Post-Insemination Infusion of Wharton’s Jelly Mesenchymal Stromal/Stem Cells-Derived Conditioned Medium: A Novel Approach for Improving Pregnancy Outcomes in Problem Mares
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
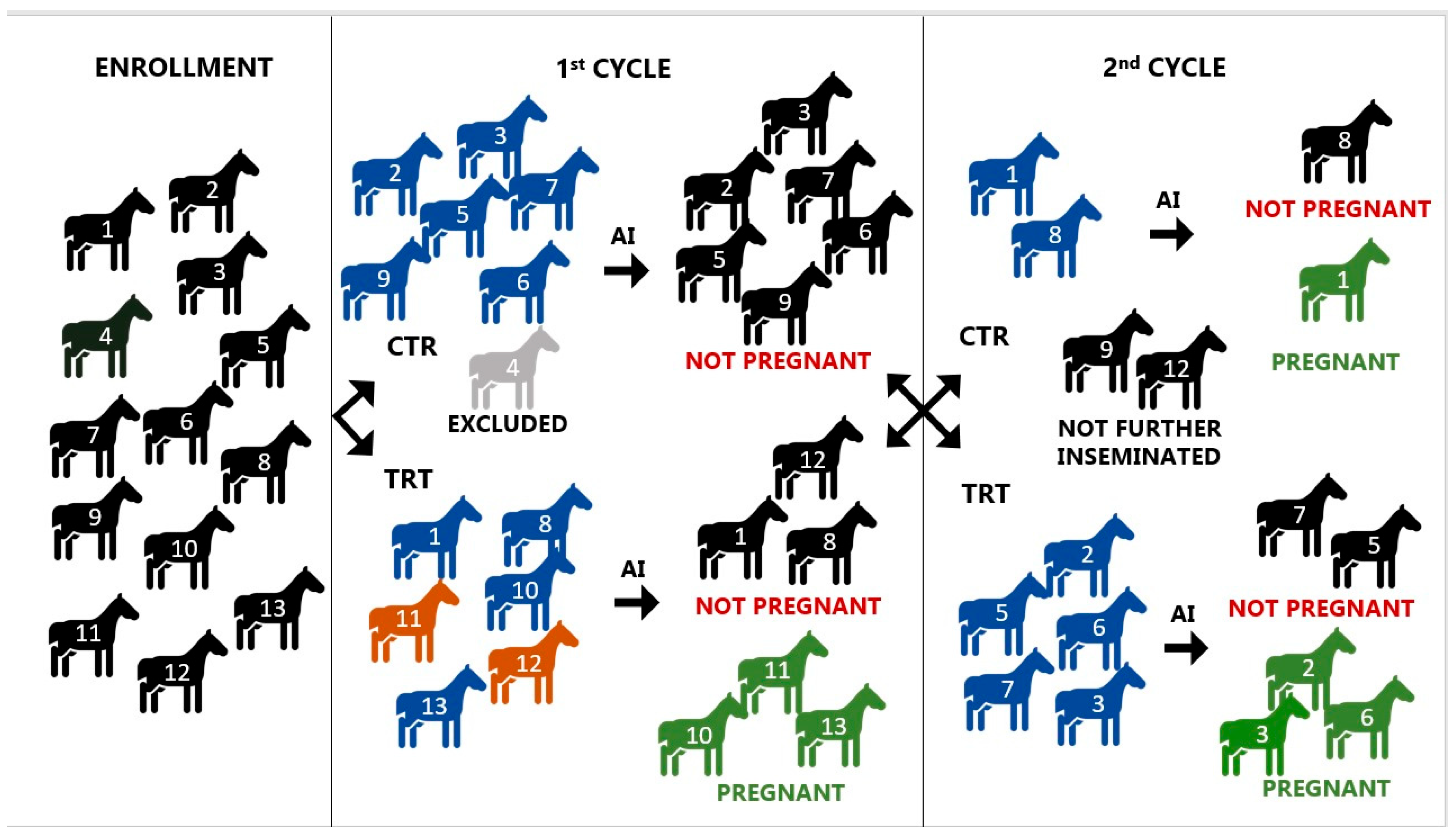
2.2. Study Design
2.3. Preparation of the Conditioned Medium
2.4. Insemination and Treatment
2.5. Analysis of LVF
2.6. Statistical Analysis
3. Results
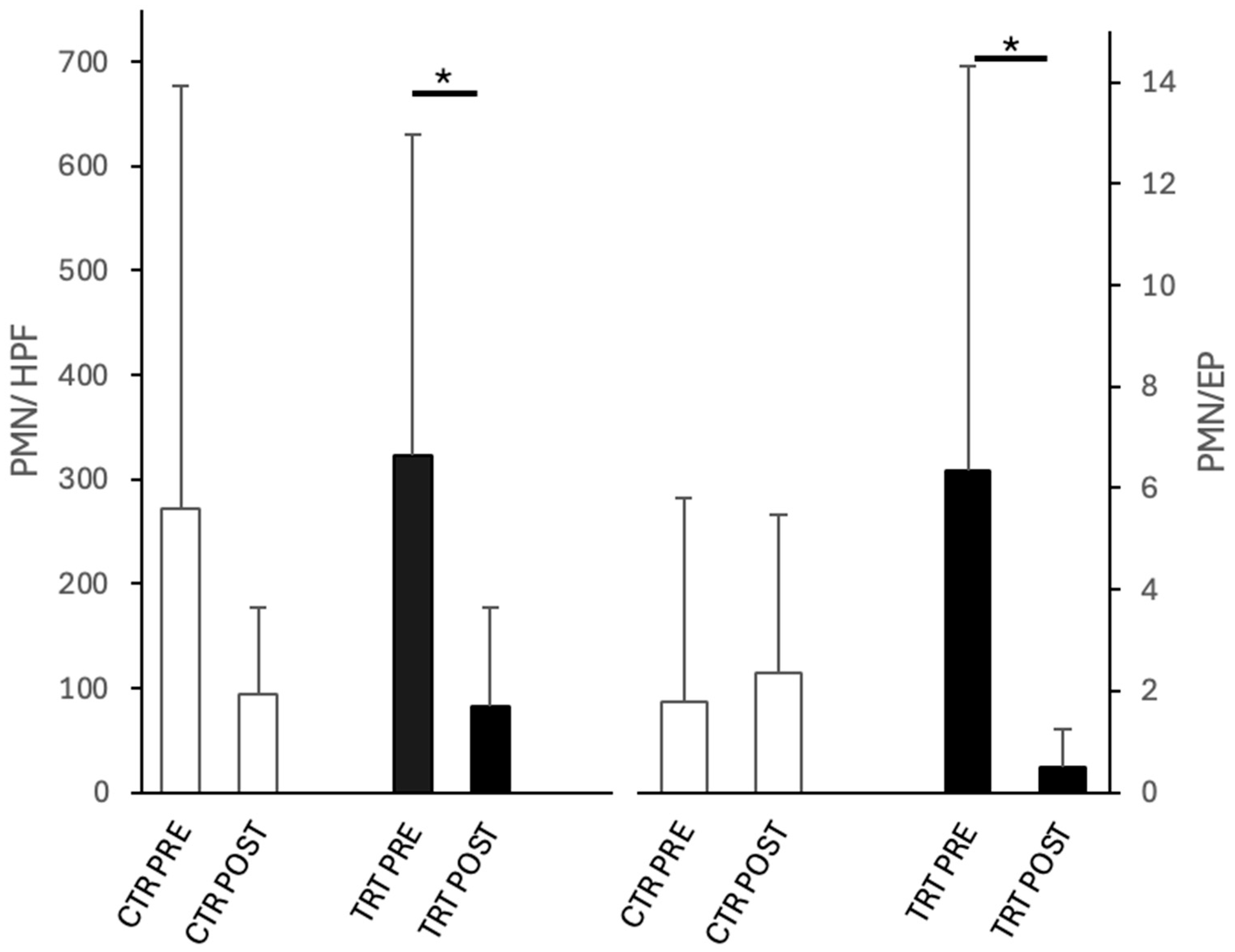
3.1. Clinical Finding
3.2. Bacterial Isolation and Identification
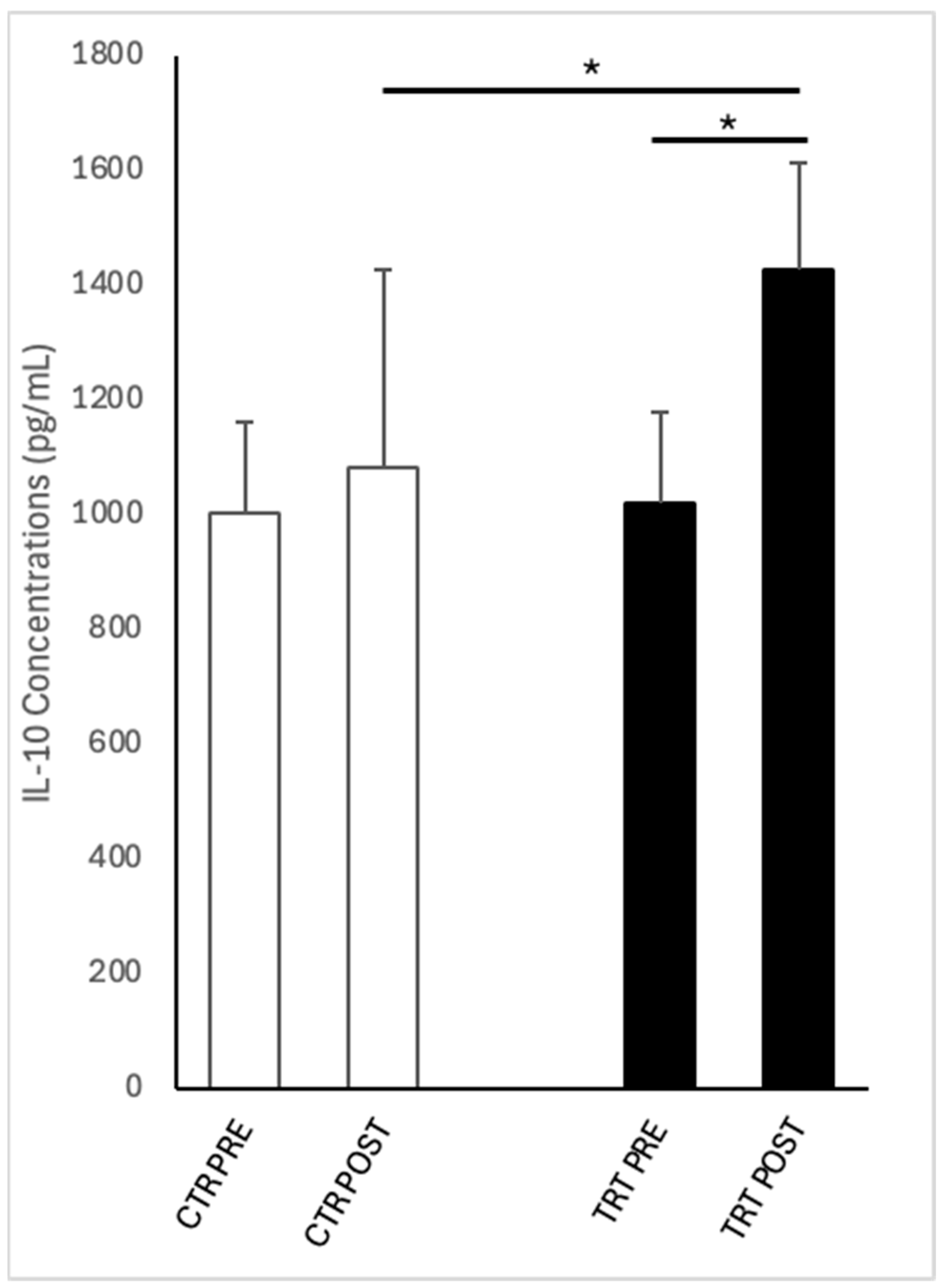
3.3. IL-10 Concentrations
3.4. Pregnancy Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | Conditioned medium |
| CTR | Control group |
| DMEM | Dulbecco’s Minimum Essential Medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| E. coli | Escherichia coli |
| EP | Epithelial cells |
| HPF | High power field |
| IL-10 | Interleukin-10 |
| PMN | Polimorphonuclear neutrophils |
| POST | After treatment |
| PRE | Before treatment |
| TRT | Treated group |
| UC | Umbilical cord |
| WJ-MSC-CM | Wharton’s jelly mesenchymal stromal/stem cell-derived medium |
References
- Troedsson, M.H.T. Uterine Clearance and Resistance to Persistent Endometritis in the Mare. Theriogenology 1999, 52, 461–471. [Google Scholar] [CrossRef]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical Problems of Adult Horses, as Ranked by Equine Practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [CrossRef]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Foster, D.N.; Carlson, C.S.; Troedsson, M.H.T. Nitric Oxide Levels and Nitric Oxide Synthase Expression in Uterine Samples from Mares Susceptible and Resistant to Persistent Breeding-Induced Endometritis. Am. J. Reprod. Immunol. 2005, 53, 230–237. [Google Scholar] [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Biol. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Liu, I.K.M.; Crabo, B.G. Sperm Transport and Survival in the Mare. Theriogenology 1998, 49, 905–915. [Google Scholar] [CrossRef]
- Troedsson, M.H.T. Therapeutic Considerations for Mating-Induced Endometritis. Pferdeheilkunde Equine Med. 1997, 13, 516–520. [Google Scholar] [CrossRef]
- Troedsson, M.H.; Liu, I.K. Uterine Clearance of Non-Antigenic Markers (51Cr) in Response to a Bacterial Challenge in Mares Potentially Susceptible and Resistant to Chronic Uterine Infections. J. Reprod. Fertil. Suppl. 1991, 44, 283–288. [Google Scholar]
