Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Viral Stock
2.3. In Vitro Infection of A-72 Cells with FCoV and CCoV
2.4. Cell Lysates
2.5. Isolation and Purification of A-72-Derived EVs Using Ultracentrifugation
2.6. Evaluate the A-72 Cell Viability Utilizing the MTT (3-(4,5-Dimethylthiazo-1-2yl)-2,5-diphenyltetrazolium Bromide) Assay
2.7. Assessment of EV Sizes and Concentrations Utilizing NanoSight Tracking Analysis (NTA)
2.8. Quantitation of Total Protein After FCoV and CCoV Infections
2.9. Analysis of Total RNA/DNA After FCoV and CCoV Infections
2.10. Dot Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. A-72 Cell Viability After FCoV and CCoV Infections
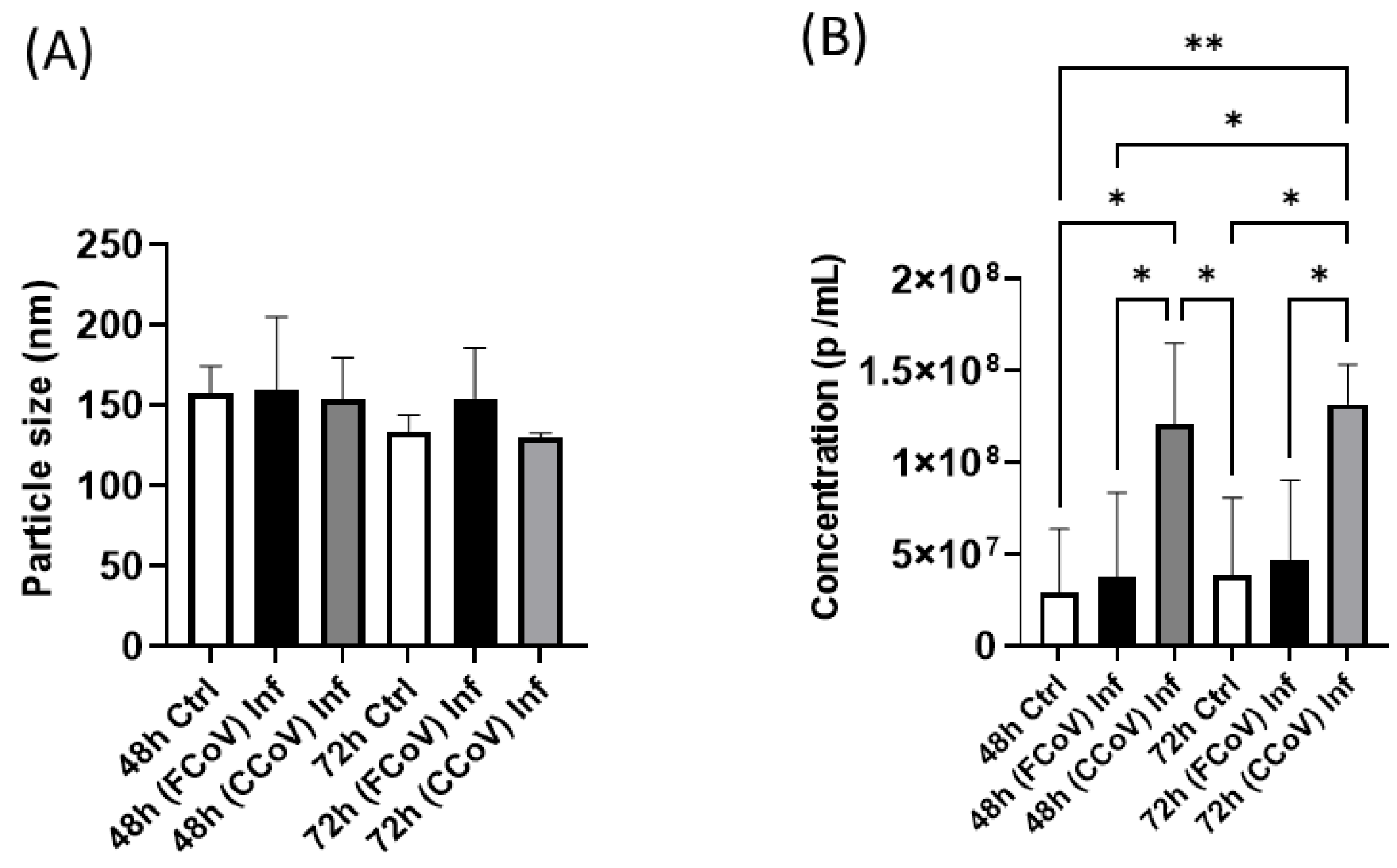
3.2. FCoV and CCoV Modified A-72-Derived EV Particle Size and Concentration
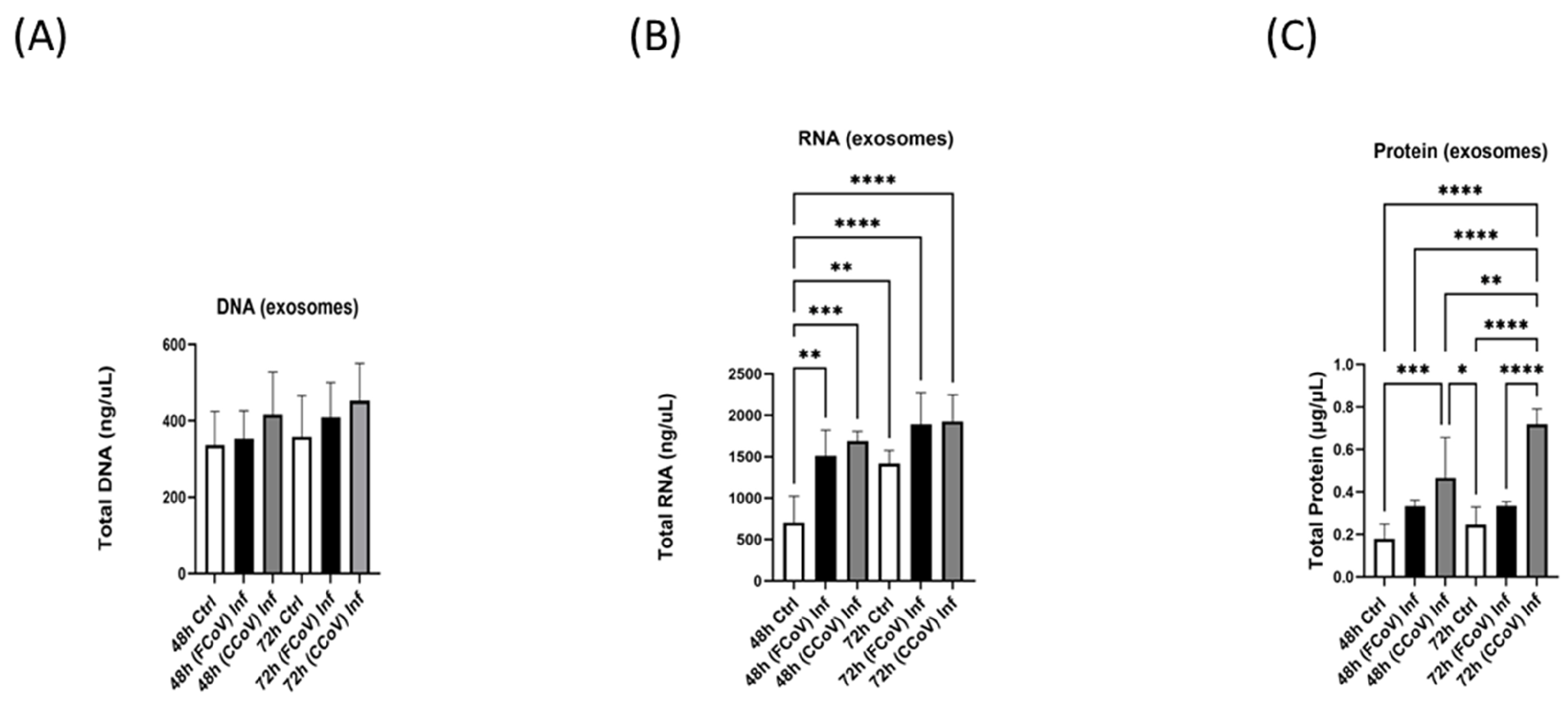
3.3. A-72 Produced EVs and Lysates Biomolecule Content Following Both FCoV and CCoV Infections
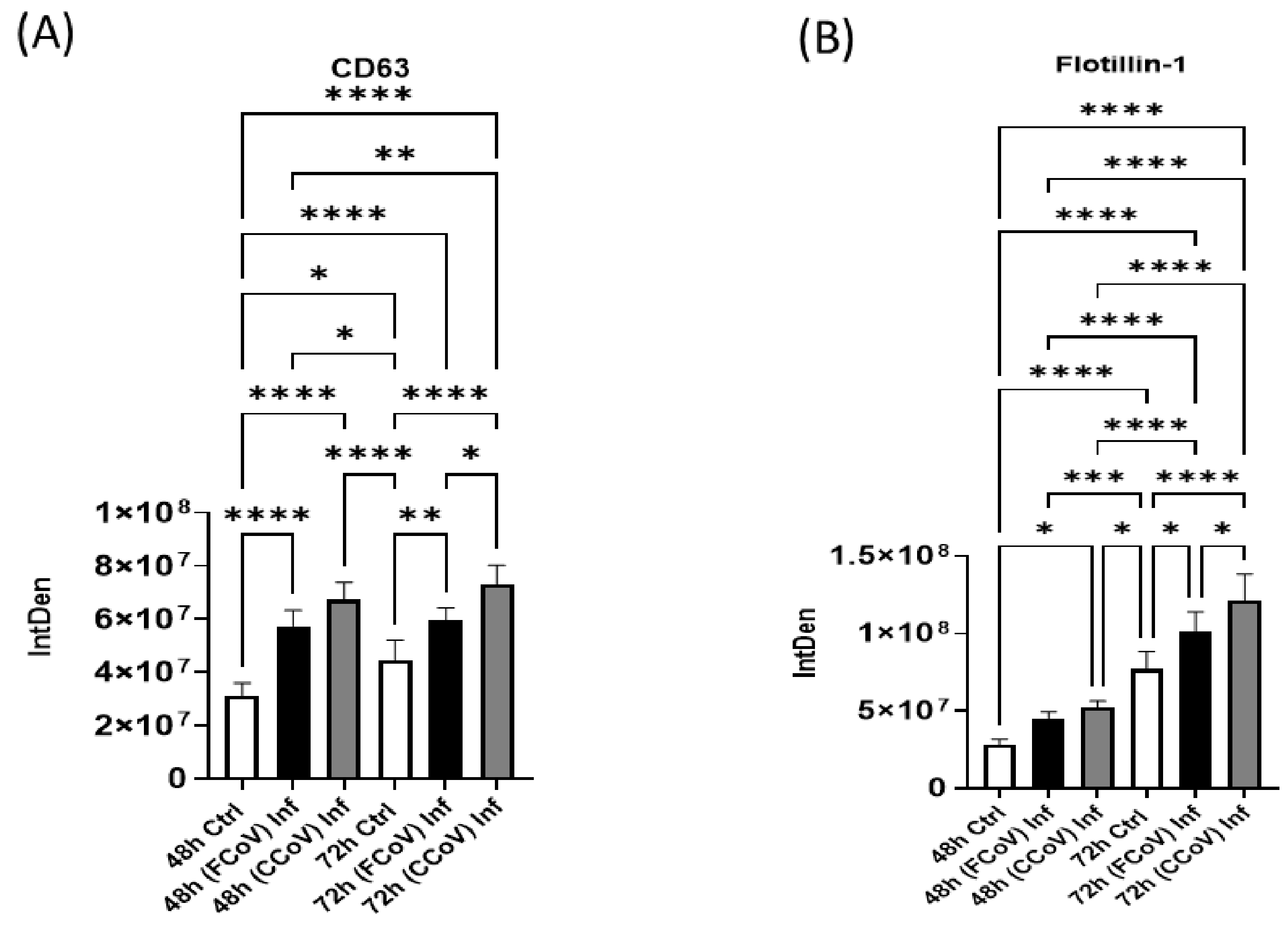
3.4. Expression of Classical Exosome Protein Elevated in Response to FCoV and CCoV Infection
3.5. Presence of Cellular Membrane Trafficking Protein After FCoV and CCoV Infection
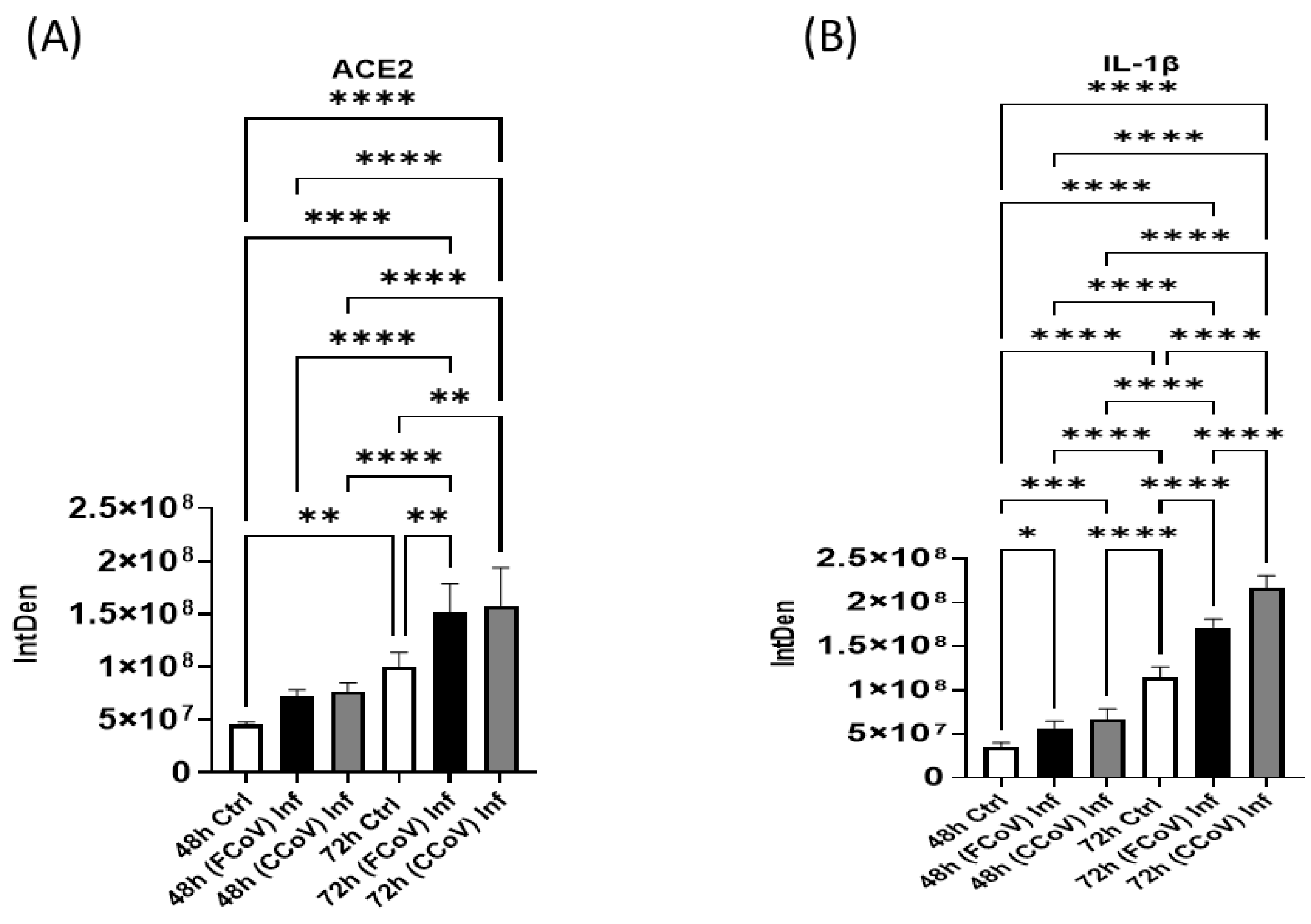
3.6. Evaluation of Host Receptor After FCoV and CCoV Infection
3.7. Expression of Pro-Inflammatory Response Post-Infection with FCoV and CCoV
3.8. Stress Response Biomarker Expression Elevated in Response to FCoV and CCoV Infections
3.9. FCoV and CCoV Infections Regulate Apoptotic Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadeghi Dousari, A.; Taati Moghadam, M.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Rabi, F.A.; Al Zoubi, M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.T.; Liang, L.T.; Rosen, J.M. COVID-19: A comparison to the 1918 influenza and how we can defeat it. Postgrad. Med. J. 2021, 97, 273–274. [Google Scholar] [CrossRef]
- Wijerathne, S.V.T.; Pandit, R.; Ipinmoroti, A.O.; Crenshaw, B.J.; Matthews, Q.L. Feline coronavirus influences the biogenesis and composition of extracellular vesicles derived from CRFK cells. Front. Vet. Sci. 2024, 11, 1388438. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, G.Z.; Zhang, Y.; Peng, L.H.; Zou, L.P.; Yang, Y.S. Coronaviruses and gastrointestinal diseases. Mil. Med. Res. 2020, 7, 49. [Google Scholar] [CrossRef]
