Infrared Thermography and Physiological Variables as Methods for Recognizing Fear in Domestic Cats (Felis catus) Using Three Pharmacological Models: Cannabidiol, Gabapentin, and Synthetic Facial Pheromones
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Temporary Housing Conditions Before the Inter-Species Interaction
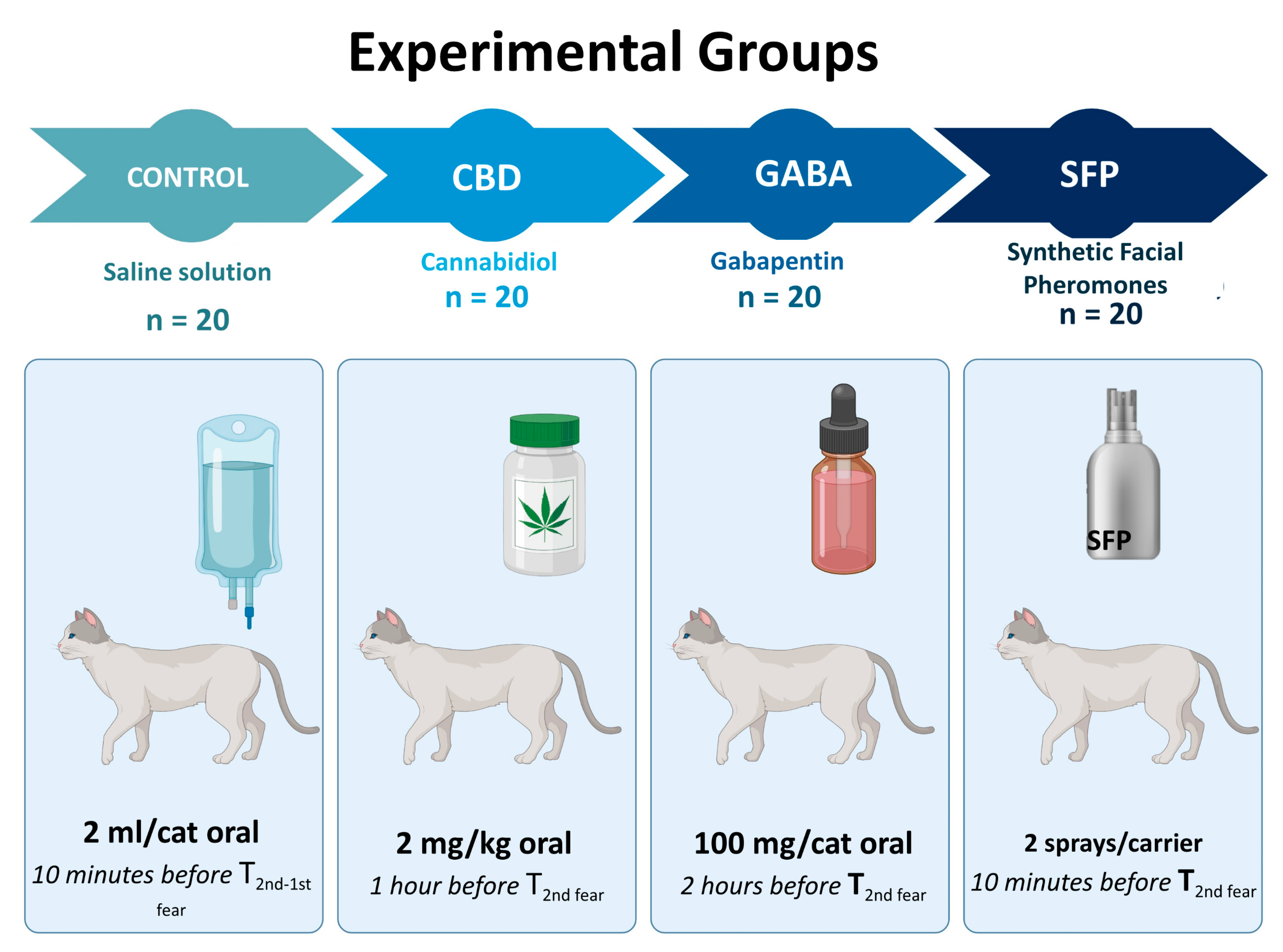
2.3. Treatments
2.4. Evaluation Times
2.5. Assessed Parameters
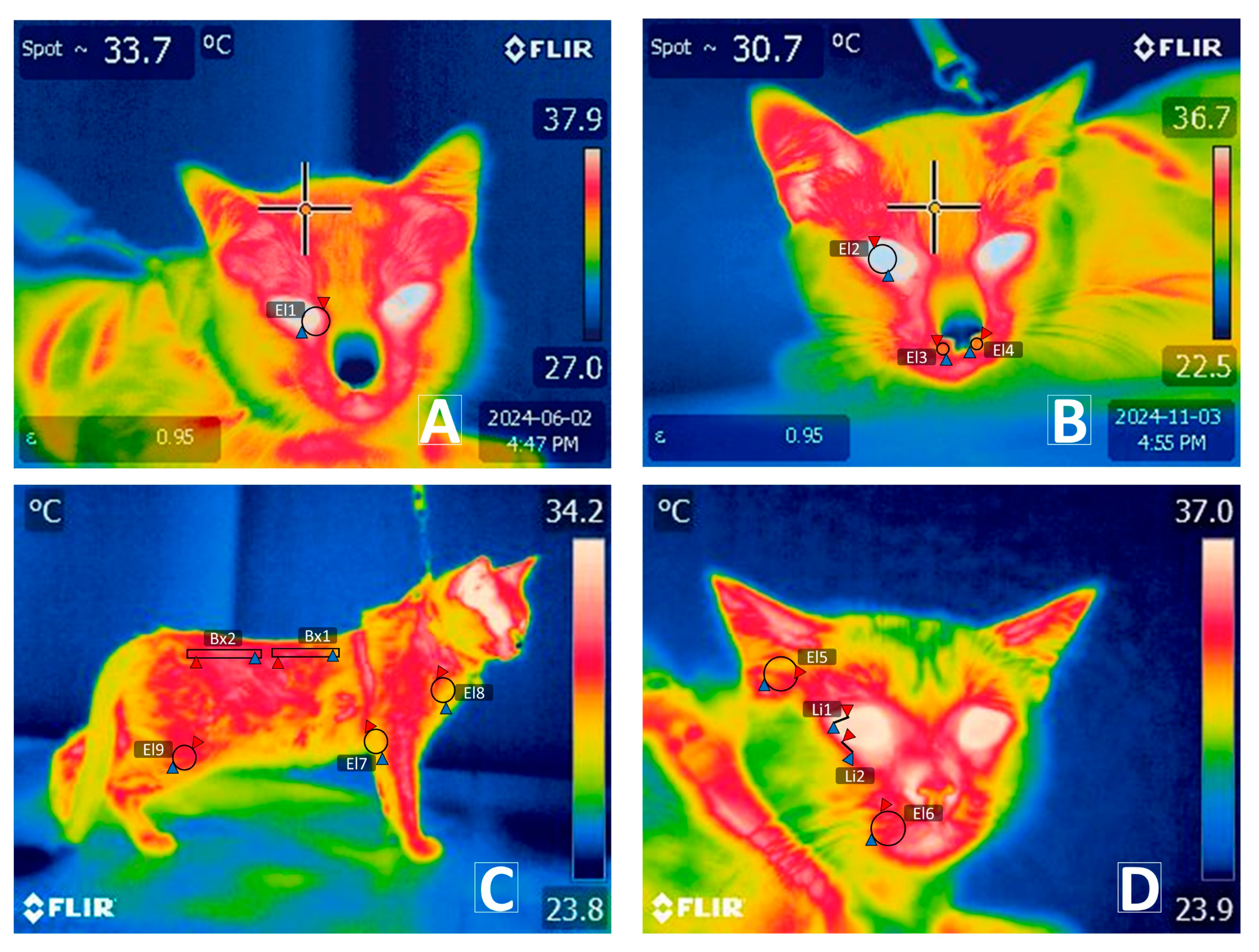
2.5.1. Infrared Thermography
2.5.2. Physiological Parameters
2.6. Procedure Description
2.7. Statistical Analysis
- Yijk = differences of thermal windows (T°leftnostril, T°rigthnostril, T°Uppereyeline, T°Lowereyeline, T°OCU, T°CAR, T°Whisk, T°EAR, T°CHEST, T°lumbar, T°TLE, T°FPL, and T°TLBB) and physiological variables (HR, RR, and T°REC);
- µ = general mean;
- αi = fixed effect (CONTROL, CBD, GABA, and SFP);
- βj = evaluation times (Tbasal−, T1stfear, T1st recovery, Tbasal+, T2ndfear, and T2ndrecovery);
- (αβ)ij = interaction between treatments and evaluation times;
- eijkl = random error.
2.8. Ethical Statement
3. Results
3.1. Upper Thermal Facial Windows
3.2. Lower Thermal Facial Windows
3.3. Dorsal and Appendicular Thermal Windows
3.4. Cardiorespiratory Parameters
4. Discussion
4.1. Effect of the Natural Stimuli
4.2. Drug Effect
4.2.1. Cannabidiol
4.2.2. Gabapentin
4.2.3. Synthetic Facial Pheromones
4.2.4. Limitations and Recommendations for Further Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tortora, G.; Derrickson, B. Principios de Anatomía y Fisiologia, 13th ed.; Editorial Médica Panamericana S.A.: Madrid, Spain, 2013. [Google Scholar]
- Driscoll, C.A.; Macdonald, D.W.; O’Brien, S.J. From Wild Animals to Domestic Pets, an Evolutionary View of Domestication. Proc. Natl. Acad. Sci. USA 2009, 106, 9971–9978. [Google Scholar] [CrossRef] [PubMed]
- Yarto-Jaramillo, E.; Rivero, J.; Çitaku, I. Exotic Animal Practice in Mexico, Central, and South America. Vet. Clin. N. Am. Exot. Anim. Pract. 2024, 27, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Riemer, S.; Heritier, C.; Windschnurer, I.; Pratsch, L.; Arhant, C.; Affenzeller, N. A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting. Animals 2021, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.; Reche-Junior, A.; Hirai, Y.; Mills, D.S. Feline Behaviour Problems in Brazil: A Review of 155 Referral Cases. Vet. Rec. 2020, 186, e9. [Google Scholar] [CrossRef]
- Furgala, N.M.; Moody, C.M.; Flint, H.E.; Gowland, S.; Niel, L. Veterinary Background Noise Elicits Fear Responses in Cats While Freely Moving in a Confined Space and during an Examination. Behav. Processes 2022, 201, 104712. [Google Scholar] [CrossRef]
- Shu, H.; Gu, X. Effect of a Synthetic Feline Facial Pheromone Product on Stress during Transport in Domestic Cats: A Randomised Controlled Pilot Study. J. Feline Med. Surg. 2022, 24, 691–699. [Google Scholar] [CrossRef]
- Pol, F.; Kling-Eveillard, F.; Champigneulle, F.; Fresnay, E.; Ducrocq, M.; Courboulay, V. Human–Animal Relationship Influences Husbandry Practices, Animal Welfare and Productivity in Pig Farming. Animal 2021, 15, 100103. [Google Scholar] [CrossRef]
- Shin, L.M.; Liberzon, I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef]
- Terburg, D.; Scheggia, D.; Triana del Rio, R.; Klumpers, F.; Ciobanu, A.C.; Morgan, B.; Montoya, E.R.; Bos, P.A.; Giobellina, G.; van den Burg, E.H.; et al. The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell 2018, 175, 723–735.e16. [Google Scholar] [CrossRef]
- Caeiro, C.C.; Burrows, A.M.; Waller, B.M. Development and aplication of CatFACS: Are human cat adopters influenced by cat facial expresions? Appl. Anim. Behav. Sci. 2017, 189, 66–78. [Google Scholar] [CrossRef]
- Powell, L.; Watson, B.; Serpell, J. Understanding Feline Feelings: An Investigation of Cat Owners’ Perceptions of Problematic Cat Behaviors. Appl. Anim. Behav. Sci. 2023, 266, 106025. [Google Scholar] [CrossRef]