- Carnevale, E.M.; Ramirez, R.J.; Squires, E.L.; Alvarenga, M.A.; Vanderwall, D.K.; McCue, P.M. Factors Affecting Pregnancy Rates and Early Embryonic Death after Equine Embryo Transfer. Theriogenology 2000, 54, 965–979. [Google Scholar] [CrossRef]
- Bucca, S.; Carli, A.; Buckley, T.; Dolci, G.; Fogarty, U. The Use of Dexamethasone Administered to Mares at Breeding Time in the Modulation of Persistent Mating Induced Endometritis. Theriogenology 2008, 70, 1093–1100. [Google Scholar] [CrossRef]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial Inflammatory Markers of the Early Immune Response in Mares Susceptible or Resistant to Persistent Breeding-Induced Endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Furzi, C.; Panzani, D.; Camillo, F. Studies on Motility and Fertility of Cooled Stallion Spermatozoa. Reprod. Domest. Anim. 2004, 39, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Riddle, W.T.; LeBlanc, M.M.; Stromberg, A.J. Relationships between Uterine Culture, Cytology and Pregnancy Rates in a Thoroughbred Practice. Theriogenology 2007, 68, 395–402. [Google Scholar] [CrossRef]
- Kareskoski, M.; Venhoranta, H.; Virtala, A.M.; Katila, T. Analysis of Factors Affecting the Pregnancy Rate of Mares after Inseminations with Cooled Transported Stallion Semen. Theriogenology 2019, 127, 7–14. [Google Scholar] [CrossRef]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of Embryo Transport through the Mare Oviduct. Theriogenology 1991, 36, 823–830. [Google Scholar] [CrossRef]
- Christoffersen, M.; Woodward, E.M.; Bojesen, A.M.; Petersen, M.R.; Squires, E.L.; Lehn-Jensen, H.; Troedsson, M.H.T. Effect of Immunomodulatory Therapy on the Endometrial Inflammatory Response to Induced Infectious Endometritis in Susceptible Mares. Theriogenology 2012, 78, 991–1004. [Google Scholar] [CrossRef]
- Friso, A.M.; Segabinazzi, L.G.T.M.; Cyrino, M.; Correal, S.B.; Freitas-Dell’Aqua, C.P.; Teoro do Carmo, M.; Dell’Aqua, J.A.; Miró, J.; Papa, F.O.; Alvarenga, M.A. Periovulatory Administration of Firocoxib Did Not Alter Ovulation Rates and Mitigated Post-Breeding Inflammatory Response in Mares. Theriogenology 2019, 138, 24–30. [Google Scholar] [CrossRef]
- Leblanc, M.; Causey, R. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Scoggin, C.F. Endometritis: Nontraditional Therapies. Vet. Clin. N. Am. Equine Pract. 2016, 32, 499–511. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Nielsen, J.M. Non-Antibiotic Treatment of Equine Endometritis. Pferdeheilkunde 2018, 34, 17–22. [Google Scholar] [CrossRef]
