching it while it is in a car (e.g., at a gas station)? F2. Does your dog exhibit aggressive behavior when a stranger approaches you or your colleagues within the team? F3. Does your dog exhibit aggressive behavior when strangers walk past its kennel? F4. Does your dog exhibit aggressive behavior when cats, birds, or other animals enter its kennel? F5. Does your dog exhibit aggressive behavior when unfamiliar people are in its kennel? |
| G. Pain or Fear-Related Aggression G1. Does your dog exhibit aggressive behavior when injured and sent for treatment? G2. Does your dog exhibit aggressive behavior when it feels afraid? G3. Does your dog exhibit aggressive behavior when forced to do something? |
| Sample | No. of OTUs | No. of Unique OTUs | Ace | Chao | Shannon | Simpson | Coverage% | |
|---|---|---|---|---|---|---|---|---|
| Non-aggression | 6710 A | 2402 A | 1326.17 A | 1232.45 A | 3.41 A | 0.11 A | 99% | |
| Aggression | 8278 A | 3970 A | 1363.63 A | 1294.92 A | 3.52 A | 0.09 A | 99% | |
| Offensive Aggression | 6613 A | 3815 A | 1195.71 A | 1110.75 A | 3.49 A | 0.10 A | 99% | |
| Defensive Aggression | 3863 A | 1065 A | 1264.02 A | 1181.87 A | 3.56 A | 0.08 A | 99% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Xie, L.; Chao, J.; Xiu, F.; Zhai, H.; Zhou, Y.; Yu, X.; Shui, Y. Study on the Correlation Between Aggressive Behavior and Gut Microbiota and Serum Serotonin (5-HT) in Working Dogs. Vet. Sci. 2025, 12, 526. https://doi.org/10.3390/vetsci12060526
Sun N, Xie L, Chao J, Xiu F, Zhai H, Zhou Y, Yu X, Shui Y. Study on the Correlation Between Aggressive Behavior and Gut Microbiota and Serum Serotonin (5-HT) in Working Dogs. Veterinary Sciences. 2025; 12(6):526. https://doi.org/10.3390/vetsci12060526
Chicago/Turabian StyleSun, Ning, Liuwei Xie, Jingjing Chao, Fuxiao Xiu, He Zhai, Yuanting Zhou, Xi Yu, and Yingyi Shui. 2025. "Study on the Correlation Between Aggressive Behavior and Gut Microbiota and Serum Serotonin (5-HT) in Working Dogs" Veterinary Sciences 12, no. 6: 526. https://doi.org/10.3390/vetsci12060526
APA StyleSun, N., Xie, L., Chao, J., Xiu, F., Zhai, H., Zhou, Y., Yu, X., & Shui, Y. (2025). Study on the Correlation Between Aggressive Behavior and Gut Microbiota and Serum Serotonin (5-HT) in Working Dogs. Veterinary Sciences, 12(6), 526. https://doi.org/10.3390/vetsci12060526





