Concentrations of Progesterone (P4), Anti-Müllerian Hormone (AMH), and Haptoglobin (Hp) in Pregnant and Non-Pregnant Ewes and Their Association with Fetal Mortality, Maternal Weight, and Twinning Rate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Examinations, and Sampling
2.2. Quantification of P4, AMH, and Hp in Sera
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hameed Ajafar, M.; Hasan Kadhim, A.; Mohammed AL-Thuwaini, T. The Reproductive Traits of Sheep and Their Influencing Factors. Rev. Agric. Sci. 2022, 10, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Allabban, M.; Erdem, H. Koyunlarda Real-Time Ultrasonografik Muayene Ile Gebelik Tanısı. J. Bahri Dagdas Anim. Res. 2020, 9, 47–55. [Google Scholar]
- Jones, A.K.; Reed, S.A. Benefits of Ultrasound Scanning during Gestation in the Small Ruminant. Small Rumin. Res. 2017, 149, 163–171. [Google Scholar] [CrossRef]
- Karen, A.; Szabados, K.; Reiczigel, J.; Beckers, J.-F.; Szenci, O. Accuracy of Transrectal Ultrasonography for Determination of Pregnancy in Sheep: Effect of Fasting and Handling of the Animals. Theriogenology 2004, 61, 1291–1298. [Google Scholar] [CrossRef]
- Chundekkad, P.; Blaszczyk, B.; Stankiewicz, T. Embryonic Mortality in Sheep: A Review. Turk. J. Vet. Anim. Sci. 2020, 44, 167–173. [Google Scholar] [CrossRef]
- Scott, P.R. Ovine Caesarean Operations: A Study of 137 Field Cases. Br. Vet. J. 1989, 145, 558–564. [Google Scholar] [CrossRef]
- Scott, P.R.; Murray, L.D.; Penny, C.D. A Preliminary Study of Serum Haptoglobin Concentration as a Prognostic Indicator of Ovine Dystocia Cases. Br. Vet. J. 1992, 148, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.; Brown, R.; Roberts, L. Bovine Haptoglobin Response in Clinically Defined Field Conditions. Vet. Rec. 1991, 128, 147–149. [Google Scholar] [CrossRef]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Ulaankhuu, A.; Lkhamjav, G.; Nakamura, Y.; Zolzaya, M. Result of Studying Some Acute Phase Proteins and Cortisol in Pregnant Ewes. Mong. J. Agric. Sci. 2017, 19, 27–31. [Google Scholar] [CrossRef][Green Version]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short Communication: Haptoglobin as an Early Indicator of Metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Burfeind, O.; Heuwieser, W. The Associations between Postpartum Serum Haptoglobin Concentration and Metabolic Status, Calving Difficulties, Retained Fetal Membranes, and Metritis. J. Dairy Sci. 2015, 98, 4544–4551. [Google Scholar] [CrossRef]
- Alward, K.J.; Bohlen, J.F. Overview of Anti-Müllerian Hormone (AMH) and Association with Fertility in Female Cattle. Reprod. Domest. Anim. 2020, 55, 3–10. [Google Scholar] [CrossRef]
- Monniaux, D.; Drouilhet, L.; Rico, C.; Estienne, A.; Jarrier, P.; Touzé, J.-L.; Sapa, J.; Phocas, F.; Dupont, J.; Dalbiès-Tran, R.; et al. Regulation of Anti-Müllerian Hormone Production in Domestic Animals. Reprod. Fertil. Dev. 2013, 25, 1–16. [Google Scholar] [CrossRef]
