Clinical Features, MRI Findings, Treatment, and Outcomes in Dogs with Haemorrhagic Myelopathy Secondary to Steroid-Responsive Meningitis-Arteritis: Nine Cases (2017–2024)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Study Designs, Inclusion, Exclusion Criteria, Medical Record Search, and Data Extraction
3. Results
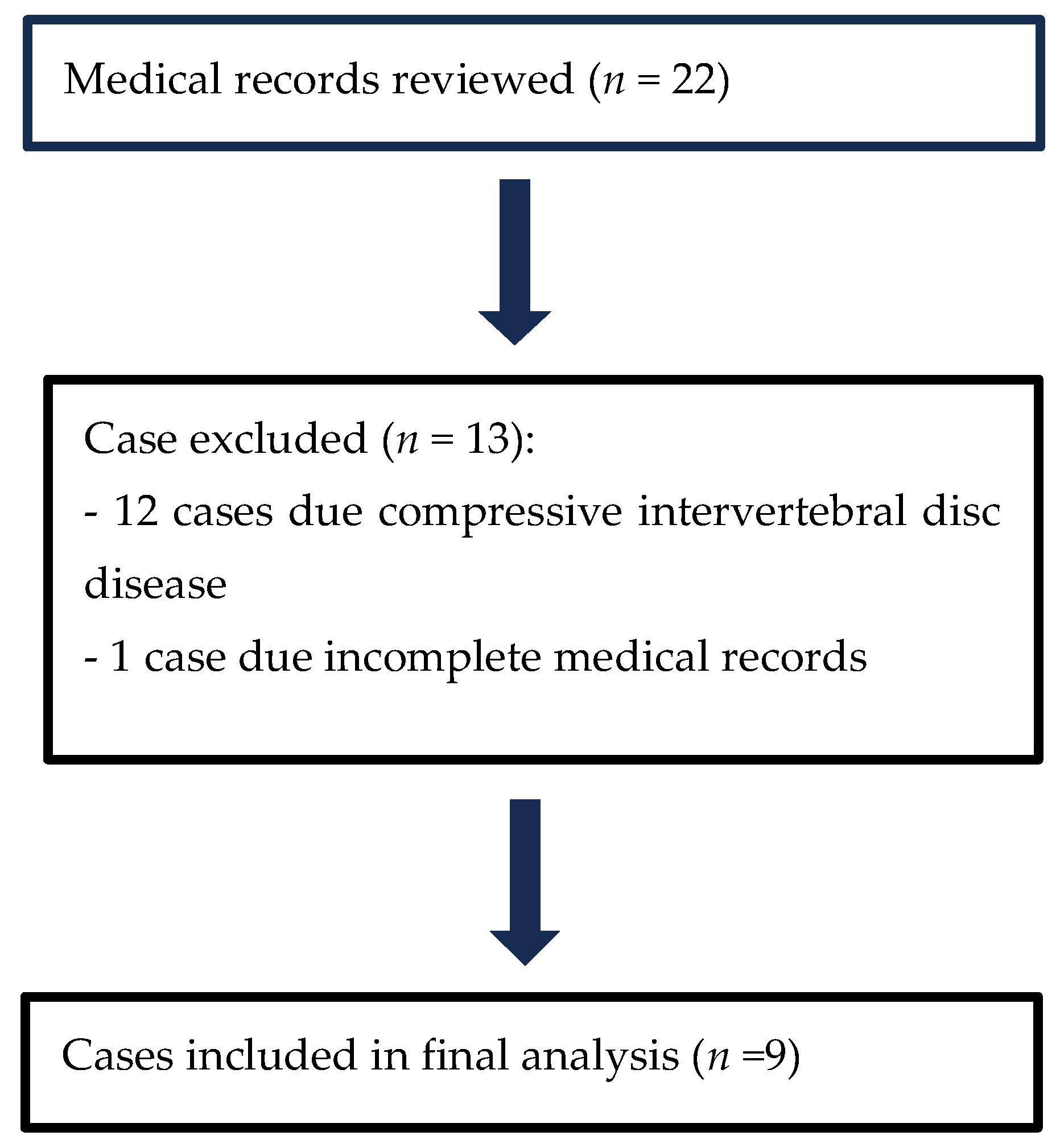
3.1. Case Selection
3.2. Cases Included
3.3. History and Findings of Clinical and Neurological Examination
3.4. Clinicopathologic Analyses
3.5. Diagnostic Imaging Findings
3.6. Diagnosis, Treatments, Outcomes, and Follow-Up
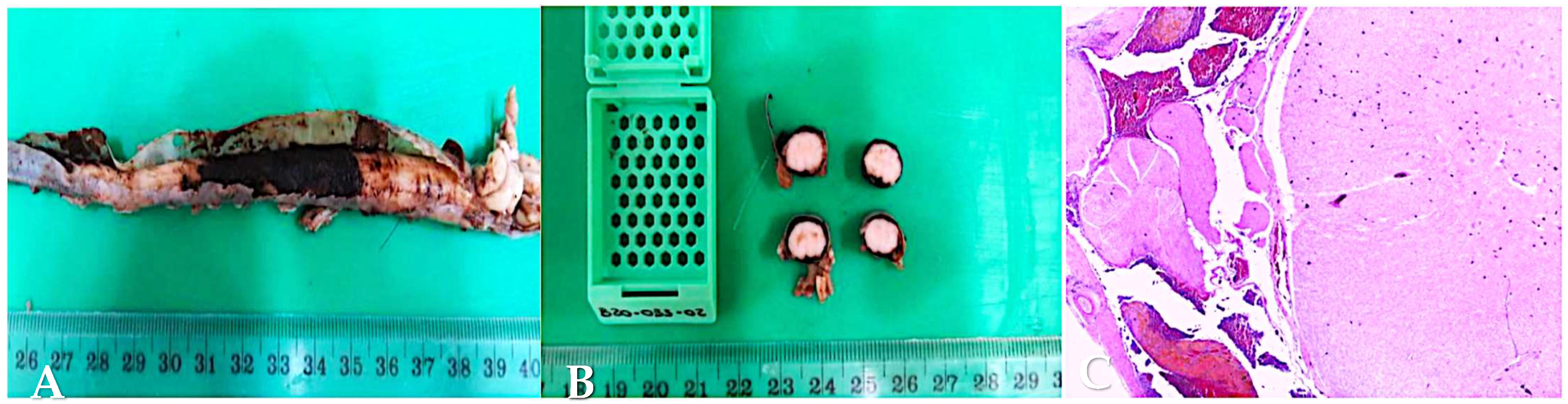
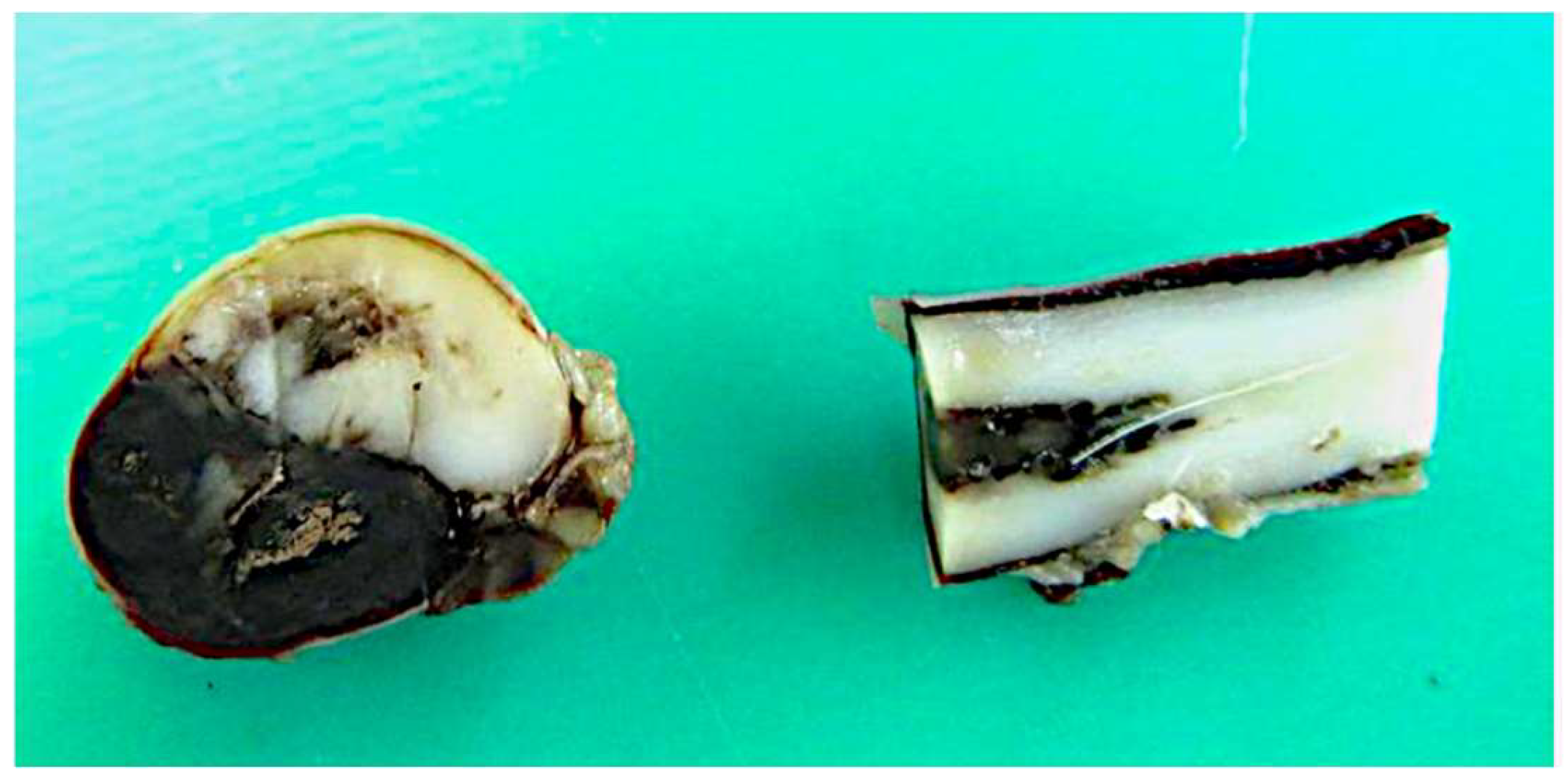
3.7. Post-Mortem Examination and Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tipold, A.; Schatzberg, S.J. An Update on Steroid Responsive Meningitis-arteritis. J. Small Anim. Pract. 2010, 51, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Nettifee, J.A.; Early, P.J.; Mariani, C.L.; Olby, N.J.; Muñana, K.R. Clinical Characteristics, Breed Differences, and Quality of Life in North American Dogs with Acute Steroid-responsive Meningitis-arteritis. J. Vet. Intern. Med. 2019, 33, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- DeLahunta, A.; Glass, E.; Kent, M. De Lahunta’s Veterinary Neuroanatomy and Clinical Neurology; Elsevier: Philadelphia, PA, USA, 2021; pp. 306–308. [Google Scholar]
- Lowrie, M.; Penderis, J.; McLaughlin, M.; Eckersall, P.D.; Anderson, T.J. Steroid Responsive Meningitis-arteritis: A Prospective Study of Potential Disease Markers, Prednisolone Treatment, and Long-term Outcome in 20 Dogs (2006–2008). J. Vet. Intern. Med. 2009, 23, 862–870. [Google Scholar] [CrossRef]
- Fluehmann, G.; Doherr, M.G.; Jaggy, A. Canine Neurological Diseases in a Referral Hospital Population between 1989 and 2000 in Switzerland. J. Small Anim. Pract. 2006, 47, 582–587. [Google Scholar] [CrossRef]
- Wohlsein, J.C.; Tipold, A. Steroid-Responsive Meningitis-Arteritis: What Have We Learned since 2010? A Narrative Review. Vet. J. 2023, 300–302, 106030. [Google Scholar] [CrossRef] [PubMed]
- Cizinauskas, S.; Jaggy, A.; Tipold, A. Long-term Treatment of Dogs with Steroid-responsive Meningitis-arteritis: Clinical, Laboratory and Therapeutic Results. J. Small Anim. Pract. 2000, 41, 295–301. [Google Scholar] [CrossRef]
- Carletti, B.E.; De Decker, S.; Rose, J.; Sanchez-Masian, D.; Bersan, E.; Cooper, C.; Szladovits, B.; Walmsley, G.; Gonçalves, R. Evaluation of Concurrent Analysis of Cerebrospinal Fluid Samples Collected from the Cerebellomedullary Cistern and Lumbar Subarachnoid Space for the Diagnosis of Steroid-Responsive Meningitis Arteritis in Dogs. J. Am. Vet. Med. Assoc. 2019, 255, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Pfister, H.; Zurbriggen, A.; Vandevelde, M. Intrathecal Synthesis of Major Immunoglobulin Classes in Inflammatory Diseases of the Canine CNS. Vet. Immunol. Immunopathol. 1994, 42, 149–159. [Google Scholar] [CrossRef]