- Del Prete, C.; Montano, C.; Cocchia, N.; de Chiara, M.; Gasparrini, B.; Pasolini, M.P. Use of Regenerative Medicine in the Treatment of Endometritis in Mares: A Systematic Review and Meta-Analysis. Theriogenology 2024, 227, 9–20. [Google Scholar] [CrossRef]
- Caplan, A.I. What’s in a Name? Tissue Eng. Part A 2010, 16, 2415–2417. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Baber, M.A.; Caplan, A.I. Influence of Adult Mesenchymal Stem Cells on In Vitro Vascular Formation. Tissue Eng. Part. A Res. Adv. 2009, 15, 1751–1761. [Google Scholar] [CrossRef]
- Mambelli, L.I.; Mattos, R.C.; Winter, G.H.Z.; Madeiro, D.S.; Morais, B.P.; Malschitzky, E.; Miglino, M.A.; Kerkis, A.; Kerkis, I. Changes in Expression Pattern of Selected Endometrial Proteins Following Mesenchymal Stem Cells Infusion in Mares with Endometrosis. PLOS ONE 2014, 9, e97889. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Van de Walle, G.R. The Mesenchymal Stromal Cell Secretome Impairs Methicillin-Resistant Staphylococcus Aureus Biofilms via Cysteine Protease Activity in the Equine Model. Stem Cells Transl. Med. 2020, 9, 746–757. [Google Scholar] [CrossRef]
- Silva-Carvalho, A.É.; Cardoso, M.H.; Alencar-Silva, T.; Bogéa, G.M.R.; Carvalho, J.L.; Franco, O.L.; Saldanha-Araujo, F. Dissecting the Relationship between Antimicrobial Peptides and Mesenchymal Stem Cells. Pharmacol. Ther. 2022, 233, 108021. [Google Scholar] [CrossRef]
- Khatibi, S.M.H.; Kheyrolahzadeh, K.; Barzegari, A.; Saadat, Y.R.; Vahed, S.Z. Medicinal Signaling Cells: A Potential Antimicrobial Drug Store. J. Cell Physiol. 2020, 235, 7731–7746. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic Applications of Adipose Cell-Free Derivatives: A Review. Stem Cell Res. Ther. 2020, 11, 312. [Google Scholar] [CrossRef]
- Meisel, R.; Brockers, S.; Heseler, K.; Degistirici; Bülle, H.; Woite, C.; Stuhlsatz, S.; Schwippert, W.; Jäger, M.; Sorg, R.; et al. Human but Not Murine Multipotent Mesenchymal Stromal Cells Exhibit Broad-Spectrum Antimicrobial Effector Function Mediated by Indoleamine 2,3-Dioxygenase. Leukemia 2011, 25, 648–654. [Google Scholar] [CrossRef]
- Alcayaga-Miranda, F.; Cuenca, J.; Khoury, M. Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies. Front. Immunol. 2017, 8, 248157. [Google Scholar] [CrossRef]