- Saviano, A.; Wrensch, F.; Ghany, M.G.; Baumert, T.F. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology 2021, 74, 1088–1100. [Google Scholar] [CrossRef]
- Brola, W.; Wilski, M. Neurological consequences of COVID-19. Pharmacol. Rep. 2022, 74, 1208–1222. [Google Scholar] [CrossRef]
- Shehata, A.A.; Attia, Y.A.; Rahman, M.T.; Basiouni, S.; El-Seedi, H.R.; Azhar, E.I.; Khafaga, A.F.; Hafez, H.M. Diversity of Coronaviruses with Particular Attention to the Interspecies Transmission of SARS-CoV-2. Animals 2022, 12, 378. [Google Scholar] [CrossRef]
- Memish, Z.A.; Perlman, S.; Van Kerkhove, M.D.; Zumla, A. Middle East respiratory syndrome. Lancet 2020, 395, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Hosie, M.J.; Hofmann-Lehmann, R.; Hartmann, K.; Egberink, H.; Truyen, U.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Anthropogenic Infection of Cats during the 2020 COVID-19 Pandemic. Viruses 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.S.; El-Sayed, A.A.; Munds, R.A.; Verma, M.S. Interactions between Humans and Dogs during the COVID-19 Pandemic: Recent Updates and Future Perspectives. Animals 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Le Poder, S. Feline and canine coronaviruses: Common genetic and pathobiological features. Adv. Virol. 2011, 2011, 609465. [Google Scholar] [CrossRef]
- Jaimes, J.A.; Whittaker, G.R. Feline coronavirus: Insights into viral pathogenesis based on the spike protein structure and function. Virology 2018, 517, 108–121. [Google Scholar] [CrossRef]
- Delaplace, M.; Huet, H.; Gambino, A.; Le Poder, S. Feline Coronavirus Antivirals: A Review. Pathogens 2021, 10, 1150. [Google Scholar] [CrossRef]
- Licitra, B.N.; Millet, J.K.; Regan, A.D.; Hamilton, B.S.; Rinaldi, V.D.; Duhamel, G.E.; Whittaker, G.R. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerg. Infect. Dis. 2013, 19, 1066–1073. [Google Scholar] [CrossRef]
- Malbon, A.J.; Fonfara, S.; Meli, M.L.; Hahn, S.; Egberink, H.; Kipar, A. Feline Infectious Peritonitis as a Systemic Inflammatory Disease: Contribution of Liver and Heart to the Pathogenesis. Viruses 2019, 11, 1144. [Google Scholar] [CrossRef]