- Lowrie, M.; Penderis, J.; Eckersall, P.D.; McLaughlin, M.; Mellor, D.; Anderson, T.J. The Role of Acute Phase Proteins in Diagnosis and Management of Steroid-Responsive Meningitis Arteritis in Dogs. Vet. J. 2009, 182, 125–130. [Google Scholar] [CrossRef]
- Rose, J.H.; Kwiatkowska, M.; Henderson, E.R.; Granger, N.; Murray, J.K.; Harcourt-Brown, T.R. The Impact of Demographic, Social, and Environmental Factors on the Development of Steroid-responsive Meningitis-arteritis (SRMA) in the United Kingdom. J. Vet. Intern. Med. 2014, 28, 1199–1202. [Google Scholar] [CrossRef]
- Hilpert, E.; Tipold, A.; Meyerhoff, N.; Schwerdt, J.; Winkler, S.; Jurina, K.; Fischer, A.; Kornberg, M.; Parzefall, B.; Flegel, T. Steroid-Responsive Meningitis-Arteritis in Dogs in Germany: Are There Epidemiological or Clinical Factors Influencing Recurrence Rate? Tierärztl. Prax. Ausg. K Kleintiere/Heimtiere 2020, 48, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Tipold, A.; Jaggy, A. Steroid Responsive Meningitis-arteritis in Dogs: Long-term Study of 32 Cases. J. Small Anim. Pract. 1994, 35, 311–316. [Google Scholar] [CrossRef]
- Harcourt, R. Polyarteritis in a Colony of Beagles. Vet. Rec. 1978, 102, 519–522. [Google Scholar] [CrossRef]
- Meric, S.M. Canine Meningitis: A Changing Emphasis. J. Vet. Intern. Med. 1988, 2, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Scott-Moncrieff, J.C.; Snyder, P.W.; Glickman, L.T.; Davis, E.L.; Felsburg, P.J. Systemic Necrotizing Vasculitis in Nine Young Beagles. J. Am. Vet. Med. Assoc. 1992, 201, 1533–1558. [Google Scholar] [CrossRef]
- Rose, J.H.; Harcourt-Brown, T.R. Screening Diagnostics to Identify Triggers in 21 Cases of Steroid-responsive Meningitis-arteritis. J. Small Anim. Pract. 2013, 54, 575–578. [Google Scholar] [CrossRef]
- Lazzerini, K.; Tipold, A.; Kornberg, M.; Silaghi, C.; Mietze, A.; Lübke-Becker, A.; Balling, A.; Pfeffer, M.; Wieler, L.H.; Pfister, K.; et al. Testing for vector transmitted microorganisms in dogs with meningitis and meningoencephalitis of unknown aetiology. J. Vet. Med. Res. 2015, 2, 1014. [Google Scholar]
- Gonçalves, R.; De Decker, S.; Walmsley, G.; Butterfield, S.; Maddox, T.W. Inflammatory Disease Affecting the Central Nervous System in Dogs: A Retrospective Study in England (2010–2019). Front. Vet. Sci. 2022, 8, 819945. [Google Scholar] [CrossRef]
- Barber, R.M.; Li, Q.; Levine, J.M.; Ruone, S.J.; Levine, G.J.; Kenny, P.; Tong, S.; Schatzberg, S.J. Screening for Viral Nucleic Acids in the Cerebrospinal Fluid of Dogs with Central Nervous System Inflammation. Front. Vet. Sci. 2022, 9, 850510. [Google Scholar] [CrossRef]