- Turgut, A.O.; Koca, D. Anti-Müllerian Hormone as a Promising Novel Biomarker for Litter Size in Romanov Sheep. Reprod. Domest. Anim. 2024, 59, e14692. [Google Scholar] [CrossRef]
- Pasciu, V.; Nieddu, M.; Baralla, E.; Porcu, C.; Sotgiu, F.; Berlinguer, F. Measurement of Progesterone in Sheep Using a Commercial ELISA Kit for Human Plasma. J. Vet. Diagn. Investig. 2022, 34, 90–93. [Google Scholar] [CrossRef]
- Rahman, A.N.M.A. Hormonal Changes in the Uterus During Pregnancy—Lessons from the Ewe: A Review. J. Agric. Rural Dev. 2006, 4, 1–7. [Google Scholar] [CrossRef]
- Berkova, N. Haptoglobin Is Present in Human Endometrium and Shows Elevated Levels in the Decidua during Pregnancy. Mol. Hum. Reprod. 2001, 7, 747–754. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Khanimov, M.; Schwartz, E.; Grazi, R.V.; Malter, H.E. Anti-Müllerian Hormone as an Independent Predictor of Twin versus Singleton Pregnancy in Fresh Cycles. Reprod. Biomed. Online 2013, 26, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Gelbaya, T.A.; Laing, I.; Nardo, L.G. The Use of Anti-Müllerian Hormone and Antral Follicle Count to Predict the Potential of Oocytes and Embryos. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, T.; MacLaughlin, D.T.; Shai, I.; Trimarchi, J.R.; Lambert-Messerlian, G.; Seifer, D.B.; Keefe, D.L.; Blazar, A.S. Müllerian Inhibiting Substance Levels at the Time of HCG Administration in IVF Cycles Predict Both Ovarian Reserve and Embryo Morphology. Hum. Reprod. 2006, 21, 159–163. [Google Scholar] [CrossRef]
- Singer, T.; Noel, M.; Huang, J.Y.; Zaninovic, N.; Davis, O.; Liu, H.; Rosenwaks, Z. Anti Mullerian Hormone Correlates With Embryo Morphology in IVF Cycles. Fertil. Steril. 2012, 97, S29. [Google Scholar] [CrossRef]
- Blazar, A.S.; Lambert-Messerlian, G.; Hackett, R.; Krotz, S.; Carson, S.A.; Robins, J.C. Use of In-Cycle Antimüllerian Hormone Levels to Predict Cycle Outcome. Am. J. Obs. Gynecol. 2011, 205, 223.e1–223.e5. [Google Scholar] [CrossRef] [PubMed]
- Buyuk, E.; Seifer, D.B.; Younger, J.; Grazi, R.V.; Lieman, H. Random Anti-Müllerian Hormone (AMH) Is a Predictor of Ovarian Response in Women with Elevated Baseline Early Follicular Follicle-Stimulating Hormone Levels. Fertil. Steril. 2011, 95, 2369–2372. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Ben-Chetrit, A.; Spitz, I.M.; Rabinowitz, R.; Markowitz, E.; Mimoni, T.; Gal, M.; Zylber-Haran, E.; Margalioth, E.J. Dynamic Assays of Inhibin B, Anti-Mullerian Hormone and Estradiol Following FSH Stimulation and Ovarian Ultrasonography as Predictors of IVF Outcome. Hum. Reprod. 2005, 20, 3178–3183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Anti-Müllerian Hormone (AMH) Defines, Independent of Age, Low versus Good Live-Birth Chances in Women with Severely Diminished Ovarian Reserve. Fertil. Steril. 2010, 94, 2824–2827. [Google Scholar] [CrossRef]
