Clinical, Histological, and Immunohistochemical Insights into a Canine Hepatic Myofibroblastic Sarcoma
Simple Summary
Abstract
1. Introduction
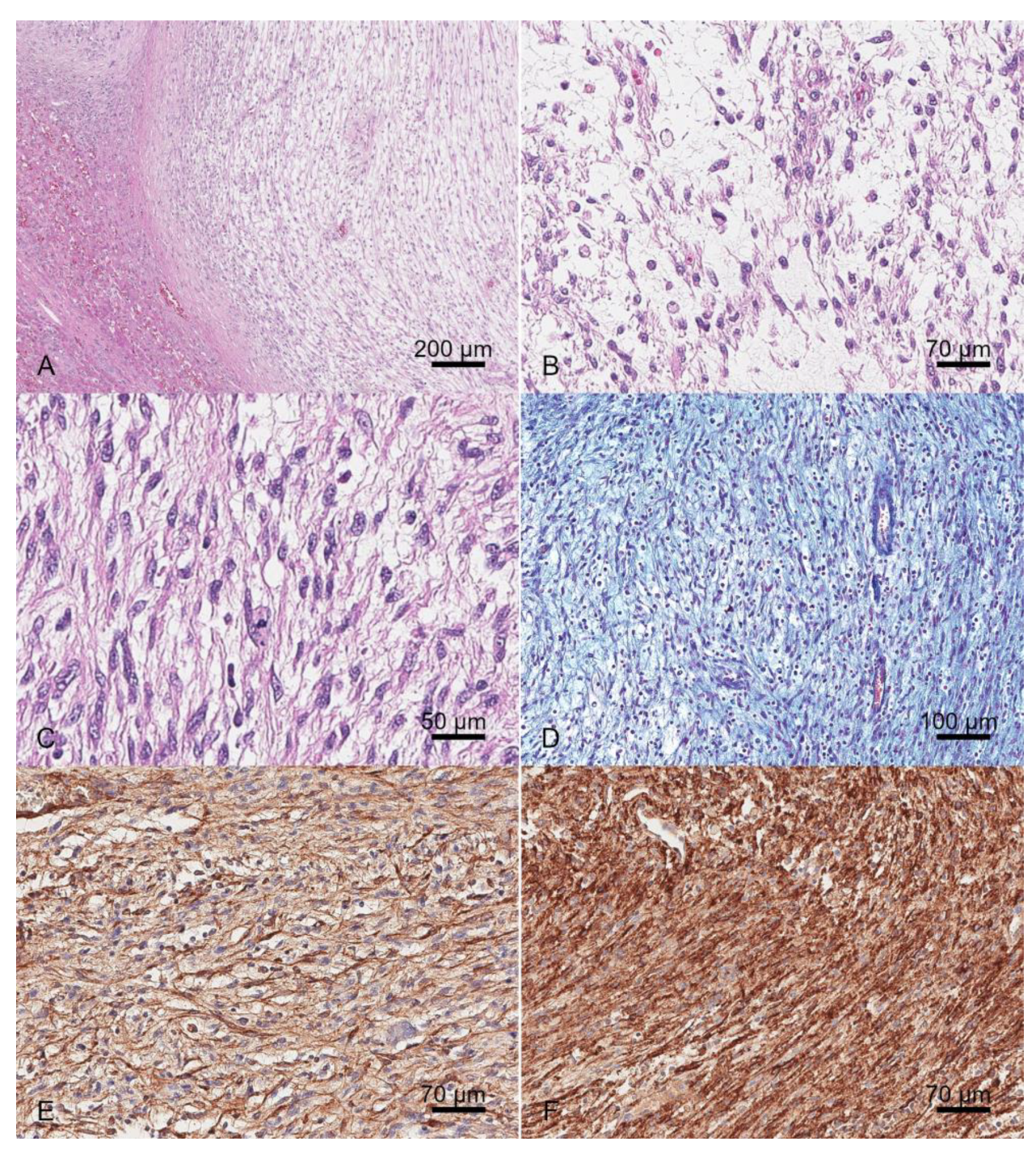
2. Case History
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawrence, H.J.; Erb, H.N.; Harvey, H.J. Nonlymphomatous Hepatobiliary Masses in Cats: 41 Cases (1972 to 1991). Vet. Surg. 1994, 23, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Mani, H.; Van Thiel, D.H. Mesenchymal Tumors of the Liver. Clin. Liver Dis. 2001, 5, 219–257, viii. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef]
- Kapatkin, A.S.; Mullen, H.S.; Matthiesen, D.T.; Patnaik, A.K. Leiomyosarcoma in Dogs: 44 Cases (1983–1988). J. Am. Vet. Med. Assoc. 1992, 201, 1077–1079. [Google Scholar] [CrossRef]
- Trigo, F.J.; Thompson, H.; Breeze, R.G.; Nash, A.S. The Pathology of Liver Tumours in the Dog. J. Comp. Pathol. 1982, 92, 21–39. [Google Scholar] [CrossRef]
- Jeraj, K.; Yano, B.; Osborne, C.A.; Wallace, L.J.; Stevens, J.B. Primary Hepatic Osteosarcoma in a Dog. J. Am. Vet. Med. Assoc. 1981, 179, 1000–1003. [Google Scholar]
- McDonald, R.K.; Helman, R.G. Hepatic Malignant Mesenchymoma in a Dog. J. Am. Vet. Med. Assoc. 1986, 188, 1052–1053. [Google Scholar] [CrossRef]
- Park, M.-K.; Sung, J.-K.; Nam, K.-H.; Kim, K.-T. Malignant Peripheral Nerve Sheath Tumor of Non-Neurofibromatosis Type I Metastasized to the Cerebrospinal Axis. J. Korean Neurosurg. Soc. 2013, 53, 190. [Google Scholar] [CrossRef]
- Jung, H.I.; Lee, H.U.; Ahn, T.S.; Lee, J.E.; Lee, H.Y.; Cho, H.D.; Lee, S.C.; Bae, S.H. Primary Hepatic Malignant Peripheral Nerve Sheath Tumor Successfully Treated with Combination Therapy: A Case Report and Literature Review. Ann. Surg. Treat. Res. 2016, 91, 327. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) Following Investigational Therapy in Dogs and Cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response Evaluation Criteria for Solid Tumours in Dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) Consensus Document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Song, W.-J.; Choi, S.; Kim, J.-H.; Jung, J.-Y.; Cheong, J.; Park, H.; Jeong, H.; Yun, Y. A Rare Case of Cecal Malignant Peripheral Nerve Sheath Tumor in a Dog. J. Vet. Med. Sci. 2022, 84, 1051. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, W.; Burgener, I.A.; Roccabianca, P.; Rytz, U.; Welle, M. Primary Splenic Peripheral Nerve Sheath Tumour in a Dog. J. Comp. Pathol. 2009, 141, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Filips, A.; Maurer, M.H.; Montani, M.; Beldi, G.; Lachenmayer, A. Inflammatory Myofibroblastic Tumor of the Liver: A Case Report and Review of Literature. World J. Hepatol. 2020, 12, 170–183. [Google Scholar] [CrossRef]