- Elbert, J.A.; Yau, W.; Rissi, D.R. Neuroinflammatory diseases of the central nervous system of dogs: A retrospective study of 207 cases (2008–2019). Can. Vet. J. 2022, 63, 178–186. [Google Scholar] [PubMed] [PubMed Central]
- Dewey, C.W.; Da Costa, R.C. (Eds.) Practical guide to canine and feline neurology; Wiley Blackwell: Chichester, UK, 2016; pp. 379–381. [Google Scholar]
- Santifort, K.M.; Platt, S. Hemorrhagic Encephalopathies and Myelopathies in Dogs and Cats: A Focus on Classification. Front. Vet. Sci. 2024, 11, 1460568. [Google Scholar] [CrossRef] [PubMed]
- West, N.; Butterfield, S.; Rusbridge, C.; Fernandez, A.; Tabanez, J.; Rudolf, N.J.; Archer, S.; Whittaker, D. Non-traumatic Hemorrhagic Myelopathy in Dogs. J. Vet. Intern. Med. 2023, 37, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.L.; Stieger-Vanegas, S.M.; Valentine, B.A. Hemorrhage in the central canal of the cervical spinal cord in a coonhound diagnosed with canine juvenile polyarteritis (steroid responsive meningitis-arteritis). Can. Vet. J. 2015, 56, 567–570. [Google Scholar] [PubMed] [PubMed Central]
- Zilli, J.; Olszewska, A.; Farke, D.; Schmidt, M.J. Successful Surgical and Medical Treatment of a Severe, Acute Epidural Bleed in a Young Dog Due to Steroid Responsive Meningitis-Arteritis. Acta Vet. Scand. 2021, 63, 27. [Google Scholar] [CrossRef]
- Wang-Leandro, A.; Huenerfauth, E.-I.; Heissl, K.; Tipold, A. MRI Findings of Early-Stage Hyperacute Hemorrhage Causing Extramedullary Compression of the Cervical Spinal Cord in a Dog with Suspected Steroid-Responsive Meningitis-Arteritis. Front. Vet. Sci. 2017, 4, 161. [Google Scholar] [CrossRef]
- Aravindan, A.; Ferreira, A. Epistaxis and Intradural-Extramedullary Haemorrhage in a Dog With Steroid Responsive Meningitis-Arteritis. Vet. Med. Sci. 2025, 11, e70148. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, D.; Antoniadis, G.; Seeling, W. Spinal Hematoma: A Literature Survey with Meta-Analysis of 613 Patients. Neurosurg. Rev. 2003, 26, 1–49. [Google Scholar] [CrossRef]
- Lubbers, C.; Beukers, M.; Bergknut, N.; Paes, G. Hemophilia a Resulting in Severe Hyperesthesia Due to Extraparenchymal Spinal Cord Hemorrhage in a Young Golden Retriever Puppy. Vet. Sci. 2022, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Mayor, C.; de La Fuente, C.; Pereira, A.; Viu, J.; Añor, S. Central Nervous System Vascular Complications Associated with the Acute Form of Steroid-Responsive Meningitis-Arteritis. Vet. J. 2025, 310, 106304. [Google Scholar] [CrossRef]