- Di Salvo, A.; Conti, M.B.; della Rocca, G. Pharmacokinetics, Efficacy, and Safety of Cannabidiol in Dogs: An Update of Current Knowledge. Front. Vet. Sci. 2023, 10, 1204526. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.E.; Flint, H.E.; Hunt, A.B.G.; Ellerby, Z.; King, T. Investigating the Effect a Single Dose of Cannabidiol Has on Measures of Stress in Cats When Being Transported in a Carrier and Meeting a Novel Person in an Unfamiliar Environment. Front. Vet. Sci. 2024, 11, 1476296. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9. [Google Scholar] [CrossRef]
- Alvarenga, I.C.; Panickar, K.S.; Hess, H.; Mcgrath, S. Scientific Validation of Cannabidiol for Management of Dog and Cat Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 227–246. [Google Scholar] [CrossRef]
- Aguiar, D.C.; Almeida-Santos, A.F.; Moreira, F.A.; Guimarães, F.S. Involvement of TRPV1 Channels in the Periaqueductal Grey on the Modulation of Innate Fear Responses. Acta Neuropsychiatr. 2015, 27, 97–105. [Google Scholar] [CrossRef]
- Gomes, F.V.; Reis, D.G.; Alves, F.H.F.; Corrêa, F.M.A.; Guimarães, F.S.; Resstel, L.B.M. Cannabidiol Injected into the Bed Nucleus of the Stria Terminalis Reduces the Expression of Contextual Fear Conditioning via 5-HT1A Receptors. J. Psychopharmacol. 2012, 26, 104–113. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of Cannabidiol (CBD) and Its Analogues: Structures, Biological Activities, and Neuroprotective Mechanisms in Epilepsy and Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef]
- Russo, M.; Graham, B.; Santarelli, D.M. Gabapentin—Friend or Foe? Pain Pract. 2023, 23, 63–69. [Google Scholar] [CrossRef]
- Davies, A.; Hendrich, J.; Van Minh, A.T.; Wratten, J.; Douglas, L.; Dolphin, A.C. Functional Biology of the Alpha(2)Delta Subunits of Voltage-Gated Calcium Channels. Trends Pharmacol. Sci. 2007, 28, 220–228. [Google Scholar] [CrossRef]
- Sarantopoulos, C.; McCallum, B.; Kwok, W.M.; Hogan, Q. Gabapentin Decreases Membrane Calcium Currents in Injured as Well as in Control Mammalian Primary Afferent Neurons. Reg. Anesth. Pain Med. 2002, 27, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.E.; Burton, M.; Mama, K.; Rao, S.; Kendall, L.V.; Desmarchelier, M.; Sadar, M.J. Behavioral and Physiologic Effects of a Single Dose of Oral Gabapentin in Rabbits (Oryctolagus Cuniculus). Top. Companion Anim. Med. 2023, 53–54, 100779. [Google Scholar] [CrossRef] [PubMed]
- van Haaften, K.A.; Eichstadt Forsythe, L.R.; Stelow, E.A.; Bain, M.J. Effects of a Single Preappointment Dose of Gabapentin on Signs of Stress in Cats during Transportation and Veterinary Examination. J. Am. Vet. Med. Assoc. 2017, 251, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, F.; Negro, V.; Ravasio, G.; Villa, R.; Draghi, S.; Cagnardi, P. Gabapentin: Clinical Use and Pharmacokinetics in Dogs, Cats, and Horses. Animals 2023, 13, 2045. [Google Scholar] [CrossRef]
- Pankratz, K.E.; Ferris, K.K.; Griffith, E.H.; Sherman, B.L. Use of Single-Dose Oral Gabapentin to Attenuate Fear Responses in Cage-Trap Confined Community Cats: A Double-Blind, Placebo-Controlled Field Trial. J. Feline Med. Surg. 2018, 20, 535–543. [Google Scholar] [CrossRef]
- Nuñez, C.R.; Ortega, A.F.; Cardenas, R.H.; Contreras, L.M.; Climaco, L.R.; Dyurich, M.M.; St, A.B.; Ct, R.A. Evaluation of the Effect of Feliway on Parameters of Parasympathetic Activity in Cats. Int. J. Curr. Adv. Res. 2020, 9, 7–10. [Google Scholar]
- Pereira, J.S.; Demirbas, Y.S.; Meppiel, L.; Endersby, S.; da Graça Pereira, G.; De Jaeger, X. Efficacy of the Feliway® Classic Diffuser in Reducing Undesirable Scratching in Cats: A Randomised, Triple-Blind, Placebo-Controlled Study. PLoS ONE 2023, 18, e0292188. [Google Scholar] [CrossRef]
- Crump, E.; Dvm, B. Effectiveness of F3 Feline Facial Pheromone Analogue for Acute Stress Reduction within Clinical Veterinary Practice. Vet. Evid. 2023, 8, 669. [Google Scholar] [CrossRef]
- Rodan, I. Understanding Feline Behavior and Application for Appropriate Handling and Management. Top. Companion Anim. Med. 2010, 25, 178–188. [Google Scholar] [CrossRef]