- Freeman, E.W.; Gracia, C.R.; Sammel, M.D.; Lin, H.; Lim, L.C.-L.; Strauss, J.F. Association of Anti-Mullerian Hormone Levels with Obesity in Late Reproductive-Age Women. Fertil. Steril. 2007, 87, 101–106. [Google Scholar] [CrossRef]
- Morris, J.; Dobson, J. Small Animal Oncology; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780470690406. [Google Scholar]
- Satterfield, M.C.; Bazer, F.W.; Spencer, T.E. Progesterone Regulation of Preimplantation Conceptus Growth and Galectin 15 (LGALS15) in the Ovine Uterus1. Biol. Reprod. 2006, 75, 289–296. [Google Scholar] [CrossRef]
- Carter, F.; Forde, N.; Duffy, P.; Wade, M.; Fair, T.; Crowe, M.A.; Evans, A.C.O.; Kenny, D.A.; Roche, J.F.; Lonergan, P. Effect of Increasing Progesterone Concentration from Day 3 of Pregnancy on Subsequent Embryo Survival and Development in Beef Heifers. Reprod. Fertil. Dev. 2008, 20, 368. [Google Scholar] [CrossRef]
- Saeed, O.A.; Ahmed, N.K.; Taha, M.M.; Abedal-Majed, M.A.; Ali, F.M.; Samsudin, A.A. Reproductive Performance and Physiological Responses in Awassi Ewes Under Intravaginal Sponges Application and Fed Selenium and Vitamin E. Trop. Anim. Sci. J. 2024, 47, 265–272. [Google Scholar] [CrossRef]
- Pool, K.R.; Rickard, J.P.; Pini, T.; de Graaf, S.P. Exogenous Melatonin Advances the Ram Breeding Season and Increases Testicular Function. Sci. Rep. 2020, 10, 9711. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F.; Li, F. An Intensive Milk Replacer Feeding Program Benefits Immune Response and Intestinal Microbiota of Lambs during Weaning. BMC Vet. Res. 2018, 14, 366. [Google Scholar] [CrossRef]
- Boscos, C.; Samartzi, F.; Lymberopoulos, A.; Stefanakis, A.; Belibasaki, S. Assessment of Progesterone Concentration Using Enzymeimmunoassay, for Early Pregnancy Diagnosis in Sheep and Goats. Reprod. Domest. Anim. 2003, 38, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Younis, L.; Akram, S. Assessing Progesterone Levels in Awassi Ewes: A Comparison between Pregnant and Non-Pregnant, Twins, and Singletons during the First Trimester. Egypt. J. Vet. Sci. 2023, 54, 1255–1263. [Google Scholar] [CrossRef]
- Hümmelchen, H.; Wagner, H.; Brügemann, K.; König, S.; Wehrend, A. Effects of Breeding for Short-Tailedness in Sheep on Parameters of Reproduction and Lamb Development. Vet. Med. Sci. 2025, 11, e70138. [Google Scholar] [CrossRef] [PubMed]
- Mukasa-Mugerwa, E.; Viviani, P. Progesterone Concentrations in Peripheral Plasma of Menz Sheep during Gestation and Parturition. Small Rumin. Res. 1992, 8, 47–53. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Solari, A.; Mirman, B. Effects of Luteectomy on the Maintenance of Pregnancy, Circulating Progesterone Concentrations and Lambing Performance in Sheep. Reprod. Fertil. Dev. 1999, 11, 317. [Google Scholar] [CrossRef]