- Jones, B.A.; Agthe, P.; Scarpante, E.; Crawford, A.; Black, V.; Espadas, I.; Formoso, S.; Fraser, A.R. Magnetic Resonance Imaging Findings in Dogs with Steroid-Responsive Meningitis-Arteritis in the UK and Their Clinical Significance: 53 Cases (2013–2021). J. Small Anim. Pract. 2024, 66, 33–42. [Google Scholar] [CrossRef]
- Ettinger, S.J.; Feldman, E.C.; Cote, E. Textbook of Veterinary Internal Medicine—Inkling E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2024; pp. 92–93+857–862. [Google Scholar]
- Grapes, N.J.; Packer, R.M.; De Decker, S. Clinical Reasoning in Canine Cervical Hyperaesthesia: Which Presenting Features Are Important? Vet. Rec. 2020, 187, 448. [Google Scholar] [CrossRef] [PubMed]
- Saastamoinen, J.; Rutter, C.R.; Jeffery, U. Subconjunctival haemorrhage in 147 dogs. J Small Anim Pract. 2019, 60, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Willesen, J.L.; Langhorn, R.; Nielsen, L.N. Hemostatic Dysfunction in Dogs Naturally Infected with Angiostrongylus vasorum-A Narrative Review. Pathogens 2022, 11, 249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Schnyder, M.; Willesen, J.L.; Potter, A.; Chandrashekar, R. Performance of the Angio Detect™ in-clinic test kit for detection of Angiostrongylus vasorum infection in dog samples from Europe. Vet. Parasitol. Reg. Stud. Rep. 2017, 7, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Ranberg, E.; Berendt, M.; Gredal, H. Biomarkers of Non-Infectious Inflammatory CNS Diseases in Dogs: Where Are We Now? Part 2—Steroid Responsive Meningitis-Arteritis. Vet. J. 2021, 273, 105692. [Google Scholar] [CrossRef] [PubMed]
- Maiolini, A.; Carlson, R.; Schwartz, M.; Gandini, G.; Tipold, A. Determination of Immunoglobulin a Concentrations in the Serum and Cerebrospinal Fluid of Dogs: An Estimation of Its Diagnostic Value in Canine Steroid-Responsive Meningitis–Arteritis. Vet. J. 2012, 191, 219–224. [Google Scholar] [CrossRef]
- Fuchs, C.; Zander, S.; Meyer-Lindenberg, A.; Tipold, A. Steroid—Responsive meningitis—Arteriitis in dogs: Computed tomography findings. In Proceedings of the European Society of Veterinary Neurology, 14th Annual Meeting, Venice, Italy, 21–23 September 2023; p. 79. [Google Scholar]
- Tipold, A.; Stein, V.M. Inflammatory Diseases of the Spine in Small Animals. Vet. Clin. North Am. Small Anim. Pract. 2010, 40, 871–879. [Google Scholar] [CrossRef]
- Remelli, C.; Martello, A.; Valentini, A.; Contiero, B.; Bernardini, M. Magnetic Resonance Imaging Highlights the Meningeal Involvement in Steroid Responsive Meningitis-Arteritis and Suggests the Inflammation of the Surrounding Tissues (70 Cases). Front. Vet. Sci. 2022, 9, 957278. [Google Scholar] [CrossRef]
- Pais, D.; Casal, D.; Arantes, M.; Casimiro, M.; O’Neill, J.G. Spinal cord arteries in Canis familiaris and their variations: Implications in experimental procedures. Braz. J. Morphol. Sci. 2007, 24, 224–228. [Google Scholar]