- Pedersen, N.C. An update on feline infectious peritonitis: Virology and immunopathogenesis. Vet. J. 2014, 201, 123–132. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. An update on canine coronaviruses: Viral evolution and pathobiology. Vet. Microbiol. 2008, 132, 221–234. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Decaro, N.; Martella, V.; Elia, G.; Campolo, M.; Desario, C.; Castagnaro, M.; Tempesta, M. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 2006, 12, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Licitra, B.N.; Duhamel, G.E.; Whittaker, G.R. Canine enteric coronaviruses: Emerging viral pathogens with distinct recombinant spike proteins. Viruses 2014, 6, 3363–3376. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C. Canine coronavirus: Not only an enteric pathogen. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1121–1132. [Google Scholar] [CrossRef]
- Hossain, M.E.; Islam, A.; Islam, S.; Rahman, M.K.; Miah, M.; Alam, M.S.; Rahman, M.Z. Detection and Molecular Characterization of Canine Alphacoronavirus in Free-Roaming Dogs, Bangladesh. Viruses 2021, 14, 67. [Google Scholar] [CrossRef]
- Decaro, N.; Mari, V.; Elia, G.; Lanave, G.; Dowgier, G.; Colaianni, M.L.; Martella, V.; Buonavoglia, C. Full-length genome analysis of canine coronavirus type I. Virus Res. 2015, 210, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Steiner, A.R.; Cattori, V.; Hofmann-Lehmann, R.; Lutz, H.; Kipar, A.; Meli, M.L. FCoV Viral Sequences of Systemically Infected Healthy Cats Lack Gene Mutations Previously Linked to the Development of FIP. Pathogens 2020, 9, 603. [Google Scholar] [CrossRef]
- Termansen, M.B.; Frische, S. Fecal-oral transmission of SARS-CoV-2: A systematic review of evidence from epidemiological and experimental studies. Am. J. Infect. Control 2023, 51, 1430–1437. [Google Scholar] [CrossRef]
- Decaro, N.; Balboni, A.; Bertolotti, L.; Martino, P.A.; Mazzei, M.; Mira, F.; Pagnini, U. SARS-CoV-2 Infection in Dogs and Cats: Facts and Speculations. Front. Vet. Sci. 2021, 8, 619207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Liang, X.Y.; Wang, Q.; Zhang, S.; Zhao, H.; Wang, K.; Hu, G.X.; Liu, W.J.; Gao, F.S. Mind the feline coronavirus: Comparison with SARS-CoV-2. Gene 2022, 825, 146443. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Toh, T.H.; Lee, J.S.; Poovorawan, Y.; Davis, P.; Azevedo, M.S.P.; Lednicky, J.A.; Saif, L.J.; Gray, G.C. Animal alphacoronaviruses found in human patients with acute respiratory illness in different countries. Emerg. Microbes Infect. 2022, 11, 699–702. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Luo, H.; Lee, J.W. Role of extracellular vesicles in lung diseases. Chin. Med. J. 2022, 135, 1765–1780. [Google Scholar] [CrossRef]
- Martins, S.T.; Alves, L.R. Extracellular Vesicles in Viral Infections: Two Sides of the Same Coin? Front. Cell. Infect. Microbiol. 2020, 10, 593170. [Google Scholar] [CrossRef]
- Kumar, A.; Kodidela, S.; Tadrous, E.; Cory, T.J.; Walker, C.M.; Smith, A.M.; Mukherjee, A.; Kumar, S. Extracellular Vesicles in Viral Replication and Pathogenesis and Their Potential Role in Therapeutic Intervention. Viruses 2020, 12, 887. [Google Scholar] [CrossRef]
- Bou, J.V.; Taguwa, S.; Matsuura, Y. Trick-or-Trap: Extracellular Vesicles and Viral Transmission. Vaccines 2023, 11, 1532. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Bakkannavar, S.; Bhat, V.R.; Sharan, K. Extracellular vesicles as novel drug delivery systems to target cancer and other diseases: Recent advancements and future perspectives. F1000Research 2023, 12, 329. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef]
- Ma, Y.; Brocchini, S.; Williams, G.R. Extracellular vesicle-embedded materials. J. Control. Release 2023, 361, 280–296. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2023, 10, 1894–1907. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.H.; Yam, J.W.P. From Exosome Biogenesis to Absorption: Key Takeaways for Cancer Research. Cancers 2023, 15, 1992. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Wen, C.; Xia, J.; Zhang, Y.; Liang, Y. Programming assembly of biomimetic exosomes: An emerging theranostic nanomedicine platform. Mater. Today Bio 2023, 22, 100760. [Google Scholar] [CrossRef]
- Kumar, S.; Crenshaw, B.J.; Williams, S.D.; Bell, C.R.; Matthews, Q.L.; Sims, B. Cocaine-Specific Effects on Exosome Biogenesis in Microglial Cells. Neurochem. Res. 2021, 46, 1006–1018. [Google Scholar] [CrossRef]