- Sammin, D.; Markey, B.; Bassett, H.; Buxton, D. The Ovine Placenta and Placentitis—A Review. Vet. Microbiol. 2009, 135, 90–97. [Google Scholar] [CrossRef]
- O’Shea, J.D.; McCoy, K. Weight, Composition, Mitosis, Cell Death and Content of Progesterone and DNA in the Corpus Luteum of Pregnancy in the Ewe. J. Reprod. Fertil. 1988, 83, 107–117. [Google Scholar] [CrossRef]
- Kaulfuss, K.H.; Giucci, E.; May, J. Influencing Factors on the Level of the Ovulation Rate in Sheep during the Main Breeding Season--an Ultrasonographic Study. Dtsch. Tierarztl. Wochenschr. 2003, 110, 445–450. [Google Scholar]
- Karen, A.; Beckers, J.-F.; Sulon, J.; de Sousa, N.M.; Szabados, K.; Reczigel, J.; Szenci, O. Early Pregnancy Diagnosis in Sheep by Progesterone and Pregnancy-Associated Glycoprotein Tests. Theriogenology 2003, 59, 1941–1948. [Google Scholar] [CrossRef]
- Al-Mousawe, A.; Ibrahim, N. Diagnosis of Pregnancy in Iraqi Awassi Ewes Through Progesterone Hormone Measurement and Ultrasonography Following Induction of Fertile Estrus with Sulpiride. Egypt. J. Vet. Sci. 2024, 55, 945–953. [Google Scholar] [CrossRef]
- Weigel, N.L.; Pousette, A.; Schrader, W.T.; O’Malley, B.W. Analysis of Chicken Progesterone Receptor Structure Using a Spontaneous Sheep Antibody. Biochemistry 1981, 20, 6798–6803. [Google Scholar] [CrossRef]
- Younis, L.; Aboud, Q. Evaluate the Effectiveness of Pregnancy-Associated Glycoprotein and Progesterone in Predicting the Gestational Status in Goats. Univ. Thi-Qar J. Agric. Res. 2023, 12, 201–210. [Google Scholar] [CrossRef]
- de Brun, V.; Meikle, A.; Fernández-Foren, A.; Forcada, F.; Palacín, I.; Menchaca, A.; Sosa, C.; Abecia, J.-A. Failure to Establish and Maintain a Pregnancy in Undernourished Recipient Ewes Is Associated with a Poor Endocrine Milieu in the Early Luteal Phase. Anim. Reprod. Sci. 2016, 173, 80–86. [Google Scholar] [CrossRef]
- Dixon, A.B.; Knights, M.; Winkler, J.L.; Marsh, D.J.; Pate, J.L.; Wilson, M.E.; Dailey, R.A.; Seidel, G.; Inskeep, E.K. Patterns of Late Embryonic and Fetal Mortality and Association with Several Factors in Sheep. J. Anim. Sci. 2007, 85, 1274–1284. [Google Scholar] [CrossRef]
- Humblot, P. Use of Pregnancy Specific Proteins and Progesterone Assays to Monitor Pregnancy and Determine the Timing, Frequencies and Sources of Embryonic Mortality in Ruminants. Theriogenology 2001, 56, 1417–1433. [Google Scholar] [CrossRef]
- Domingues, R.R.; Andrade, J.P.N.; Cunha, T.O.; Madureira, G.; Moallem, U.; Gomez-Leon, V.; Martins, J.P.N.; Wiltbank, M.C. Is Pregnancy Loss Initiated by Embryonic Death or Luteal Regression? Profiles of Pregnancy-Associated Glycoproteins during Elevated Progesterone and Pregnancy Loss. JDS Commun. 2023, 4, 149–154. [Google Scholar] [CrossRef]