- Mazensky, D.; Flesarova, S.; Sulla, I. Arterial Blood Supply to the Spinal Cord in Animal Models of Spinal Cord Injury. A Review. Anat. Rec. 2017, 300, 2091–2106. [Google Scholar] [CrossRef]
- Hague, D.W.; Joslyn, S.; Bush, W.W.; Glass, E.N.; Durham, A.C. Clinical, Magnetic Resonance Imaging, and Histopathologic Findings in 6 Dogs with Surgically Resected Extraparenchymal Spinal Cord Hematomas. J. Vet. Intern. Med. 2015, 29, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Herring, J.; McMichael, M. Diagnostic Approach to Small Animal Bleeding Disorders. Top. Companion Anim. Med. 2012, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Turrentine, M.A.; Kraus, K.H.; Johnson, G.S. Buccal mucosa bleeding times of healthy dogs and of dogs in various pathologic states, including thrombocytopenia, uremia, and von Willebrand’s disease. Am. J. Vet. Res. 1987, 48, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Steffen, F.; Alder, D.S.; Beatrice, L.; Geigy, C.; Beckmann, K. Evaluating the Use of Cytosine Arabinoside for Treatment for Recurrent Canine Steroid-responsive Meningitis-arteritis. Vet. Rec. 2020, 187, e7. [Google Scholar] [CrossRef]
- Behr, S.; Cauzinille, L. Aseptic Suppurative Meningitis in Juvenile Boxer Dogs: Retrospective Study of 12 Cases. J. Am. Anim. Hosp. Assoc. 2006, 42, 277–282. [Google Scholar] [CrossRef]
- Presthus, J. Aseptic suppurative meningitis in Bernese Mountain dogs. Eur. J. Companion Anim. Pract. 1991, 1, 24–28. [Google Scholar]

| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Breed | Crossbreed | Beagle | Whippet | Border Collie | Boxer | Greyhound | Whippet | Beagle | Whippet |
| Sex | Male/ Neutered | Female/ Entire | Female/Entire | Female/Entire | Male/ Neutered | Female/ Neutered | Female/ Neutered | Female/Entire | Female/ Neutered |
| Age (months) | 9 | 16 | 13 | 9 | 14 | 78 | 10 | 11 | 12 |
| Weight (kg) | 23.5 | 12.6 | 11.2 | 21.1 | 27.3 | 28.4 | 12.0 | 10.8 | 10.6 |
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Presenting complains | |||||||||
| Pyrexia | X | X | X | X | X | X | |||
| Hyperesthesia | X | X | X | X | X | X | X | ||
| Lethargy | X | X | X | X | X | X | X | ||
| Inappetence | X | ||||||||
| Tremors | X | ||||||||
| Bilateral scleral haemorrhage | X | ||||||||
| Spastic paresis | X | ||||||||
| Seizures like episodes | X | X | |||||||
| Monolateral scleral haemorrhage of the left eye | X | ||||||||
| Cervical hyperesthesia that progressed to tetraplegia | X | ||||||||
| Ambulatory tetraparesis | X | ||||||||
| Hemiparesis | X | X | |||||||
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Physical examination abnormalities | |||||||||