- Ayariga, J.A.; Matthews, Q.L. Commentary on “Exosome-mediated stable epigenetic repression of HIV-1”. ExRNA 2022, 4, 1–4. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, X.; Hu, L.; Wang, L.; Zhu, Q. Harnessing crosstalk between extracellular vesicles and viruses for disease diagnostics and therapeutics. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 358–370. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, Y.; Chen, Q.; Chen, Y.; Mao, L. Dual roles and potential applications of exosomes in HCV infections. Front. Microbiol. 2022, 13, 1044832. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Hoang, A.; Dragoljevic, D.; Dubrovsky, L.; Pushkarsky, T.; Low, H.; Ditiatkovski, M.; Fu, Y.; Ohkawa, R.; Meikle, P.J.; et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019, 15, e1007907. [Google Scholar] [CrossRef] [PubMed]
- Sviridov, D.; Mukhamedova, N.; Makarov, A.A.; Adzhubei, A.; Bukrinsky, M. Comorbidities of HIV infection: Role of Nef-induced impairment of cholesterol metabolism and lipid raft functionality. AIDS 2020, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Brichacek, B.; Prashant, N.M.; Pushkarsky, T.; Mukhamedova, N.; Fleetwood, A.J.; Xu, Y.; Dragoljevic, D.; Fitzgerald, M.; Horvath, A.; et al. Extracellular vesicles carrying HIV-1 Nef induce long-term hyperreactivity of myeloid cells. Cell Rep. 2022, 41, 111674. [Google Scholar] [CrossRef]
- Horn, M.D.; MacLean, A.G. Extracellular Vesicles as a Means of Viral Immune Evasion, CNS Invasion, and Glia-Induced Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 695899. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, P.; Lin, L.; Liu, X.; Ma, F.; An, H.; Wang, Z.; Cao, X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009, 183, 2150–2158. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Khoo, A.S.; Peh, S.C. Pathogenic Role of Exosomes in Epstein-Barr Virus (EBV)-Associated Cancers. Int. J. Biol. Sci. 2017, 13, 1276–1286. [Google Scholar] [CrossRef]
- Xia, X.; Yuan, P.; Liu, Y.; Wang, Y.; Cao, W.; Zheng, J.C. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology 2021, 163, 416–430. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Yao, M.; Feng, Z.; Yuan, G.; Ye, F.; Nguyen, K.; Karn, J.; McComsey, G.A.; McIntyre, T.M.; et al. COVID-19 plasma exosomes promote proinflammatory immune responses in peripheral blood mononuclear cells. Sci. Rep. 2022, 12, 21779. [Google Scholar] [CrossRef]