- Cortés-Araya, Y.; Amilon, K.; Rink, B.E.; Black, G.; Lisowski, Z.; Donadeu, F.X.; Esteves, C.L. Comparison of Antibacterial and Immunological Properties of Mesenchymal Stem/Stromal Cells from Equine Bone Marrow, Endometrium, and Adipose Tissue. Stem Cells Dev. 2018, 27, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Kim, H.O.; Choi, S.M.; Kim, H.S. Mesenchymal Stem Cell-Derived Secretome and Microvesicles as a Cell-Free Therapeutics for Neurodegenerative Disorders. Tissue. Eng. Regen. Med. 2013, 10, 93–101. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-Inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv. Exp. Med. Biol. 2019, 1084, 187–206. [Google Scholar] [CrossRef]
- Lanci, A.; Merlo, B.; Grandis, A.; Mariella, J.; Castagnetti, C.; Iacono, E. Gross and Histological Examination of Wharton’s Jelly in the Equine Umbilical Cord. Theriogenology 2023, 209, 184–192. [Google Scholar] [CrossRef]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, Characterization and Differentiation of Mesenchymal Stem Cells from Amniotic Fluid, Umbilical Cord Blood and Wharton’s Jelly in the Horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef]
- Merlo, B.; Pirondi, S.; Iacono, E.; Rossi, B.; Ricci, F.; Mari, G. Viability, in Vitro Differentiation and Molecular Characterization of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Cryopreserved in Serum and Serum-Free Medium. CryoLetters 2016, 37, 243–252. [Google Scholar]
- Samper, J.C. A Review of a Practitioner’s Perspective on Endometrial Edema. Pferdeheilkunde 2010, 26, 14–18. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Petersen, M.R.; Bojesen, A.M.; Pedersen, H.G.; Lehn-Jensen, H.; Christoffersen, M. Equine Infectious Endometritis—Clinical and Subclinical Cases. J. Equine Vet. Sci. 2015, 35, 95–104. [Google Scholar] [CrossRef]
- Leblanc, M.M. How to Perform and Interpret Findings From a Low-Volume Uterine Flush. In Proceedings of the 57th Annual Convention of the American Association of Equine Practitioners, San Antonio, TX, USA, 18–22 November 2011; Volume 57, pp. 32–36. [Google Scholar]
- Nocera, F.P.; D’eletto, E.; Ambrosio, M.; Fiorito, F.; De Martino, L.; De Martino, L. Occurrence and Antimicrobial Susceptibility Profiles of Streptococcus Equi Subsp. Zooepidemicus Strains Isolated from Mares with Fertility Problems. Antibiotics 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Cocchia, N.; Paciello, O.; Auletta, L.; Uccello, V.; Silvestro, L.; Mallardo, K.; Paraggio, G.; Pasolini, M.P. Comparison of the Cytobrush, Cottonswab, and Low-Volume Uterine Flush Techniques to Evaluate Endometrial Cytology for Diagnosing Endometritis in Chronically Infertile Mares. Theriogenology 2012, 77, 89–98. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and Scale-up of Wharton’s Jelly-Derived Mesenchymal Stem Cells for Clinical Applications. Stem Cell Res. 2010, 5, 244–254. [Google Scholar] [CrossRef]