| Pyrexia | X | X | X | X | X | ||||
| Bilateral scleral haemorrhages | X | X | |||||||
| Monolateral scleral haemorrhage of the left eye | X | ||||||||
| Unremarkable | X | ||||||||
| Neurological signs | |||||||||
| Normal neurological examination | X | ||||||||
| Obtunded mentation | X | ||||||||
| Low head carriage | X | X | |||||||
| Cervical hyperesthesia | X | X | X | X | X | X | X | ||
| Ambulatory paraparetic with delayed postural reaction | X | ||||||||
| Paraplegic with intact nociception | X | ||||||||
| Paraplegic with absent nociception | O | X | |||||||
| Ambulatory tetraparetic | X | ||||||||
| Tetraplegic with intact nociception | X | ||||||||
| Proprioceptive ataxia | X | ||||||||
| Spinal reflexes reduced front limbs | X | ||||||||
| Anisocoria. The direct and indirect PLR was normal in the right eye | X | ||||||||
| Neuroanatomical localisation | (-) | (-) | + | (-) | Neck region | C1–C5 spinal cord segments | (-) | C6-T2 spinal cord segments | T3-L3 spinal cord segments |
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Neutrophil | 25.5 × 109/L; (RI 2.9–13.6 × 109/L) | 24.8 × 109/; (RI 3.0–11.5 × 109/L) | 19.09 × 109/L; (RI 3.0–12.0 × 109/L) | 12.51 × 109/L; (RI 2.95–11.64 × 109/L) | 12.92 × 109/L; (RI 2.95–11.64 × 109/L) | (-) | 12.14 × 109/L; (RI 3.0–11.5) | 19.32 × 109/L; (RI 3.0–11.5) | (-) |
| Monocyte | 5.4 × 109/L; (RI 0.0 1.3 × 109/L) | 2.4 × 109/L; (RI 0.0–1.3 × 109/L) | 1.64 × 109/L; (RI 0.2–1.5 × 109/L) | 1.45 × 109/L; (RI 0.16–1.12 × 109/L) | 1.55 × 109/L; (RI 0.16–1.12 × 109/L) | (-) | 2.34 × 109/L; (RI 0.2–1.4) | (-) | 2.34 × 109/L; (RI 0.2–1.4) |
| Eosinophil | (-) | (-) | (-) | (-) | (-) | 0.02 × 109/L; (RI 0.06–1.23 × 109/L) | (-) | (-) | (-) |
| CK | 1061 U/L; (RI 0–350 U/L) | (-) | (-) | (-) | (-) | (-) | 697 IU/L; (RI 0–190) | (-) | (-) |
| ALT | (-) | 170 U/L; (RI 13–78 U/L) | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| ALP | (-) | 179 U/L; (RI 12–83 U/L) | (-) | (-) | (-) | (-) | 224 IU/L; (RI 14–105) | 444 IU/L; (14–105) | 233 IU/L; (RI 14–105) |
| CRP | 214 mg/L; (RI < 10.0 mg/L) | 174.34 mg/L; (RI < 10.0 mg/L) | >82.8 mg/L; (RI < 10.0 mg/L) | >100 mg/L; (RI < 10.0 mg/L) | >100 mg/L; (RI < 10.0 mg/L) | * | * | 336 mg/L; (RI < 10.0 mg/L) | * |
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Location CSF tap | |||||||||
| Cisterna magna | X | X | X | X | X | ||||
| Lumbar | X | ||||||||
| Both sites | X | ||||||||
| Unsuccessful | X | X | |||||||
| Neutrophilic pleocytosis (RI: cells/μL) | |||||||||
| Marked | 4896 | ||||||||
| Moderate | 455 | C: 262, L: 539 | 475 | 455 | 23 | 20 | |||
| RBC count (RI: cells/μL) | |||||||||
| Elevated | 39,200 | (^) | 4056 | 3920 | 21,024 | ||||
| Protein concentration (RI: Cisterna < 30 mg/dL, Lumbar < 45 mg/dL) | |||||||||