- Nicholson, S.L.; O’Carroll, R.Á. Development of an Ethogram/Guide for Identifying Feline Emotions: A New Approach to Feline Interactions and Welfare Assessment in Practice. Ir. Vet. J. 2021, 74, 8. [Google Scholar] [CrossRef]
- Scott, L.; Florkiewicz, B.N. Feline Faces: Unraveling the Social Function of Domestic Cat Facial Signals. Behav. Processes 2023, 213, 104959. [Google Scholar] [CrossRef] [PubMed]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a Non-Invasive Measure of Stress and Fear of Humans in Sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, K.; Nakamura, K. Facial Temperature and Pupil Size as Indicators of Internal State in Primates. Neurosci. Res. 2022, 175, 25–37. [Google Scholar] [CrossRef]
- Szenczi, P.; Jim, A.; Urrutia, A.; Hudson, R. Non-Invasive, Real-Time Stress Measurement: Vocalization Compared with Thermal Imaging in Kittens of the Domestic Cat in Response to Social Separation. Behav. Process. 2023, 213, 104955. [Google Scholar] [CrossRef]
- Foster, S.; Ijichi, C. The Association between Infrared Thermal Imagery of Core Eye Temperature, Personality, Age and Housing in Cats. Appl. Anim. Behav. Sci. 2017, 189, 79–84. [Google Scholar] [CrossRef]
- Urrutia, A.; Bánszegi, O.; Szenczi, P.; Hudson, R. Scaredy-Cat: Assessment of Individual Differences in Response to an Acute Everyday Stressor across Development in the Domestic Cat. Appl. Anim. Behav. Sci. 2022, 256, 105771. [Google Scholar] [CrossRef]
- Vianna, D.M.L.; Carrive, P. Changes in Cutaneous and Body Temperature during and after Conditioned Fear to Context in the Rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Lezama-García, K.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Casas-Alvarado, A.; Torres-Bernal, F.; Martínez-Burnes, J. Neurobiology of Emotions in Animal Relationships: Facial Expressions and Their Biological Functions in Mammals. J. Anim. Behav. Biometeorol. 2023, 11, e2023ss01. [Google Scholar] [CrossRef]
- Gómez-Prado, J.; Pereira, A.M.F.; Wang, D.; Villanueva-García, D.; Domínguez-Oliva, A.; Mora-Medina, P.; Hernández-Avalos, I.; Martínez-Burnes, J.; Casas-Alvarado, A.; Olmos-Hernández, A.; et al. Thermoregulation Mechanisms and Perspectives for Validating Thermal Windows in Pigs with Hypothermia and Hyperthermia: An Overview. Front. Vet. Sci. 2022, 9, 1023294. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal Homeostasis in the Newborn Puppy: Behavioral and Physiological Responses. J. Anim. Behav. Biometeorol. 2021, 9, e2112. [Google Scholar] [CrossRef]
- Giannetto, C.; Di Pietro, S.; Falcone, A.; Pennisi, M.; Giudice, E.; Piccione, G.; Acri, G. Thermographic Ocular Temperature Correlated with Rectal Temperature in Cats. J. Therm. Biol. 2021, 102, 103104. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W. Reduction of Animal Use: Experimental Design and Quality of Experiments. Lab. Anim. 1994, 28, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Edgar, E.; Albert-Georg, L.; Axel, B. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Rozental, A.J.; Gustafson, D.L.; Kusick, B.R.; Bartner, L.R.; Cruz, S.C.; McGrath, S. Pharmacokinetics of Escalating Single-Dose Administration of Cannabidiol to Cats. J. Vet. Pharmacol. Ther. 2022, 46, 25–35. [Google Scholar] [CrossRef]
- Masataka, N. Is Cannabidiol (CBD) Effective to Ease Separation Anxiety? Heliyon 2024, 10, e25851. [Google Scholar] [CrossRef]
- Derek, A.; Papich, M.G.; Baynes, R.; Stafford, E.; Lascelles, B.D.X. The Pharmacokinetics of Gabapentin in Cats. J. Vet. Intern. Med. 2018, 32, 1996–2002. [Google Scholar] [CrossRef]
- Siao, K.T.; Pypendop, B.H.; Ilkiw, J.E. Pharmacokinetics of Gabapentin in Cats. Am. J. Vet. Res. 2010, 71, 817–821. [Google Scholar] [CrossRef]
- Prior, M.R.; Mills, D.S. Cats vs. Dogs: The Efficacy of Feliway FriendsTM and AdaptilTM Products in Multispecies Homes. Front. Vet. Sci. 2020, 7, 399. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef]