- Higuchi, O.; Okabe, M.; Yoshida, T.; Fathy, M.; Saito, S.; Miyawaki, T.; Nikaido, T. Stemness of Human Wharton’s Jelly Mesenchymal Cells Is Maintained by Floating Cultivation. Cell Reprogr. 2012, 14, 448–455. [Google Scholar] [CrossRef]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Mareschi, K.; Castiglia, S.; Sanavio, F.; Rustichelli, D.; Muraro, M.; Defedele, D.; Bergallo, M.; Fagioli, F. Immunoregulatory Effects on T Lymphocytes by Human Mesenchymal Stromal Cells Isolated from Bone Marrow, Amniotic Fluid, and Placenta. Exp. Hematol. 2016, 44, 138–150.e1. [Google Scholar] [CrossRef]
- Bárcia, R.N.; Santos, J.M.; Filipe, M.; Teixeira, M.; Martins, J.P.; Almeida, J.; Água-Doce, A.; Almeida, S.C.P.; Varela, A.; Pohl, S.; et al. What Makes Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int. 2015, 2015, 583984. [Google Scholar] [CrossRef]
- Fong, C.Y.; Gauthaman, K.; Cheyyatraivendran, S.; Lin, H.D.; Biswas, A.; Bongso, A. Human Umbilical Cord Wharton’s Jelly Stem Cells and Its Conditioned Medium Support Hematopoietic Stem Cell Expansion Ex Vivo. J. Cell Biochem. 2012, 113, 658–668. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Kalluri, R. Emerging Role of Bacterial Extracellular Vesicles in Cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, F.K.; Hanlon, D.W.; Hou, W.; Tasma, Z.; Damani, T.; Bouma, G.J.; Murtazina, D.A.; Chamley, L. Use of Equine Embryo -Derived Mesenchymal Stromal Cells and Their Extracellular Vesicles as a Treatment for Persistent Breeding-Induced Endometritis in Susceptible Mares. J. Equine Vet. Sci. 2024, 139, 105079. [Google Scholar] [CrossRef] [PubMed]
- Laroye, C.; Boufenzer, A.; Jolly, L.; Cunat, L.; Alauzet, C.; Merlin, J.L.; Yguel, C.; Bensoussan, D.; Reppel, L.; Gibot, S. Bone Marrow vs Wharton’s Jelly Mesenchymal Stem Cells in Experimental Sepsis: A Comparative Study. Stem Cell Res. Ther. 2019, 10, 192. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Funghi, F.; Cantile, C.; Idda, A.; Cremonesi, F.; Riccaboni, P. Case Report: Use of Amniotic Microvesicles for Regenerative Medicine Treatment of a Mare With Chronic Endometritis. Front. Vet. Sci. 2020, 7, 529611. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Abdelnaby, E.A.; Abdallah, A.N.; Anwar, I.M.; El-Tookhy, O.S.; Shamaa, A.A. The Therapeutic Effect of Stem Cell-Derived Exosomes in the Treatment of Chronic Endometritis as Assessed by Histopathological, Doppler and Hormonal Expression in Arabian Mares. Equine Vet. Educ. 2024, 36, 347–356. [Google Scholar] [CrossRef]
- Cyktor, J.C.; Turner, J. Interleukin-10 and Immunity against Prokaryotic and Eukaryotic Intracellular Pathogens. Infect. Immun. 2011, 79, 2964–2973. [Google Scholar] [CrossRef]
- Ferris, R.A.; Frisbie, D.D.; McCue, P.M. Use of Mesenchymal Stem Cells or Autologous Conditioned Serum to Modulate the Inflammatory Response to Spermatozoa in Mares. Theriogenology 2014, 82, 36–42. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Gaspari, G.; Funghi, F.; Capra, E.; Cretich, M.; Frigerio, R.; Bosi, G.; Cremonesi, F. Amniotic Mesenchymal-Derived Extracellular Vesicles and Their Role in the Prevention of Persistent Post-Breeding Induced Endometritis. Int. J. Mol. Sci. 2023, 24, 5166. [Google Scholar] [CrossRef]
- de Tongu, E.A.O.; Segabinazzi, L.G.T.M.; Alvarenga, M.L.; Monteiro, A.; Papa, F.O.; Alvarenga, M.A. Allogenic Mesenchymal Stem Cell-Conditioned Medium Does Not Affect Sperm Parameters and Mitigates Early Endometrial Inflammatory Responses in Mares. Theriogenology 2021, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Stewart, J.; da Silva, M.A.C. Endometritis: Managing Persistent Post-Breeding Endometritis. Vet. Clin. N. Am. Equine Pr. 2016, 32, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Neuberg, K.P.; Failing, K.; Wehrend, A. Cytological Diagnosis of Endometritis in the Mare: Investigations of Sampling Techniques and Relation to Bacteriological Results. Anim. Reprod. Sci. 2012, 132, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Beltaire, K.A.; Cheong, S.H.; da Silva, M.A.C. Retrospective Study on Equine Uterine Fungal Isolates and Antifungal Susceptibility Patterns (1999–2011). Equine Vet. J. Suppl. 2012, 44, 84–87. [Google Scholar] [CrossRef]
- Del Prete, C.; Nocera, F.P.; Piegari, G.; Palumbo, V.; De Martino, L.; Cocchia, N.; Paciello, O.; Montano, C.; Pasolini, M.P. Use of Cytobrush for Bacteriological and Cytological Diagnosis of Endometritis in Mares. Vet. World 2024, 17, 398. [Google Scholar] [CrossRef]