- Gleason, B.C.; Hornick, J.L. Inflammatory Myofibroblastic Tumours: Where Are We Now? J. Clin. Pathol. 2008, 61, 428–437. [Google Scholar] [CrossRef]
- Ijzer, J.; Roskams, T.; Molenbeek, R.F.; Ultee, T.; Penning, L.C.; Rothuizen, J.; van den Ingh, T.S.G.A.M. Morphological Characterisation of Portal Myofibroblasts and Hepatic Stellate Cells in the Normal Dog Liver. Comp. Hepatol. 2006, 5, 7. [Google Scholar] [CrossRef]
- Cullen, J.M.; Stalker, M.J. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals: Volume 2, 6th ed.; Maxie, M.G., Ed.; Saunders Ltd.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Stroebel, P.; Mayer, F.; Zerban, H.; Bannasch, P. Spongiotic Pericytoma: A Benign Neoplasm Deriving from the Perisinusoidal (Ito) Cells in Rat Liver. Am. J. Pathol. 1995, 146, 903–913. [Google Scholar]
- Tillmann, T.; Kamino, K.; Dasenbrock, C.; Germann, P.G.; Kohler, M.; Morawietz, G.; Campo, E.; Cardesa, A.; Tomatis, L.; Mohr, U. Ito Cell Tumor: Immunohistochemical Investigations of a Rare Lesion in the Liver of Mice. Toxicol. Pathol. 1999, 27, 364–369. [Google Scholar] [CrossRef]
- Cassiman, D.; Roskams, T. Beauty Is in the Eye of the Beholder: Emerging Concepts and Pitfalls in Hepatic Stellate Cell Research. J. Hepatol. 2002, 37, 527–535. [Google Scholar] [CrossRef]
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical Evaluation of Canine Peripheral Nerve Sheath Tumors and Other Soft Tissue Sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Dundr, P.; Povýsil, C.; Tvrdík, D. Actin Expression in Neural Crest Cell-Derived Tumors Including Schwannomas, Malignant Peripheral Nerve Sheath Tumors, Neurofibromas and Melanocytic Tumors. Pathol. Int. 2009, 59, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Amorim, I.; Rêma, A.; Faria, F.; Gärtner, F. Molecular Heterogeneity of Canine Cutaneous Peripheral Nerve Sheath Tumors: A Drawback in the Diagnosis Refinement. In Vivo 2016, 30, 819–827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogilvie, G.K.; Powers, B.E.; Mallinckrodt, C.H.; Withrow, S.J. Surgery and Doxorubicin in Dogs with Hemangiosarcoma. J. Vet. Intern. Med. 1996, 10, 379–384. [Google Scholar] [CrossRef]
- Selting, K.A.; Powers, B.E.; Thompson, L.J.; Mittleman, E.; Tyler, J.W.; Lafferty, M.H.; Withrow, S.J. Outcome of Dogs with High-Grade Soft Tissue Sarcomas Treated with and without Adjuvant Doxorubicin Chemotherapy: 39 Cases (1996–2004). J. Am. Vet. Med. Assoc. 2005, 227, 1442–1448. [Google Scholar] [CrossRef]