- Riggio, G.; Borrelli, C.; Piotti, P.; Grondona, A.; Gazzano, A.; Di Iacovo, F.P.; Fatjó, J.; Bowen, J.E.; Mota-Rojas, D.; Pirrone, F.; et al. Cat–Owner Relationship and Cat Behaviour: Effects of the COVID-19 Confinement and Implications for Feline Management. Vet. Sci. 2022, 9, 369. [Google Scholar] [CrossRef]
- Bertoni, V.; Regaiolli, B.; Cozzi, A.; Vaglio, S.; Spiezio, C. Can an Enrichment Programme with Novel Manipulative and Scent Stimuli Change the Behaviour of Zoo-Housed European Wildcats? A Case Study. Animals 2023, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Croney, C.; Udell, M.; Delgado, M.; Ekenstedt, K.; Shoveller, A.K. CATastrophic Myths Part 1: Common Misconceptions about the Social Behavior of Domestic Cats and Implications for Their Health, Welfare, and Management. Vet. J. 2023, 300–302, 106028. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Adolphs, R. A Framework for Studying Emotions across Species. Cell 2014, 157, 187–200. [Google Scholar] [CrossRef]
- Ermatinger, F.A.; Brügger, R.K.; Burkart El, J.M. Uso de Termografía Infrarroja Para Investigar Las Emociones En Titíes Comunes—ScienceDirect. Physiol. Behav. 2019, 211, 112672. [Google Scholar] [CrossRef]
- Bennett, V.; Gourkow, N.; Mills, D. Facial Correlates of Emotional Behaviour in the Domestic Cat (Felis Catus). Behav. Process 2017, 141, 342–350. [Google Scholar] [CrossRef]
- Daldrup, T.; Remmes, J.; Lesting, J.; Gaburro, S.; Fendt, M.; Meuth, P.; Kloke, V.; Pape, H.C.; Seidenbecher, T. Expression of Freezing and Fear-Potentiated Startle during Sustained Fear in Mice. Genes Brain Behav. 2015, 14, 281–291. [Google Scholar] [CrossRef]
- Chalak, L.F.; Sánchez, P.J.; Adams-Huet, B.; Laptook, A.R.; Heyne, R.J.; Rosenfeld, C.R. Biomarkers for Severity of Neonatal Hypoxic-Ischemic Encephalopathy and Outcomes in Newborns Receiving Hypothermia Therapy. J. Pediatr. 2014, 164, 468–474.e1. [Google Scholar] [CrossRef]
- Adams, D.B.; Baccelli, G.; Mancia, G.; Zanchetti, A. Cardiovascular Changes during Preparation for Fighting Behaviour in the Cat. Nature 1968, 220, 1239–1240. [Google Scholar] [CrossRef]
- Learn, A. Reducing Fear, Anxiety, and Stress in Veterinary Clinics. Feline Behav. Welf. 2025, 11, 139–160. [Google Scholar] [CrossRef]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala Inhibitory Circuits and the Control of Fear Memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef]
- Paré, D.; Collins, D.R. Neuronal Correlates of Fear in the Lateral Amygdala: Multiple Extracellular Recordings in Conscious Cats. J. Neurosci. 2000, 20, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.; Dayan, P. Freezing Revisited: Coordinated Autonomic and Central Optimization of Threat Coping. Nat. Rev. Neurosci. 2022, 23, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Gregg, T.R.; Siegel, A. Brain Structures and Neurotansmitters Regulating Aggression in Cats: Implications for Human Aggression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 91–140. [Google Scholar] [CrossRef]
- Watson, T.C.; Cerminara, N.L.; Lumb, B.M.; Apps, R. Neural Correlates of Fear in the Periaqueductal Gray. J. Neurosci. 2016, 36, 12707–12719. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Jacobs, B.L. Single-Unit Response of Noradrenergic Neurons in the Locus Coeruleus of Freely Moving Cats. I. Acutely Presented Stressful and Nonstressful Stimuli. J. Neurosci. 1987, 7, 2837. [Google Scholar] [CrossRef]
- Choi, E.A.; Leman, S.; Vianna, D.M.L.; Waite, P.M.E.; Carrive, P. Expression of Cardiovascular and Behavioural Components of Conditioned Fear to Context in T4 Spinally Transected Rats. Auton. Neurosci. 2005, 120, 26–34. [Google Scholar] [CrossRef]
- Levine, E.S.; Joseph Litto, W.; Jacobs, B.L. Activity of Cat Locus Coeruleus Noradrenergic Neurons during the Defense Reaction. Brain Res. 1990, 531, 189–195. [Google Scholar] [CrossRef]
- Ruffolo, R.R. Distribution and Function of Peripheral α-Adrenoceptors in the Cardiovascular System. Pharmacol. Biochem. Behav. 1985, 22, 827–833. [Google Scholar] [CrossRef]