- Ahmad, N.; Townsend, E.C.; Dailey, R.A.; Inskeep, E.K. Relationships of Hormonal Patterns and Fertility to Occurrence of Two or Three Waves of Ovarian Follicles, before and after Breeding, in Beef Cows and Heifers. Anim. Reprod. Sci. 1997, 49, 13–28. [Google Scholar] [CrossRef]
- Starbuck, G.R.; Darwash, A.O.; Mann, G.E.; Lamming, G.E. The Detection and Treatment of Post Insemination Progesterone Insufficiency in Dairy Cows. BSAP Occas. Publ. 2001, 26, 447–450. [Google Scholar] [CrossRef]
- Kaskous, S.; Gottschalk, J.; Hippel, T.; Grün, E. The Behavior of Growth-Influencing and Steroid Hormones in the Blood Plasma during Pregnancy of Awassi Sheep in Syria. Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 108–116. [Google Scholar] [PubMed]
- Kulcsár, M.; Dankó, G.; Magdy, H.G.I.; Reiczigel, J.; Forgach, T.; Proháczik, A.; Delavaud, C.; Magyar, K.; Chilliard, Y.; Solti, L.; et al. Pregnancy Stage and Number of Fetuses May Influence Maternal Plasma Leptin in Ewes. Acta Vet. Hung. 2006, 54, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Gür, S.; Türk, G.; Demirci, E.; Yüce, A.; Sönmez, M.; Özer, Ş.; Aksu, E. Effect of Pregnancy and Foetal Number on Diameter of Corpus Luteum, Maternal Progesterone Concentration and Oxidant/Antioxidant Balance in Ewes. Reprod. Domest. Anim. 2011, 46, 289–295. [Google Scholar] [CrossRef]
- Manalu, W.; Sumaryadi, M.Y.; Kusumorini, N. Effect of Fetal Number on the Concentrations of Circulating Maternal Serum Progesterone and Estradiol of Does during Late Pregnancy. Small Rumin. Res. 1997, 23, 117–124. [Google Scholar] [CrossRef]
- Takarkhede, R.C.; Honmode, J.D.; Kadu, M.S. Seasonal Variations in Estradiol-17f3 and Progesterone Level during Oestrus Cycle in Malpura Ewes. Indian J. Anim. Sci. 2002, 72, 296–299. [Google Scholar]
- Spencer, T.E.; Burghardt, R.C.; Johnson, G.A.; Bazer, F.W. Conceptus Signals for Establishment and Maintenance of Pregnancy. Anim. Reprod. Sci. 2004, 82–83, 537–550. [Google Scholar] [CrossRef]
- Cushman, R.A.; Yake, H.K.; Snider, A.P.; Lents, C.A.; Murphy, T.W.; Freking, B.A. An Extreme Model of Fertility in Sheep Demonstrates the Basis of Controversies Surrounding Antral Follicle Count and Circulating Concentrations of Anti-Müllerian Hormone as Predictors of Fertility in Ruminants. Anim. Reprod. Sci. 2023, 259, 107364. [Google Scholar] [CrossRef]
- Hannerz, J.; Greitz, D.; Ericson, K. Is There a Relationship between Obesity and Intracranial Hypertension? Int. J. Obes. Relat. Metab. Disord. 1995, 19, 240–244. [Google Scholar]
- Eurell, T.E.; Bane, D.P.; Hall, W.F.; Schaeffer, D.J. Serum Haptoglobin Concentration as an Indicator of Weight Gain in Pigs. Can. J. Vet. Res. 1992, 56, 6–9. [Google Scholar]