| Elevated | 115 | C: 73.8 L: 70.6 | 191.95 | 115 | 214 | ||||
| CK (RI:0–40 IU/L) | |||||||||
| Elevated | 314 | 324 | |||||||
| Bacterial culture | |||||||||
| Negative | X | X | (*) | X | (*) | X | (*) | ||
| PCR Toxoplasma gondii/Neospora caninum | |||||||||
| Negative | X | X | (*) | X | (*) | X | (*) | ||
| PCR distemper | |||||||||
| Negative | (*) | (*) | (*) | (*) | (*) | X | (*) | ||
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | RI |
|---|---|---|---|---|---|---|---|---|---|---|
| PT | (-) | (-) | (-) | (-) | * | (-) | (-) | (-) | (-) | 12.0–17.0 s |
| APTT | (-) | (-) | (-) | (-) | * | (-) | (-) | (-) | (-) | 96–116 s |
| BMBT | 5 min | * | * | * | * | * | * | (-) | * | <4 min |
| Case No. | Localisation of Haemorrhage | Haemorrhage Type | Side of Haemorrhage | T1W/T1W Post-GAD | T2W | T2*W |
|---|---|---|---|---|---|---|
| 1 | T12-L1 | Intradural– Extramedullary | Right-sided | Hypointense/poor enhancement | Hyperintense | Hypointense |
| 2 | T4–T5 | Intramedullary | Diffuse | Hyperintense/no enhancement | Hyperintense | Hypointense |
| 3 | C7-T1 | Extramedullary | Ventral and left-sided | Hyperintense/poor enhancement | Hypointense to isointense | Hypointense |
| 4 | C1–C3 | Intradural– Extramedullary | Ventral | Hyperintense to isointense/poor enhancement | Hyperintense to isointense | Hypointense to isointense |
| 5 | T1–T2 | Extramedullary | Right-sided | Hypointense/poor enhancement | Hyperintense to isointense | (*) |
| 6 | C3 | Intradural– Extramedullary | Left-sided | Hypointense/poor enhancement | Hyperintense | Hypointense |
| 7 | T9-L3 | Intradural– Extramedullary | Dorsal and right-sided | Hyperintense/poor enhancement | Hyperintense | Hypointense |
| 8 | C5 | Intradural– Extramedullary | Ventral and left-sided | Hyperintense/poor enhancement | Hyperintense | Shows peripheral susceptibility artefacts |
| 9 | T11-L3 | Extradural | Dorsal and right-sided | Hyperintense/poor enhancement | Hyperintense | Diffuse signal void surrounding the cord |
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Immunosuppressive treatments | |||||||||
| Prednisolone | 1 mg/kg PO q24h from D 1 | 1.25 mg/kg PO q12h from D 2 | 1 mg/kg PO q12h for 1 M | 2.2 mg/kg PO q24h for 1 M | 2.2 mg/kg PO q24h for 1 M | 2 mg/kg PO q12 started 4 M after discontinuation | |||
| Dexamethasone | 0.2 mg/kg IV from D 1 (case 2) | 0.166 mg/kg PO q24 for 6 M | 0.166 mg/kg PO q24h for 5 D | 0.2 mg/kg PO q24h for 5 M | |||||
| Cytarabine | 200 mg/m2 CRI every 3 W | 200 mg/m2 SC single dose | 200 mg/m2 CRI single dose | ||||||
| Mycophenolate | 12 mg/kg PO q24h from D 4 | ||||||||
| Ciclosporine | 5 mg/kg PO q12h for 7 M, tapered after 5 M | ||||||||
| Azathioprine | 2 mg/kg PO q24h from D 2 | ||||||||