- Koss, M.C. Characterization of Adrenoceptor Subtypes in Cat Cutaneous Vasculature. J. Pharmacol. Exp. Ther. 1990, 254, 221–227. [Google Scholar] [CrossRef]
- Liu, Y.; Rodenkirch, C.; Moskowitz, N.; Schriver, B.; Wang, Q. Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Rep. 2017, 20, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.; Harbin, K.; Macpherson, J.; Rundle, K.; Overall, K.L. A Review of Pre-Appointment Medications to Reduce Fear and Anxiety in Dogs and Cats at Veterinary Visits. Can. Vet. J. 2021, 62, 952. [Google Scholar] [PubMed]
- Gamble, L.-J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Tucker, L.E.; Sanchez, A.; Valverde, A.; Blois, S.; Monteith, G.; Longworth, P.; Downie, A.; Gu, Y.; Johnson, R. Evaluation of the Sedative Properties of Oral Trazodone, Gabapentin or Their Combination in Healthy Cats. J. Feline Med. Surg. 2024, 26, 1098612X241281481. [Google Scholar] [CrossRef]
- Strzelczuk, M.; Romaniuk, A. Fear Induced by the Blockade of GABAA-Ergic Transmission in the Hypothalamus of the Cat: Behavioral and Neurochemical Study. Behav. Brain Res. 1995, 72, 63–71. [Google Scholar] [CrossRef]
- Gaudin-Chazal, G.; Daszuta, A.; Faudon, M.; Ternaux, J.P. 5-HT Concentration in Cat’s Brain. Brain Res. 1979, 160, 281–293. [Google Scholar] [CrossRef]
- Meissner, H.; Cascella, M. Cannabidiol (CBD); StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Assareh, N.; Gururajan, A.; Zhou, C.; Luo, J.L.; Kevin, R.C.; Arnold, J.C. Cannabidiol Disrupts Conditioned Fear Expression and Cannabidiolic Acid Reduces Trauma-Induced Anxiety-Related Behaviour in Mice. Behav. Pharmacol. 2020, 31, 591–596. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Morris, E.M.; Kitts-Morgan, S.E.; Spangler, D.M.; McLeod, K.R.; Costa, J.H.C.; Harmon, D.L. The Impact of Feeding Cannabidiol (CBD) Containing Treats on Canine Response to a Noise-Induced Fear Response Test. Front. Vet. Sci. 2020, 7, 569565. [Google Scholar] [CrossRef]
- Draeger, A.L.; Thomas, E.P.; Jones, K.A.; Davis, A.J.; Porr, C.A.S. The Effects of Pelleted Cannabidiol Supplementation on Heart Rate and Reaction Scores in Horses. J. Vet. Behav. 2021, 46, 97–100. [Google Scholar] [CrossRef]
- Saito, S.; Shingai, R. Evolution of ThermoTRP Ion Channel Homologs in Vertebrates. Physiol. Genom. 2006, 27, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Versteg, N.; Dias, T.P.; de Freitas, V.R.; das Neves, V.B.; Gomes, M.R.; Meinerz, A.R.M.; Jorge, S.; Rondelli, M.C.H.; Cleff, M.B. A Comparative Study between Integrative Practices and Preappointement Gabapentin on Serum Cortisol in Cats. Vet. Res. Commun. 2024, 48, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.; Gower, L. Randomised Clinical Trial Evaluating the Effect of a Single Preappointment Dose of Gabapentin on Signs of Stress in Hyperthyroid Cats. J. Feline Med. Surg. 2022, 24, e85–e89. [Google Scholar] [CrossRef]
- Maren, S. Neurobiology of Pavlovian Fear Conditioning. Annu. Rev. Neurosci. 2001, 24, 897–931. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Ververidis, C.; Mylonakis, M.E.; Savvas, I.; Kazakos, G. Orally Administered Gabapentin and Alprazolam Induce Comparable Levels of Anxiolysis and Sedation in Cats. J. Am. Vet. Med. Assoc. 2024, 262, 904–908. [Google Scholar] [CrossRef]
- McPeake, K.; Sparkes, A.; Billy, C.; Endersby, S.; Collin, J.F.; De Jaeger, X. Development of a Cat Behaviour Issues Assessment Scale (CABIAS) Assessing Problem Behaviours in Cats. Animals 2023, 13, 2992. [Google Scholar] [CrossRef]
- Pereira, J.S.; Fragoso, S.; Beck, A.; Lavigne, S.; Varejão, A.S.; da Graça Pereira, G. Improving the Feline Veterinary Consultation: The Usefulness of Feliway Spray in Reducing Cats’ Stress. J. Feline Med. Surg. 2016, 18, 959–964. [Google Scholar] [CrossRef]
- Kronen, P.W.; Ludders, J.W.; Erb, H.N.; Moon, P.F.; Gleed, R.D.; Koski, S. A Synthetic Fraction of Feline Facial Pheromones Calms but Does Not Reduce Struggling in Cats before Venous Catheterization. Vet. Anaesth. Analg. 2006, 33, 258–265. [Google Scholar] [CrossRef]