- Albihn, A.; Båverud, V.; Magnusson, U. Uterine Microbiology and Antimicrobial Susceptibility in Isolated Bacteria from Mares with Fertility Problems. Acta Vet. Scand. 2003, 44, 121–129. [Google Scholar] [CrossRef]
- Frontoso, R.; De Carlo, E.; Pasolini, M.P.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective Study of Bacterial Isolates and Their Antimicrobial Susceptibilities in Equine Uteri during Fertility Problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Nocera, F.P.; Capozzi, L.; Simone, D.; Pizzano, F.; Iovane, V.; Bianco, A.; Parisi, A.; De Martino, L. Multi-Locus Sequence Typing and in Vitro Antimicrobial Resistance of Equine Streptococcus Equi Subspecies Zooepidemicus Strains. Vet. Res. Commun. 2024, 48, 215–224. [Google Scholar] [CrossRef]
- Nocera, F.P.; Ambrosio, M.; Conte, A.; Di Palma, T.; Castaldo, S.; Pasolini, M.P.; Fiorito, F.; De Martino, L. Importance of Broth-Enrichment Culture in Equine Endometritis Diagnosis. New Microbiol 2021, 44, 19–23. [Google Scholar]
- Däubener, W.; Schmidt, S.K.; Heseler, K.; Spekker, K.H.; MacKenzie, C.R. Antimicrobial and Immunoregulatory Effector Mechanisms in Human Endothelial Cells Indoleamine 2,3-Dioxygenase versus Inducible Nitric Oxide Synthase. Thromb. Haemost. 2009, 102, 1110–1116. [Google Scholar] [CrossRef]
- Marx, C.; Gardner, S.; Harman, R.M.; Wagner, B.; Van de Walle, G.R. Mesenchymal Stromal Cell-Secreted CCL2 Promotes Antibacterial Defense Mechanisms through Increased Antimicrobial Peptide Expression in Keratinocytes. Stem Cells Transl. Med. 2021, 10, 1666–1679. [Google Scholar] [CrossRef]
| Group | ID Mares | Cycle | PRE | POST | Pregnancy |
|---|---|---|---|---|---|
| CTR | 2 | 1st | Escherichia coli | Escherichia coli | N |
| 3 | 1st | Streptococcus equinus; Escherichia coli | Streptococcus equinus; Escherichia coli | N | |
| 5 | 1st | Pseudomonas putida | Pseudomonas putida | N | |
| 6 | 1st | Staphylococcus aureus | Staphylococcus aureus | N | |
| 7 | 1st | Deftia tsurunatensis | Escherichia coli | N | |
| 9 | 1st | Escherichia coli | Escherichia coli | N | |
| 1 | 2nd | Streptococcus equi subsp. zooepidemicus; Escherichia coli | Streptococcus equi subsp. zooepidemicus; Escherichia coli | Y | |
| 8 | 2nd | Enterococcus faecalis; Escherichia coli | Enterococcus faecalis; Escherichia coli | N | |
| TRT | 1 | 1st | Escherichia coli; Streptococcus equi subsp. zooepidemicus | Escherichia coli; Streptococcus equi subsp. zooepidemicus | N |
| 8 | 1st | - | Escherichia coli; Streptococcus equi subsp. zooepidemicus | N | |