- Daly, J.; Smith, H.; McGrice, H.A.; Kind, K.L.; van Wettere, W.H.E.J. Towards Improving the Outcomes of Assisted Reproductive Technologies of Cattle and Sheep, with Particular Focus on Recipient Management. Animals 2020, 10, 293. [Google Scholar] [CrossRef]
- Ireland, J.L.H.; Scheetz, D.; Jimenez-Krassel, F.; Themmen, A.P.N.; Ward, F.; Lonergan, P.; Smith, G.W.; Perez, G.I.; Evans, A.C.O.; Ireland, J.J. Antral Follicle Count Reliably Predicts Number of Morphologically Healthy Oocytes and Follicles in Ovaries of Young Adult Cattle1. Biol. Reprod. 2008, 79, 1219–1225. [Google Scholar] [CrossRef]
- Karakas Alkan, K.; Alkan, H.; Kaymaz, M. The Effect of Anti-Müllerian Hormone and Progesterone Concentrations on Superovulation Response and Embryo Yield in Goats. Theriogenology 2020, 143, 1–9. [Google Scholar] [CrossRef]
- Mossa, F.; Carter, F.; Walsh, S.W.; Kenny, D.A.; Smith, G.W.; Ireland, J.L.H.; Hildebrandt, T.B.; Lonergan, P.; Ireland, J.J.; Evans, A.C.O. Maternal Undernutrition in Cows Impairs Ovarian and Cardiovascular Systems in Their Offspring. Biol. Reprod. 2013, 88, 92. [Google Scholar] [CrossRef]
- Mossa, F.; Evans, A.C.O. Review: The Ovarian Follicular Reserve—Implications for Fertility in Ruminants. Animal 2023, 17, 100744. [Google Scholar] [CrossRef]
- Hollinshead, F.; Walker, C.; Hanlon, D. Determination of the Normal Reference Interval for Anti-Müllerian Hormone (AMH) in Bitches and Use of AMH as a Potential Predictor of Litter Size. Reprod. Domest. Anim. 2017, 52, 35–40. [Google Scholar] [CrossRef]
- Evci, E.C.; Aslan, S.; Schäfer-Somi, S.; Ergene, O.; Sayıner, S.; Darbaz, I.; Seyrek-İntaş, K.; Wehrend, A. Monitoring of Canine Pregnancy by Considering Anti-Mullerian Hormone, C-Reactive Protein, Progesterone and Complete Blood Count in Pregnant and Non-Pregnant Dogs. Theriogenology 2023, 195, 69–76. [Google Scholar] [CrossRef]
- Satué, K.; Marcilla, M.; Medica, P.; Ferlazzo, A.; Fazio, E. Sequential Concentrations of Placental Growth Factor and Haptoglobin, and Their Relation to Oestrone Sulphate and Progesterone in Pregnant Spanish Purebred Mare. Theriogenology 2018, 115, 77–83. [Google Scholar] [CrossRef]
- Skinner, J.; Roberts, L. Haptoglobin as an Indicator of Infection in Sheep. Vet. Rec. 1994, 134, 33–36. [Google Scholar] [CrossRef]
- González, J.F.; Hernández, Á.; Meeusen, E.N.T.; Rodríguez, F.; Molina, J.M.; Jaber, J.R.; Raadsma, H.W.; Piedrafita, D. Fecundity in Adult Haemonchus Contortus Parasites Is Correlated with Abomasal Tissue Eosinophils and Γδ T Cells in Resistant Canaria Hair Breed Sheep. Vet. Parasitol. 2011, 178, 286–292. [Google Scholar] [CrossRef]
- Albay, M.K.; Karakurum, M.C.; Sahinduran, S.; Sezer, K.; Yildiz, R.; Buyukoglu, T. Selected Serum Biochemical Parameters and Acute Phase Protein Levels in a Herd of Saanen Goats Showing Signs of Pregnancy Toxaemia. Vet. Med. 2014, 59, 336–342. [Google Scholar] [CrossRef]