| Supportive therapy | |||||||||
| Paracetamol | 15 mg/kg IV q8h from D 1 | 10 mg/kg PO q8h for 1 M | 15 mg/kg PO q8h for 2 W | 15 mg/kg PO q8h for 1 M | |||||
| Amoxicillin | 12 mg/kg PO q8h from D 3 | ||||||||
| Tranexamic acid | 25 mg/kg PO q8h from D 4 (case 1) | ||||||||
| Levetiracetam | 30 mg/kg PO q8h ongoing | ||||||||
| Surgery | Right-sided haemilaminectomy at T11–T12 on D 1 | ||||||||
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| Complications | |||||||||
| Paraplegia with absent nociception | Developed on D 3 | Persistent on W 5 | |||||||
| Paraplegia with nociception | Initially, but regained ambulation by D 14 | ||||||||
| Severe hypoventilation | Developed on D 5 | ||||||||
| Relapse | 4 M after prednisolone discontinuation | ||||||||
| Mild ataxia | Present still on D 30 | ||||||||
| Follow-up Findings | |||||||||
| Complete recovery | In 1 M | In 4 M | In 4 M | In 1M | In 6 M | Regained ambulation in 2 W | |||
| No recovery | X | X | X | ||||||
| Repeated MRI | At W 11 showed resolution of lesions | ||||||||
| Outcome | |||||||||
| Euthanised | X | X | X | ||||||
| Full recovery | X | X | X | X | X | X | |||
| Prognosis | |||||||||
| Poor | X | X | X | ||||||
| Good | X | X | X | X | X | X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitello, G.; Carletti, B.E.; Gomes, S.A.; Motta, L.; Colverde, A.; Holmes, A.; Mariscoli, M. Clinical Features, MRI Findings, Treatment, and Outcomes in Dogs with Haemorrhagic Myelopathy Secondary to Steroid-Responsive Meningitis-Arteritis: Nine Cases (2017–2024). Vet. Sci. 2025, 12, 476. https://doi.org/10.3390/vetsci12050476
Vitello G, Carletti BE, Gomes SA, Motta L, Colverde A, Holmes A, Mariscoli M. Clinical Features, MRI Findings, Treatment, and Outcomes in Dogs with Haemorrhagic Myelopathy Secondary to Steroid-Responsive Meningitis-Arteritis: Nine Cases (2017–2024). Veterinary Sciences. 2025; 12(5):476. https://doi.org/10.3390/vetsci12050476
Chicago/Turabian StyleVitello, Giuseppe, Beatrice Enrica Carletti, Sergio A. Gomes, Luca Motta, Alessia Colverde, Andrea Holmes, and Massimo Mariscoli. 2025. "Clinical Features, MRI Findings, Treatment, and Outcomes in Dogs with Haemorrhagic Myelopathy Secondary to Steroid-Responsive Meningitis-Arteritis: Nine Cases (2017–2024)" Veterinary Sciences 12, no. 5: 476. https://doi.org/10.3390/vetsci12050476
APA StyleVitello, G., Carletti, B. E., Gomes, S. A., Motta, L., Colverde, A., Holmes, A., & Mariscoli, M. (2025). Clinical Features, MRI Findings, Treatment, and Outcomes in Dogs with Haemorrhagic Myelopathy Secondary to Steroid-Responsive Meningitis-Arteritis: Nine Cases (2017–2024). Veterinary Sciences, 12(5), 476. https://doi.org/10.3390/vetsci12050476