- Thakur, A. Shedding Lights on the Extracellular Vesicles as Functional Mediator and Therapeutic Decoy for COVID-19. Life 2023, 13, 840. [Google Scholar] [CrossRef]
- Taşlı, N.P.; Gönen, Z.B.; Kırbaş, O.K.; Gökdemir, N.S.; Bozkurt, B.T.; Bayrakcı, B.; Sağraç, D.; Taşkan, E.; Demir, S.; Ekimci Gürcan, N.; et al. Preclinical Studies on Convalescent Human Immune Plasma-Derived Exosome: Omics and Antiviral Properties to SARS-CoV-2. Front. Immunol. 2022, 13, 824378. [Google Scholar] [CrossRef]
- El-Shennawy, L.; Hoffmann, A.D.; Dashzeveg, N.K.; McAndrews, K.M.; Mehl, P.J.; Cornish, D.; Yu, Z.; Tokars, V.L.; Nicolaescu, V.; Tomatsidou, A.; et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 2022, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Gniffke, E.P.; Harrington, W.E.; Dambrauskas, N.; Jiang, Y.; Trakhimets, O.; Vigdorovich, V.; Frenkel, L.; Sather, D.N.; Smith, S.E.P. Plasma From Recovered COVID-19 Patients Inhibits Spike Protein Binding to ACE2 in a Microsphere-Based Inhibition Assay. J. Infect. Dis. 2020, 222, 1965–1973. [Google Scholar] [CrossRef]
- Moulin, C.; Crupi, M.J.F.; Ilkow, C.S.; Bell, J.C.; Boulton, S. Extracellular Vesicles and Viruses: Two Intertwined Entities. Int. J. Mol. Sci. 2023, 24, 1036. [Google Scholar] [CrossRef] [PubMed]
- Binn, L.N.; Marchwicki, R.H.; Stephenson, E.H. Establishment of a canine cell line: Derivation, characterization, and viral spectrum. Am. J. Vet. Res. 1980, 41, 855–860. [Google Scholar] [CrossRef]
- Razzuoli, E.; Chirullo, B.; De Ciucis, C.G.; Mecocci, S.; Martini, I.; Zoccola, R.; Campanella, C.; Varello, K.; Petrucci, P.; Di Meo, A.; et al. Animal models of Soft Tissue Sarcoma for alternative anticancer therapy studies: Characterization of the A-72 Canine Cell Line. Vet. Res. Commun. 2023, 47, 1615–1627. [Google Scholar] [CrossRef]
- Abdelgadir, A.; Vlasova, A.N.; Gray, G.C. Susceptibility of different cell lines to the novel canine coronavirus CCoV-HuPn-2018. Influenza Other Respir. Viruses 2021, 15, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef]
- TRIzolTM Reagent: Thermo Fisher Scientific. 2016. Available online: https://tools.thermofisher.com/content/sfs/manuals/trizol_reagent.pdf (accessed on 1 November 2024).