- Narin, K.; Aytekin, İ. Paraoxonase, Haptoglobin, Serum Amyloid A, Tumor Necrosis Factor and Acetylcholinesterase Levels in Ewes with Pregnancy Toxemia. Turk. J. Vet. Res. 2024, 8, 1–6. [Google Scholar] [CrossRef]
- Greguła-Kania, M.; Kosior-Korzecka, U.; Hahaj-Siembida, A.; Kania, K.; Szysiak, N.; Junkuszew, A. Age-Related Changes in Acute Phase Reaction, Cortisol, and Haematological Parameters in Ewes in the Periparturient Period. Animals 2021, 11, 3459. [Google Scholar] [CrossRef] [PubMed]
- Iliev, P.; Georgieva, T. Acute Phase Proteins in Sheep and Goats—Function, Reference Ranges and Assessment Methods: An Overview. Bulg. J. Vet. Med. 2018, 21, 1–16. [Google Scholar] [CrossRef]


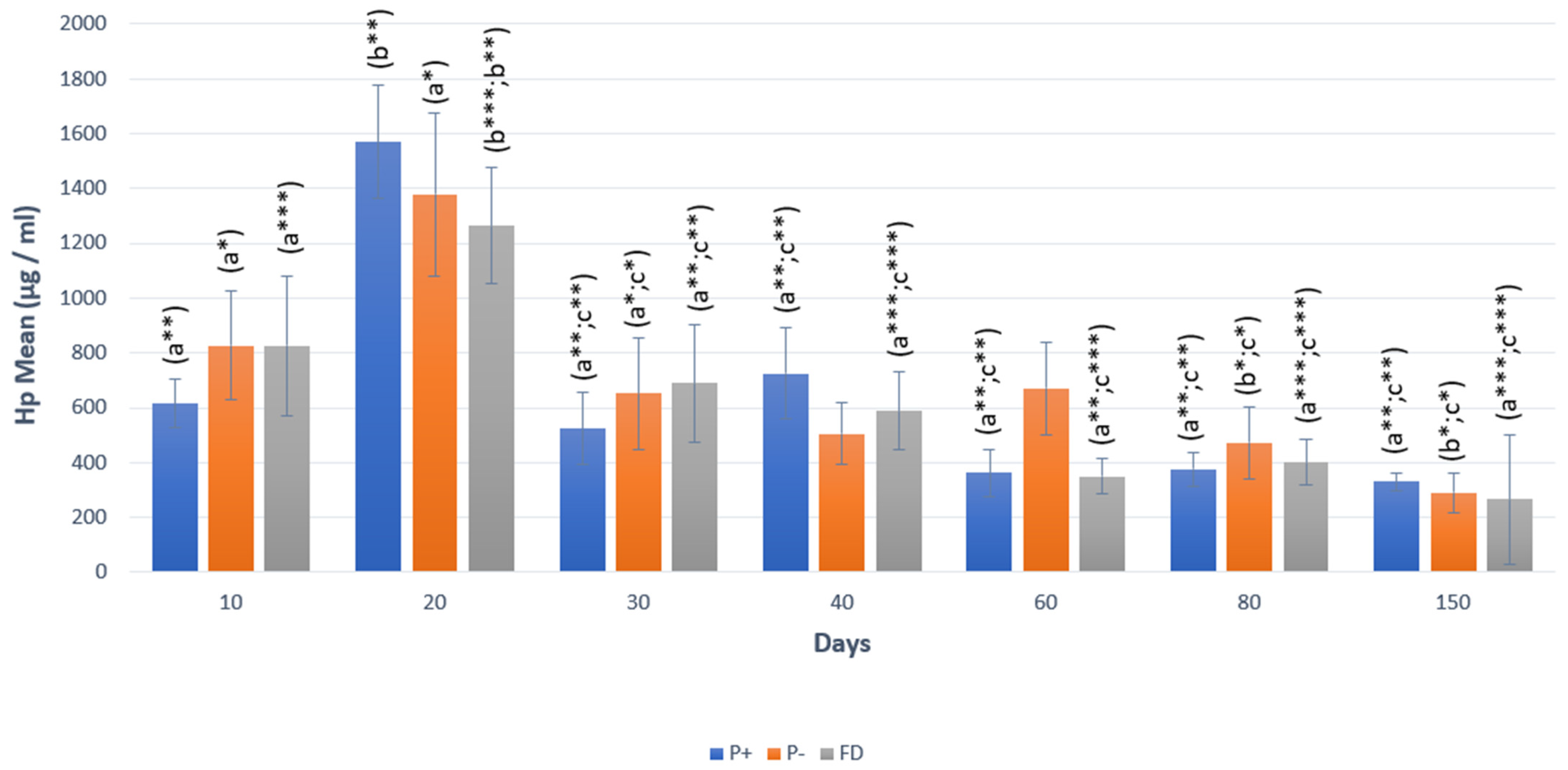

| P4 (ng/mL) | AMH (ng/mL) | Hp (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | P+ | P− | p | P+ | P− | p | P+ | P− | p |
| 10 | 4.97 ± 0.50 | 3.99 ± 0.74 | n.s. | 2.33 ± 0.36 | 2.00 ± 0.48 | n.s. | 613.92 ± 88.46 | 827.42 ± 196.72 | n.s. |
| 20 | 4.46 ± 0.57 (a***) | 0.73 ± 0.39 (b***) | *** p < 0.0001 | 3.02 ± 0.79 | 1.55 ± 0.38 | n.s. | 1570.51 ± 206.54 | 1378.27 ± 296.17 | n.s. |
| 30 | 3.88 ± 0.23 (a***) | 0.63 ± 0.46 (b***) | *** p < 0.0001 | 3.04 ± 0.68 (s**) | 1.11 ± 0.29 (b**) | p < 0.01 | 523.13 ± 131.71 | 650.83 ± 202.15 | n.s. |
| 40 | 3.57 ± 0.24 (a***) | 0.07 ± 0.01 (b***) | *** p < 0.0001 | 2.52 ± 0.58 | 1.14 ± 0.29 | n.s. | 724.33 ± 166.61 | 505.48 ± 110.85 | n.s. |
| 60 | 5.05 ± 0.63 (a**) | 1.70 ± 0.75 (b**) | ** p < 0.001 | 2.54 ± 0.67 | 2.29 ± 0.91 | n.s. | 362.65 ± 85.75 | 671.17 ± 167.98 | n.s. |
| 80 | 5.77 ± 0.67 (a***) | 0.32 ± 0.16 (b***) | *** p < 0.0001 | 1.28 ± 0.29 | 0.61 ± 0.16 | n.s. | 375.72 ± 60.59 | 471.86 ± 130.36 | n.s. |
| 150 | 8.15 ± 1.67 (a***) | 0.26 ± 0.09 (b***) | *** p < 0.0001 | 1.52 ± 0.37 | 0.94 ± 0.22 | n.s. | 329.65 ± 31.65 | 289.90 ± 72.63 | n.s. |
| P4 (ng/mL) | AMH (ng/mL) | Hp (µg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | P− | FD | p | P− | FD | p | P− | FD | p |
| 10 | 3.99 ± 0.74 | 3.83 ± 0.59 | n.s | 2.00 ± 0.48 | 1.46 ± 0.35 | n.s | 827.42 ± 196.72 | 826.76 ± 255.12 | n.s |
| 20 | 0.73 ± 0.39 (a*) | 1.73 ± 0.4 (b*) | p < 0.05 | 1.55 ± 0.38 | 1.85 ± 0.73 | n.s | 1378.27 ± 296.17 | 1262.95 ± 211.74 | n.s |
| 30 | 0.63 ± 0.46 (a**) | 2.06 ± 0.40 (b**) | p < 0.01 | 1.11 ± 0.29 | 1.60 ± 0.52 | n.s | 650.83 ± 202.15 | 688.91 ± 214.04 | n.s |
| 40 | 0.07 ± 0.01 (a**) | 1.29 ± 0.36 (b**) | p < 0.01 | 1.14 ± 0.2 | 1.57 ± 0.43 | n.s | 505.48 ± 110.85 | 586.84 ± 142.42 | n.s |
| 60 | 1.70 ± 0.75 (a**) | 1.51 ± 0.43 (b**) | p < 0.01 | 2.29 ± 0.91 | 2.56 ± 1.00 | n.s | 671.17 ± 167.98 | 350.08 ± 64.88 | n.s |
| 80 | 0.32 ± 0.16 | 0.88 ± 0.37 | n.s | 0.61 ± 0.16 | 1.02 ± 0.15 | n.s | 471.86 ± 130.36 | 399.90 ± 82.29 | n.s |
| 150 | 0.26 ± 0.09 | 0.23 ± 0.06 | n.s | 0.94 ± 0.22 | 1.35 ± 0.27 | n.s | 289.90 ± 72.63 | 264.96 ± 34.59 | n.s |