- DePorter, T.L.; Bledsoe, D.L.; Beck, A.; Ollivier, E. Evaluation of the Efficacy of an Appeasing Pheromone Diffuser Product vs Placebo for Management of Feline Aggression in Multi-Cat Households: A Pilot Study. J. Feline Med. Surg. 2019, 21, 293–305. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Talwar, V.; Lin, D. Neural Circuits of Social Behaviors: Innate yet Flexible. Neuron 2021, 109, 1600–1620. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, A.F.; Veronezi, T.M.; Zardo, I.L.; Ferronatto, J.V.B.; Franck, K.R.; Spiering, A.G.; Nunes, L.N.; da Costa, F.V.A. Does Preappointment Gabapentin Affect Neurological Examination Findings? A Prospective, Randomized and Blinded Study in Healthy Cats. J. Feline Med. Surg. 2023, 25, 1098612X221149384. [Google Scholar] [CrossRef]
- Masataka, N. Possible Effects of Cannabidiol (CBD) Administration on the Vocal Activity of Healthy Domestic Dogs upon Their Temporary Separation from Caregivers. Heliyon 2024, 10, e25548. [Google Scholar] [CrossRef]
- Ruviaro Tuleski, G.L.; Silveira, M.F.; Bastos, R.F.; Pscheidt, M.J.G.R.; Prieto, W.d.S.; Sousa, M.G. Behavioral and Cardiovascular Effects of a Single Dose of Gabapentin or Melatonin in Cats: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Feline Med. Surg. 2022, 24, e524–e534. [Google Scholar] [CrossRef]
- Frank, D.; Beauchamp, G.; Palestrini, C. Systematic Review of the Use of Pheromones for Treatment of Undesirable Behavior in Cats and Dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1308–1316. [Google Scholar] [CrossRef]



| Thermal Windows | Groups | Tbasal− | T1stfear | T1strecovery | Tbasal+ | T2ndfear | T2ndrecovery | p-Value |
|---|---|---|---|---|---|---|---|---|
| T°OCU | CONTROL (n = 20) | 36.74 ± 0.15 a,1 | 35.59 ± 0.19 b,1 | 36.60 ± 0.13 a,1 | 36.61 ± 0.09 a,1 | 35.73 ± 0.16 b,1 | 36.73 ± 0.15 a,1 | p = 0.0004 |
| CBD (n = 20) | 36.95 ± 0.19 a,b,1 | 36.18 ± 0.18 a,1 | 37.0 ± 0.18 b,1 | 37.06 ± 0.17 a,b,1 | 36.95 ± 0.14 a,b,2 | 37.12 ± 0.16 b,1 | p = 0.0117 | |
| GABA (n = 20) | 37.0 ± 0.18 a,1 | 36.01 ± 0.26 b,1 | 37.03 ± 0.22 a,1 | 36.97 ± 0.19 a,b,1 | 36.79 ± 0.14 a,b,2 | 36.78 ± 0.14 a,b,1 | p = 0.0013 | |
| SFP (n = 20) | 36.96 ± 0.20 a,1 | 36.22 ± 0.21 b,1 | 35.27 ± 1.86 a,b,1 | 37.18 ± 0.14 a,1 | 36.91 ± 0.14 a,b,2 | 36.98 ± 0.14 a,b,1 | p = 0.0003 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0067 | p > 0.05 | ||
| T°CAR | CONTROL (n = 20) | 36.1 ± 0.20 a,1 | 35.18 ± 0.22 b,1 | 36.13 ± 0.26 a,1 | 36.33 ± 0.17 a,1 | 35.05 ± 0.20 b,1 | 36.64 ± 0.21 a,1 | p < 0.0001 |
| CBD (n = 20) | 36.62 ± 0.24 a,1 | 35.34 ± 0.18 b,1 | 36.92 ± 0.23 a,1 | 37 ± 0.24 a,1 | 36.95 ± 0.21 a,2 | 36.88 ± 0.18 a,1 | p < 0.0001 | |
| GABA (n = 20) | 36.89 ± 0.16 a,1 | 34.93 ± 0.20 b,1 | 37.12 ± 0.19 a,1 | 36.83 ± 0.26 a,1 | 36.58 ± 0.21 a,2 | 36.67 ± 0.22 a,1 | p < 0.0001 | |
| SFP (n = 20) | 36.6 ± 0.20 a,1 | 35.51 ± 0.22 b,1 | 36.91 ± 0.18 a,1 | 36.87 ± 0.16 a,1 | 36.52 ± 0.20 a,2 | 36.74 ± 0.18 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0008 | p > 0.05 | ||
| T°Uppereyeline | CONTROL (n = 20) | 35.39 ± 0.22 a,1 | 36.18 ± 0.14 b,1 | 35.38 ± 0.20 a,1 | 35.17 ± 0.16 a,1 | 35.72 ± 0.12 b,1 | 35.30 ± 0.21 a,1 | p = 0.0004 |
| CBD (n = 20) | 34.85 ± 0.28 a,1 | 36.05 ± 0.16 b,1 | 35.29 ± 0.25 a,1 | 35.26 ± 0.22 a,1 | 35.12 ± 0.22 a,1 | 35.23 ± 0.23 a,1 | p = 0.0041 | |
| GABA (n = 20) | 35.17 ± 0.15 a,1 | 35.88 ± 0.17 b,1 | 34.94 ± 0.20 a,b,1 | 34.85 ± 0.25 a,b,1 | 34.89 ± 0.18 a,1 | 34.93 ± 0.18 a,1 | p = 0.0237 | |
| SFP (n = 20) | 34.76 ± 0.23 a,1 | 35.81 ± 0.20 b,1 | 34.76 ± 0.21 a,1 | 34.78 ± 0.16 a,1 | 34.76 ± 0.21 a,1 | 34.74 ± 0.20 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0672 | p > 0.05 | ||
| T°Lowereyeline | CONTROL (n = 20) | 35.13 ± 0.17 a,1 | 36.05 ± 0.12 b,1 | 35.31 ± 0.22 a,1 | 35.28 ± 0.16 a,1 | 35.02 ± 0.52 a,1 | 35.06 ± 0.15 a,1 | p = 0.0004 |