- Meyer, M.; Seetharam, M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr. Treat Options Oncol. 2019, 20, 6. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A Systematic Meta-Analysis of Randomized Controlled Trials of Adjuvant Chemotherapy for Localized Resectable Soft-Tissue Sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Elmslie, R.E.; Glawe, P.; Dow, S.W. Metronomic Therapy with Cyclophosphamide and Piroxicam Effectively Delays Tumor Recurrence in Dogs with Incompletely Resected Soft Tissue Sarcomas. J. Vet. Intern. Med. 2008, 22, 1373–1379. [Google Scholar] [CrossRef]
- Thway, K.; Folpe, A.L. Update on Selected Advances in the Immunohistochemical and Molecular Genetic Analysis of Soft Tissue Tumors. Virchows Arch. 2020, 476, 3–15. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Tsuchiya, H. Sarcoma: Molecular Pathology, Diagnostics, and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5833. [Google Scholar] [CrossRef]
- Bovée, J.V.M.G.; Hogendoorn, P.C.W. Molecular Pathology of Sarcomas: Concepts and Clinical Implications. Virchows Arch. 2010, 456, 193–199. [Google Scholar] [CrossRef]

| Parameters | Value | Range |
|---|---|---|
| Hemoglobin | 6.3 | 12–18 g/dL |
| Hematocrit | 18.4 | 37.0–55.0% |
| Red Blood Cells | 2,640,000 | 5,500,000–8,500,000/mm3 |
| Platelets | 145,000 | 160,000–500,000/mm3 |
| White Blood Cells | 12,950 | 6000–17,000/mm3 |
| Mean Corpuscular Volume | 69.6 | 60.0–77.0 fL |
| Mean Platelet Volume | 31.6 | 6.6–10.9 fL |
| Mean Corpuscular Hemoglobin | 34 | 32.0–38.0 g/dL |
| Mean Corpuscular Hemoglobin Concentration | 23.7 | 19.5–24.5 pgr |
| Red Cell Distribution Width | 28.8 | 13.0–15.7% |
| Lymphocytes | 4278 | 1000–4800/mm3 |
| Monocytes | 924 | 100–1400/mm3 |
| Neutrophils | 7003 | 3000–12,000/mm3 |
| Eosinophils | 100 | 0–750/mm3 |
| Basophils | 12 | 0–180/mm3 |
| Reticulocytes | 14,410 | 0–60,000/mm3 |
| Reticulocyte Percentage | 5.45% | 0–1.5% |
| Corrected Reticulocyte Percentage | 2.20% | 0–1% |
| Glucose | 100 mg/dL | 70–125 |
| Urea | 50 mg/dL | 18–55 |
| Creatinine | 1.1 mg/dL | 0.65–1.35 |
| Total Bilirubin | 0.2 mg/dL | 0.07–0.34 |
| AST | 356 U/L | 20–42 |
| ALT | 574 U/L | 20–55 |
| DGGR Lipase | 60 U/L | 10–130 |
| GGT | 5 U/L | 0–5.8 |
| CK | 300 U/L | 50–290 |
| Calcium | 10.5 mg/dL | 9.0–11.8 |
| Corrected Calcium | 10.4 mg/dL | 9.0–11.8 |
| Phosphorus | 3.9 mg/dL | 2.6–4.9 |
| Total Proteins | 6.6 mg/dL | 5.3–7.9 |
| Cholesterol | 300 mg/dL | 140–350 |
| Triglycerides | 230 mg/dL | 30–120 |
| Albumin | 3.6 g/dL | 2.5–3.7 |
| Sodium | 144 mEq/L | 143–154 |
| Potassium | 4.1 mEq/L | 3.9–5.3 |
| Chloride | 110 mEq/L | 108–118 |
| Corrected Chloride | 112 mEq/L | 108–118 |
| ALP | 1500 U/L | 42–180 |
| Iron | 248 μg/dL | 72–168 |
| UIBC | 201 μg/dL | 140–296 |