| 10 | 1st | Streptococcus equinus | Streptococcus equinus | Y | |
| 11 | 1st | - | - | Y | |
| 12 | 1st | - | - | N | |
| 13 | 1st | Escherichia coli; Enterococcus faecalis; Staphylococcus aureus | Escherichia coli; Enterococcus faecalis; Staphylococcus aureus | Y | |
| 2 | 2nd | Pseudomonas spp. | Escherichia coli | Y | |
| 3 | 2nd | Escherichia coli; Staphylococcus schleiferi | Escherichia coli; Staphylococcus schleiferi | Y | |
| 5 | 2nd | Streptococcus dysgalactiae; Klebsiella pneumoniae | Streptococcus dysgalactiae | N | |
| 6 | 2nd | Staphylococcus aureus; Streptococcus dysgalactiae; Escherichia coli | Staphylococcus aureus; Streptococcus dysgalactiae; Escherichia coli | Y | |
| 7 | 2nd | - | Escherichia coli | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Prete, C.; Attolini, E.; Merlo, B.; Iacono, E.; Nocera, F.P.; De Martino, L.; Longobardi, C.; Damiano, S.; Longobardi, V.; Cocchia, N.; et al. Post-Insemination Infusion of Wharton’s Jelly Mesenchymal Stromal/Stem Cells-Derived Conditioned Medium: A Novel Approach for Improving Pregnancy Outcomes in Problem Mares. Vet. Sci. 2025, 12, 482. https://doi.org/10.3390/vetsci12050482
Del Prete C, Attolini E, Merlo B, Iacono E, Nocera FP, De Martino L, Longobardi C, Damiano S, Longobardi V, Cocchia N, et al. Post-Insemination Infusion of Wharton’s Jelly Mesenchymal Stromal/Stem Cells-Derived Conditioned Medium: A Novel Approach for Improving Pregnancy Outcomes in Problem Mares. Veterinary Sciences. 2025; 12(5):482. https://doi.org/10.3390/vetsci12050482
Chicago/Turabian StyleDel Prete, Chiara, Emilia Attolini, Barbara Merlo, Eleonora Iacono, Francesca Paola Nocera, Luisa De Martino, Consiglia Longobardi, Sara Damiano, Valentina Longobardi, Natascia Cocchia, and et al. 2025. "Post-Insemination Infusion of Wharton’s Jelly Mesenchymal Stromal/Stem Cells-Derived Conditioned Medium: A Novel Approach for Improving Pregnancy Outcomes in Problem Mares" Veterinary Sciences 12, no. 5: 482. https://doi.org/10.3390/vetsci12050482
APA StyleDel Prete, C., Attolini, E., Merlo, B., Iacono, E., Nocera, F. P., De Martino, L., Longobardi, C., Damiano, S., Longobardi, V., Cocchia, N., & Pasolini, M. P. (2025). Post-Insemination Infusion of Wharton’s Jelly Mesenchymal Stromal/Stem Cells-Derived Conditioned Medium: A Novel Approach for Improving Pregnancy Outcomes in Problem Mares. Veterinary Sciences, 12(5), 482. https://doi.org/10.3390/vetsci12050482