| CBD (n = 20) | 34.92 ± 0.25 a,1 | 36.16 ± 0.17 b,1 | 35.44 ± 0.26 a,b,1 | 35.31 ± 0.21 a,b,1 | 35.03 ± 0.21a,1 | 35.23 ± 0.22 a,1 | p = 0.0002 | |
| GABA (n = 20) | 35.39 ± 0.20 a,1 | 36.38 ± 0.21 b,1 | 35.39 ± 0.22 a,1 | 35.67 ± 0.16 a,b,1 | 35.33 ± 0.15 a,1 | 35.51 ± 0.15 a,1 | p = 0.036 | |
| SFP (n = 20) | 34.68 ± 0.23 a,1 | 35.99 ± 0.18 b,1 | 34.90 ± 0.27 a,1 | 35.03 ± 0.16 a,1 | 34.99 ± 0.22 a,1 | 35.01 ± 0.19 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | ||
| T°RigthNostril | CONTROL (n = 20) | 31.27 ± 0.35 a,1 | 29.03 ± 0.43 b,1 | 31.67 ± 0.44 a,1 | 31.45 ± 0.41 a,1 | 29.66 ± 0.42 b,1 | 31.78 ± 0.40 a,1 | p < 0.0001 |
| CBD (n = 20) | 32.85 ± 0.56 a,1 | 30.67 ± 0.51 b,1 | 32.82 ± 0.69 a,1 | 33.08 ± 0.47 a,1 | 33.11 ± 0.51 a,2 | 32.86 ± 0.46 a,1 | p < 0.0001 | |
| GABA (n = 20) | 34.84 ± 0.33 a,1 | 31.90 ± 0.62 b,1 | 35.03 ± 0.47 a,1 | 34.47 ± 0.39 a,2 | 34.20 ± 0.47 a,2 | 34.47 ± 0.45 a,2 | p < 0.0001 | |
| SFP (n = 20) | 32.90 ± 0.51 a,1 | 31.31 ± 0.47 b,1 | 33.66 ± 0.46 a,1 | 33.27 ± 0.35 a,1 | 33.01 ± 0.48 a,2 | 33.17 ± 0.40 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0007 | p = 0.0002 | p = 0.0254 | ||
| T°LeftNostril | CONTROL (n = 20) | 31.17 ± 0.39 a,1 | 29.18 ± 0.47 b,1 | 31.24 ± 0.54 a,1 | 31.12 ± 0.45 a,1 | 29.62 ± 0.46 b,1 | 31.64 ± 0.45 a,1 | p < 0.0001 |
| CBD (n = 20) | 33.02 ± 0.51 a,1 | 30.55 ± 0.39 b,1 | 32.72 ± 0.63 a,1 | 33.02 ± 0.52 a,1 | 32.88 ± 0.49 a,2 | 33.08 ± 0.48 a,1 | p = 0.0028 | |
| GABA (n = 20) | 34.0 ± 0.23 a,1 | 30.78 ± 0.47 b,1 | 34.67 ± 0.32 a,1 | 34.09 ± 0.29 a,2 | 33.78 ± 0.34 a,2 | 33.64 ± 0.32 a,1 | p = 0.0005 | |
| SFP (n = 20) | 33.38 ± 0.47 a,1 | 31.82 ± 0.54 b,1 | 33.76 ± 0.48 a,1 | 33.45 ± 0.40 a,1 | 33.16 ± 0.45 a,2 | 33.40 ± 0.41 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0343 | p = 0.0033 | p > 0.05 | ||
| T°Whisk | CONTROL (n = 20) | 33.39 ± 0.33 a,1 | 32.49 ± 0.33 a,1 | 31.51 ± 0.27 a,1 | 33.22 ± 0.27 a,1 | 32.63 ± 0.31 a,1 | 33.43 ± 0.24 a,1 | p = 0.081 |
| CBD(n = 20) | 34.27 ± 0.32 a,b,1 | 33.48 ± 0.34 a,1 | 34.40 ± 0.33 b,1 | 34.44 ± 0.32 a,b,1 | 34.30 ± 0.31 a,b,1 | 34.39 ± 0.30 a,b,1 | p = 0.0023 | |
| GABA (n = 20) | 34.88 ± 0.29 a,1 | 33.55 ± 0.47 b,1 | 35.01 ± 0.35 a,1 | 34.55 ± 0.44 a,b,1 | 34.30 ± 0.32 a,b,1 | 34.48 ± 0.36 a,b,1 | p = 0.0012 | |
| SFP (n = 20) | 34.21 ± 0.29 a,1 | 33.48 ± 0.32 b,1 | 34.31 ± 0.31 a,1 | 34.19 ± 0.24 a,b,1 | 34.05 ± 0.24 a,b,1 | 34.09 ± 0.25 a,b,1 | p = 0.0004 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.129 | p > 0.05 | ||
| T°EAR | CONTROL (n = 20) | 35.14 ± 0.29 a,1 | 32.28 ± 0.39 b,1 | 33.39 ± 0.34 a,1 | 33.95 ± 0.28 a,1 | 32.12 ± 0.33 b,1 | 34.01 ± 0.35 a,1 | p < 0.0001 |
| CBD (n = 20) | 34.79 ± 0.43 a,1 | 32.69 ± 0.28 b,1 | 34.24 ± 0.35 a,1 | 34.69 ± 0.39 a,1 | 34.91 ± 0.35 a,2 | 34.74 ± 0.35 a,1 | p < 0.0001 | |
| GABA (n = 20) | 35.24 ± 0.27 a,1 | 33.26 ± 0.40 b,1 | 35.36 ± 0.31 a,1 | 35.02 ± 0.31 a,1 | 35.19 ± 0.22 a,2 | 34.91 ± 0.27 a,1 | p < 0.0001 | |
| SFP (n = 20) | 35.34 ± 0.19 a,1 | 32.80 ± 0.34 b,1 | 34.0 ± 0.57 a,1 | 34.72 ± 0.31 a,1 | 35.0 ± 0.28 a,2 | 34.61 ± 0.33 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0002 | p > 0.05 | ||
| T°CHEST | CONTROL (n = 20) | 29.03 ± 0.21 a,1 | 27.45 ± 0.27 b,1 | 28.28 ± 0.31 a,b,1 | 28.63 ± 0.26 a,1 | 27.51 ± 0.28 b,1 | 28.96 ± 0.28 a,1 | p < 0.0001 |
| CBD (n = 20) | 30.51 ± 0.42 a,1 | 29.12 ± 0.41 b,1 | 29.91 ± 0.41 a,1 | 30.37 ± 0.38 a,2 | 30.39 ± 0.40 a,2 | 30.32 ± 0.39 a,1 | p = 0.0001 | |
| GABA (n = 20) | 29.83 ± 0.34 a,1 | 28.33 ± 0.40 b,1 | 29.89 ± 0.41 a,1 | 29.64 ± 0.42 a,1 | 29.85 ± 0.39 a,2 | 29.87 ± 0.40 a,1 | p = 0.0024 | |
| SFP (n = 20) | 29.12 ± 0.27 a,b,1 | 28.66 ± 0.41 b,1 | 29.10 ± 0.39 a,b,1 | 29.06 ± 0.36 a,b,1 | 29.14 ± 0.39 a,b,1 | 29.22 ± 0.38 a,1 | p = 0.0469 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0296 | p = 0.0002 | p > 0.05 |
| Parameters | Groups | Tbasal− | T1stfear | T1strecovery | Tbasal+ | T2ndfear | T2ndrecovery | p-Value |
|---|---|---|---|---|---|---|---|---|