- Matthews, Q.L.; Thweatt, I.N.; Wijerathne, S.V.; Efa, B.B.; Ezeuko, C.C.; Ipinmoroti, A.O.; Pandit, R.; Xu, J.; Fluker, K.A.; Ajayi, O. Characterization of cannabis plant-derived extracellular vesicles for biomedical applications. Med. Res. Arch. 2024, 12, 1–6. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Ivanusic, D.; Denner, J. The large extracellular loop is important for recruiting CD63 to exosomes. Micropubl. Biol. 2023, 2023. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Matveichuk, O.V.; Fronk, J.; Ciesielska, A. Flotillins: At the Intersection of Protein S-Palmitoylation and Lipid-Mediated Signaling. Int. J. Mol. Sci. 2020, 21, 2283. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Shirbhate, E.; Pandey, J.; Patel, V.K.; Kamal, M.; Jawaid, T.; Gorain, B.; Kesharwani, P.; Rajak, H. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: A potential approach for therapeutic intervention. Pharmacol. Rep. 2021, 73, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Nejat, R.; Torshizi, M.F.; Najafi, D.J. S Protein, ACE2 and Host Cell Proteases in SARS-CoV-2 Cell Entry and Infectivity; Is Soluble ACE2 a Two Blade Sword? A Narrative Review. Vaccines 2023, 11, 204. [Google Scholar] [CrossRef]
- Ensor, C.M.; AlSiraj, Y.; Shoemaker, R.; Sturgill, J.; Keshavamurthy, S.; Gordon, E.M.; Dong, B.E.; Waters, C.; Cassis, L.A. SARS-CoV-2 Spike Protein Regulation of Angiotensin Converting Enzyme 2 and Tissue Renin-Angiotensin Systems: Influence of Biologic Sex. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Garlanda, C.; Jaillon, S. The Interleukin-1 Family. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 438–446. [Google Scholar]
- Makaremi, S.; Asgarzadeh, A.; Kianfar, H.; Mohammadnia, A.; Asghariazar, V.; Safarzadeh, E. The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. Inflamm. Res. 2022, 71, 923–947. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Szyller, J.; Bil-Lula, I. Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxidative Med. Cell. Longev. 2021, 2021, 6678457. [Google Scholar] [CrossRef]
- Rutledge, B.S.; Choy, W.Y.; Duennwald, M.L. Folding or holding?-Hsp70 and Hsp90 chaperoning of misfolded proteins in neurodegenerative disease. J. Biol. Chem. 2022, 298, 101905. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Y.; Jia, Y.; Chen, X.; Niu, T.; Chatterjee, A.; He, P.; Hou, G. Heat shock protein 90: Biological functions, diseases, and therapeutic targets. MedComm 2024, 5, e470. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Gurung, P.; Kanneganti, T.-D. Novel Roles for Caspase-8 in IL-1β and Inflammasome Regulation. Am. J. Pathol. 2015, 185, 17–25. [Google Scholar] [CrossRef]
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. [Google Scholar] [CrossRef]
- Serretiello, E.; Ballini, A.; Smimmo, A.; Acunzo, M.; Raimo, M.; Cantore, S.; Di Domenico, M. Extracellular Vesicles as a Translational Approach for the Treatment of COVID-19 Disease: An Updated Overview. Viruses 2023, 15, 1976. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Robatzek, S. Functions of Extracellular Vesicles in Immunity and Virulence. Plant Physiol. 2019, 179, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Ripa, I.; López-Guerrero, J.A. Extracellular Vesicles in Viral Spread and Antiviral Response. Viruses 2020, 12, 623. [Google Scholar] [CrossRef]
- Martin, C.; Ligat, G.; Malnou, C.E. The Yin and the Yang of extracellular vesicles during viral infections. Biomed. J. 2023, 47, 100659. [Google Scholar] [CrossRef]
- Guo, B.C.; Wu, K.H.; Chen, C.Y.; Lin, W.Y.; Chang, Y.J.; Lee, T.A.; Lin, M.J.; Wu, H.P. Mesenchymal Stem Cells in the Treatment of COVID-19. Int. J. Mol. Sci. 2023, 24, 4800. [Google Scholar] [CrossRef]
- Hu, J.C.; Zheng, C.X.; Sui, B.D.; Liu, W.J.; Jin, Y. Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration. World J. Stem Cells 2022, 14, 318–329. [Google Scholar] [CrossRef]
- Khushman, M.; Bhardwaj, A.; Patel, G.K.; Laurini, J.A.; Roveda, K.; Tan, M.C.; Patton, M.C.; Singh, S.; Taylor, W.; Singh, A.P. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Yeung, L.; Hickey, M.J.; Wright, M.D. The Many and Varied Roles of Tetraspanins in Immune Cell Recruitment and Migration. Front. Immunol. 2018, 9, 1644. [Google Scholar] [CrossRef]
- Pfistershammer, K.; Majdic, O.; Stöckl, J.; Zlabinger, G.; Kirchberger, S.; Steinberger, P.; Knapp, W. CD63 as an activation-linked T cell costimulatory element. J. Immunol. 2004, 173, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Mangala, L.S.; Hu, W.; Bayaktar, E.; Yokoi, A.; Hu, W.; Pradeep, S.; Lee, S.; Piehowski, P.D.; Villar-Prados, A.; et al. CD63-mediated cloaking of VEGF in small extracellular vesicles contributes to anti-VEGF therapy resistance. Cell Rep. 2021, 36, 109549. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef]
- Ruiz-Mateos, E.; Pelchen-Matthews, A.; Deneka, M.; Marsh, M. CD63 is not required for production of infectious human immunodeficiency virus type 1 in human macrophages. J. Virol. 2008, 82, 4751–4761. [Google Scholar] [CrossRef]