| C-Reactive Protein | 5.2 mg/dL | 0.0–1 |
| Na/K Ratio | 35 | |
| Globulins | 3 g/dL | 2.5–4.5 |
| A/G Ratio | 1.2 | 0.6–1.3 |
| TIBC | 449 μg/dL | 268–404 |
| Antigen | Dilutions | Source | Epitope Retrieval | Positive Control |
|---|---|---|---|---|
| Vimentin | 1:1000 | Agilent Dako; Santa Clara, CA, United States | Heat-induced epitope retrieval, pH 6, 10′ | Vascular tunica media |
| Pan-cytokeratins (CKAE1-AE3) | 1:1000 | Agilent Dako; Santa Clara, CA, United States | Enzymatic digestion, 10′ | Bile duct |
| Cytokeratin-7 (CK7) | 1:200 | Agilent Dako; Santa Clara, CA, United States | Heat-induced epitope retrieval, pH 6, 15′ at 97 °C | Bile duct |
| Smooth muscle actin (SMA) | 1:2000 | Agilent Dako; Santa Clara, CA, United States | None | Vascular tunica media |
| Muscular actin (HHF35) | 1:500 | Agilent Dako; Santa Clara, CA, United States | Heat-induced epitope retrieval, pH 6, 10′ at 97 °C | Vascular tunica media |
| Desmin | 1:200 | D33; Histo-line, Pantigliate, Itay | Heat-induced epitope retrieval, pH 6, 20′ at 97 °C | Vascular tunica media |
| S100 | 1:100 | Leica; Wetzlar, Germany | None | Nerve |
| Glial fibrillary acidic protein (GFAP) | 1:3000 | Agilent Dako; Santa Clara, CA, United States | Heat-induced epitope retrieval, pH 6, 20′ | Nerve |
| Nerve growth factor receptor (NGFR) | 1:4000 | Invitrogen, Thermo Fisher; Waltham, MA, United States | Heat-induced epitope retrieval, pH 6, 20′ | Nerve |
| CD117 | 1:500 | Agilent Dako; Santa Clara, CA, United States | Heat-induced epitope retrieval, pH 9, 20′ | Mast cell tumor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, V.; Nordio, L.; Vignoli, M.; Masci, S.; Ressel, L.; Crisi, P.E. Clinical, Histological, and Immunohistochemical Insights into a Canine Hepatic Myofibroblastic Sarcoma. Vet. Sci. 2025, 12, 521. https://doi.org/10.3390/vetsci12060521
Rinaldi V, Nordio L, Vignoli M, Masci S, Ressel L, Crisi PE. Clinical, Histological, and Immunohistochemical Insights into a Canine Hepatic Myofibroblastic Sarcoma. Veterinary Sciences. 2025; 12(6):521. https://doi.org/10.3390/vetsci12060521
Chicago/Turabian StyleRinaldi, Valentina, Laura Nordio, Massimo Vignoli, Stefano Masci, Lorenzo Ressel, and Paolo Emidio Crisi. 2025. "Clinical, Histological, and Immunohistochemical Insights into a Canine Hepatic Myofibroblastic Sarcoma" Veterinary Sciences 12, no. 6: 521. https://doi.org/10.3390/vetsci12060521
APA StyleRinaldi, V., Nordio, L., Vignoli, M., Masci, S., Ressel, L., & Crisi, P. E. (2025). Clinical, Histological, and Immunohistochemical Insights into a Canine Hepatic Myofibroblastic Sarcoma. Veterinary Sciences, 12(6), 521. https://doi.org/10.3390/vetsci12060521