| Heart rate (bpm) | CONTROL (n = 20) | 189 ± 8.00 a,1 | 240 ± 5.64 b,1 | 194.5 ± 7.73 a,1 | 202.1 ± 6.53 a,b,1 | 230.6 ± 6.74 b,1 | 199.9 ± 5.06 a,1 | p < 0.0001 |
| CBD (n = 20) | 176.1 ± 4.71 a,1 | 222.4 ± 6.35 b,1 | 181.4 ± 7.44 a,1 | 177.8 ± 4.86 a,1 | 183.6 ± 4.85 a,2 | 179.2 ± 4.81 a,1 | p < 0.0001 | |
| GABA (n = 20) | 173 ± 6.94 a,1 | 226.8 ± 7.95 b,1 | 184.4 ± 9.65 a,1 | 174.3 ± 7.35 a,1 | 177.9 ± 7.44 a,2 | 177.8 ± 7.07 a,1 | p < 0.0001 | |
| SFP (n = 20) | 178.4 ± 6.60 a,1 | 229.7 ± 7.61 b,1 | 186.8 ± 5.94 a,1 | 183 ± 5.05 a,1 | 185.8 ± 5.04 a,2 | 185.7 ± 4.49 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0058 | p > 0.05 | ||
| Respiratory rate (bpm) | CONTROL (n = 20) | 52.50 ± 3.15 a,1 | 71.85 ± 3.89 b,1 | 54.45 ± 4.38 a,1 | 51.75 ± 3.08 a,1 | 67.9 ± 3.56 b,1 | 53.45 ± 3.73 a,1 | p < 0.0001 |
| CBD (n = 20) | 53.15 ± 4.46 a,1 | 81.60 ± 5.59 b,1 | 64.95 ± 6.10 a,1 | 51.50 ± 3.98 a,1 | 51.20 ± 3.52 a,2 | 47.30 ± 3.30 a,1 | p < 0.0001 | |
| GABA (n = 20) | 60.15 ± 3.95 a,c,1 | 93.80 ± 5.17 b,1 | 74.20 ± 5.93 a,c,1 | 54.15 ± 4.22 a,1 | 52.85 ± 4.10 a,2 | 51.85 ± 4.34 a,1 | p < 0.0001 | |
| SFP (n = 20) | 59.40 ± 3.40 a,1 | 88.40 ± 3.25 b,1 | 64.70 ± 2.80 a,1 | 60.15 ± 5.45 a,1 | 62.60 ± 5.33 a,1 | 62.60 ± 5.48 a,1 | p = 0.0004 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0108 | p > 0.05 | ||
| Rectal temperature (°C) | CONTROL (n = 20) | 38.69 ± 0.16 a,1 | 37.83 ± 0.13 b,1 | 38.29 ± 0.17 a,1 | 38.58 ± 0.17 a,1 | 38.02 ± 0.12 b,1 | 38.39 ± 0.12 a,b,1 | p = 0.0003 |
| CBD (n = 20) | 38.90 ± 0.11 a,1 | 37.95 ± 0.12 b,1 | 38.50 ± 0.09 a,1 | 38.54 ± 0.11 a,1 | 38.62 ± 0.10 a,2 | 38.45 ± 0.10 a,1 | p < 0.0001 | |
| GABA (n = 20) | 38.93 ± 0.11 a,1 | 37.91 ± 0.13 b,1 | 38.62 ± 0.13 a,1 | 38.81 ± 0.08 a,1 | 38.84 ± 0.10 a,2 | 38.63 ± 0.12 a,1 | p < 0.0001 | |
| SFP (n = 20) | 39.21 ± 0.09 a,1 | 38.18 ± 0.15 b,1 | 38.93 ± 0.10 a,1 | 39.03 ± 0.08 a,1 | 38.97 ± 0.10 a,2 | 38.98 ± 0.08 a,1 | p < 0.0001 | |
| p-Value | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p = 0.0002 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Bernal, F.; Martínez-Burnes, J.; Hernández-Avalos, I.; Olmos-Hernández, A.; Domínguez-OIiva, A.; Reyes-Sotelo, B.; González-López, C.; Villanueva-Pereyra, D.; Mota-Rojas, D. Infrared Thermography and Physiological Variables as Methods for Recognizing Fear in Domestic Cats (Felis catus) Using Three Pharmacological Models: Cannabidiol, Gabapentin, and Synthetic Facial Pheromones. Vet. Sci. 2025, 12, 523. https://doi.org/10.3390/vetsci12060523
Torres-Bernal F, Martínez-Burnes J, Hernández-Avalos I, Olmos-Hernández A, Domínguez-OIiva A, Reyes-Sotelo B, González-López C, Villanueva-Pereyra D, Mota-Rojas D. Infrared Thermography and Physiological Variables as Methods for Recognizing Fear in Domestic Cats (Felis catus) Using Three Pharmacological Models: Cannabidiol, Gabapentin, and Synthetic Facial Pheromones. Veterinary Sciences. 2025; 12(6):523. https://doi.org/10.3390/vetsci12060523
Chicago/Turabian StyleTorres-Bernal, Fabiola, Julio Martínez-Burnes, Ismael Hernández-Avalos, Adriana Olmos-Hernández, Adriana Domínguez-OIiva, Brenda Reyes-Sotelo, Cynthia González-López, Diana Villanueva-Pereyra, and Daniel Mota-Rojas. 2025. "Infrared Thermography and Physiological Variables as Methods for Recognizing Fear in Domestic Cats (Felis catus) Using Three Pharmacological Models: Cannabidiol, Gabapentin, and Synthetic Facial Pheromones" Veterinary Sciences 12, no. 6: 523. https://doi.org/10.3390/vetsci12060523
APA StyleTorres-Bernal, F., Martínez-Burnes, J., Hernández-Avalos, I., Olmos-Hernández, A., Domínguez-OIiva, A., Reyes-Sotelo, B., González-López, C., Villanueva-Pereyra, D., & Mota-Rojas, D. (2025). Infrared Thermography and Physiological Variables as Methods for Recognizing Fear in Domestic Cats (Felis catus) Using Three Pharmacological Models: Cannabidiol, Gabapentin, and Synthetic Facial Pheromones. Veterinary Sciences, 12(6), 523. https://doi.org/10.3390/vetsci12060523