- Scott, T.A.; Supramaniam, A.; Idris, A.; Cardoso, A.A.; Shrivastava, S.; Kelly, G.; Grepo, N.A.; Soemardy, C.; Ray, R.M.; McMillan, N.A.J.; et al. Engineered extracellular vesicles directed to the spike protein inhibit SARS-CoV-2. Mol. Ther.-Methods Clin. Dev. 2022, 24, 355–366. [Google Scholar] [CrossRef]
- Zhan, Z.; Ye, M.; Jin, X. The roles of FLOT1 in human diseases (Review). Mol. Med. Rep. 2023, 28, 212. [Google Scholar] [CrossRef]
- Cremona, M.L.; Matthies, H.J.; Pau, K.; Bowton, E.; Speed, N.; Lute, B.J.; Anderson, M.; Sen, N.; Robertson, S.D.; Vaughan, R.A.; et al. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci. 2011, 14, 469–477. [Google Scholar] [CrossRef]
- Negi, G.; Sharma, A.; Dey, M.; Dhanawat, G.; Parveen, N. Membrane attachment and fusion of HIV-1, influenza A, and SARS-CoV-2: Resolving the mechanisms with biophysical methods. Biophys. Rev. 2022, 14, 1109–1140. [Google Scholar] [CrossRef] [PubMed]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife 2020, 9, e61390. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Chauhan, D.; Nguyen, A.; Ramalingam, M.; Camci-Unal, G. Advances in Targeting ACE2 for Developing COVID-19 Therapeutics. Ann. Biomed. Eng. 2022, 50, 1734–1749. [Google Scholar] [CrossRef]
- Wang, C.W.; Chuang, H.C.; Tan, T.H. ACE2 in chronic disease and COVID-19: Gene regulation and post-translational modification. J. Biomed. Sci. 2023, 30, 71. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Lu, X. Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19? Front. Endocrinol. 2021, 12, 725967. [Google Scholar] [CrossRef]
- Tey, S.K.; Lam, H.; Wong, S.W.K.; Zhao, H.; To, K.K.; Yam, J.W.P. ACE2-enriched extracellular vesicles enhance infectivity of live SARS-CoV-2 virus. J. Extracell. Vesicles 2022, 11, e12231. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.U.; Lee, S.; Kim, D.Y.; Lyu, J.; Yoon, G.Y.; Kim, K.D.; Ku, K.B.; Ko, J.; Kwon, Y.C. Zika Virus Infection Induces Interleukin-1β-Mediated Inflammatory Responses by Macrophages in the Brain of an Adult Mouse Model. J. Virol. 2023, 97, e0055623. [Google Scholar] [CrossRef]
- Declercq, J.; De Leeuw, E.; Lambrecht, B.N. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine 2022, 157, 155934. [Google Scholar] [CrossRef]
- Fawzy, S.; Ahmed, M.M.; Alsayed, B.A.; Mir, R.; Amle, D. IL-2 and IL-1β Patient Immune Responses Are Critical Factors in SARS-CoV-2 Infection Outcomes. J. Pers. Med. 2022, 12, 1729. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.S.; Jalal, P.J. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann. Med. Surg. 2023, 85, 2291–2297. [Google Scholar] [CrossRef]
- Cahill, C.M.; Rogers, J.T. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008, 283, 25900–25912. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Zhang, Y.; Bouchard, R.; Mahaffey, G. Induction of an inflammatory loop by interleukin-1β and tumor necrosis factor-α involves NF-kB and STAT-1 in differentiated human neuroprogenitor cells. PLoS ONE 2013, 8, e69585. [Google Scholar] [CrossRef]
- Mansour, H.M.; Mohamed, A.F.; Khattab, M.M.; El-Khatib, A.S. Heat Shock Protein 90 in Parkinson’s Disease: Profile of a Serial Killer. Neuroscience 2024, 537, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Song, D.; Li, H.; He, M.L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct. Target. Ther. 2020, 5, 125. [Google Scholar] [CrossRef]
- Pang, J.; Vince, J.E. The role of caspase-8 in inflammatory signalling and pyroptotic cell death. Semin. Immunol. 2023, 70, 101832. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2021, 109, 121–141. [Google Scholar] [CrossRef]
- Bader, S.M.; Cooney, J.P.; Pellegrini, M.; Doerflinger, M. Programmed cell death: The pathways to severe COVID-19? Biochem. J. 2022, 479, 609–628. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X.; Shen, J.; Zhou, Y.; Shi, Z.L.; Zhou, P.; et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef]
- Pandit, R.; Ipinmoroti, A.O.; Crenshaw, B.J.; Li, T.; Matthews, Q.L. Canine Coronavirus Infection Modulates the Biogenesis and Composition of Cell-Derived Extracellular Vesicles. Biomedicines 2023, 11, 976. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijerathne, S.V.T.; Pandit, R.; Ezeuko, C.C.; Matthews, Q.L. Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line. Vet. Sci. 2025, 12, 477. https://doi.org/10.3390/vetsci12050477
Wijerathne SVT, Pandit R, Ezeuko CC, Matthews QL. Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line. Veterinary Sciences. 2025; 12(5):477. https://doi.org/10.3390/vetsci12050477
Chicago/Turabian StyleWijerathne, Sandani V. T., Rachana Pandit, Chioma C. Ezeuko, and Qiana L. Matthews. 2025. "Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line" Veterinary Sciences 12, no. 5: 477. https://doi.org/10.3390/vetsci12050477
APA StyleWijerathne, S. V. T., Pandit, R., Ezeuko, C. C., & Matthews, Q. L. (2025). Comparative Examination of Feline Coronavirus and Canine Coronavirus Effects on Extracellular Vesicles Acquired from A-72 Canine Fibrosarcoma Cell Line. Veterinary Sciences, 12(5), 477. https://doi.org/10.3390/vetsci12050477