| P4 (ng/mL) | AMH (ng/mL) | Hp (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | P+ | FD | p | P+ | FD | p | P+ | FD | p |
| 10 | 4.97 ± 0.50 | 3.83 ± 0.59 | n.s | 2.33 ± 0.36 (a*) | 1.46 ± 0.35 (b) | n.s | 613.92 ± 88.46 | 826.76 ± 255.12 | n.s |
| 20 | 4.46 ± 0.57 (a**) | 1.73 ± 0.47 (b**) | ** p < 0.01 | 3.02 ± 0.79 (a*) | 1.85 ± 0.73 (b) | n.s | 1570.51 ± 206.54 | 1262.95 ± 211.74 | n.s |
| 30 | 3.88 ± 0.23 (a***) | 2.05 ± 0.40 (b***) | *** p < 0.001 | 3.04 ± 0.68 (a*) | 1.60 ± 0.52 (b*) | * p < 0.01 | 523.13 ± 131.71 | 688.91 ± 214.04 | n.s |
| 40 | 3.57 ± 0.24 (a***) | 1.28 ± 0.36 (b***) | **** p < 0.0001 | 2.52 ± 0.58 | 1.57 ± 0.43 | n.s | 724.33 ± 166.61 | 586.84 ± 142.42 | n.s |
| 60 | 5.05 ± 0.63 (a***) | 1.51 ± 0.43 (b***) | **** p < 0.0001 | 2.54 ± 0.67 | 2.56 ± 1.00 | n.s | 362.65 ± 85.75 | 350.08 ± 64.88 | n.s |
| 80 | 5.77 ± 0.67 (a***) | 0.88 ± 0.37 (b***) | **** p < 0.0001 | 1.28 ± 0.29 | 1.02 ± 0.15 | n.s | 375.72 ± 60.59 | 399.90 ± 82.29 | n.s |
| 150 | 8.15 ± 1.67 (a***) | 0.23 ± 0.06 (b***) | **** p < 0.0001 | 1.52 ± 0.37 | 1.35 ± 0.27 | n.s | 329.65 ± 31.65 | 264.96 ± 34.59 | n.s |
| P4 (ng/mL) | AMH (ng/mL) | Hp (µg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Months | P+ | P− | FD | P+ | P− | FD | P+ | P− | FD |
| A2 | 4.46 ± 0.19 (a***) | 1.35 ± 0.3 (a) | 2.23 ± 0.26 (a**,a***) | 2.52 ± 0.29 (a*, a***) | 1.5 ± 0.19 (a) | 1.62 ± 0.26 (a) | 857.97 ± 89.78 (a***) | 840.50 ± 116.90 (a;b**) | 841.37 ± 108.23 (a*) |
| A4 | 5.56 ± 0.45 (b***) | 1.01 ± 0.41 (a) | 1.20 ± 0.29 (b**) | 1.91 ± 0.38 (b*) | 1.5 ± 0.50 (a) | 1.79 ± 0.52 (a) | 369.19 ± 51.79 (b***) | 571.52 ± 105.88 (a) | 374.99 ± 51.51 (b*) |
| A6 | 8.60 ± 1.71 (b***,c**) | 0.26 ± 0.09 (a) | 0.23 ± 0.06 (b***) | 8.15 ± 1.67 (b*,c***) | 0.9 ± 0.22 (a) | 1.36 ± 0.27 (a) | 329.65 ± 31.65 (b***, c**) | 289.90 ± 72.63 (c**) | 264.96 ± 34.59 (b*; c*) |
| Weight Measurement Days (days) | Weights (kg) | P4 (ng/mL) | AMH (ng/mL) | Hp (µg/mL) |
|---|---|---|---|---|
| 10 | 67.26 ± 6.71 | 4.97 ± 0.50 (a*) | 2.39 ± 0.36 (a***;a**) | 613.91 ± 88.46 (a*;a**) |
| 80 | 76.18 ± 8.98 | 5.77 ± 0.66 (a*) | 1.27 ± 0.29 (b***) | 375.72 ± 60.58 (b*) |
| 150 | 86.15 ± 9.14 | 8.15 ± 1.67 (a*) | 1.52 ± 0.37 (b**) | 329.64 ± 31.64 (b*;b**) |
| p | p > 0.05 | p > 0.05 | a**:b** p < 0.01 a***:b*** p < 0.001 | a*:b* p < 0.05 a**:b** p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturan, H.G.; Aslan, S.; Zabitler Tepik, F.; Darbaz, I.; Sayiner, S.; Wehrend, A. Concentrations of Progesterone (P4), Anti-Müllerian Hormone (AMH), and Haptoglobin (Hp) in Pregnant and Non-Pregnant Ewes and Their Association with Fetal Mortality, Maternal Weight, and Twinning Rate. Vet. Sci. 2025, 12, 463. https://doi.org/10.3390/vetsci12050463
Ozturan HG, Aslan S, Zabitler Tepik F, Darbaz I, Sayiner S, Wehrend A. Concentrations of Progesterone (P4), Anti-Müllerian Hormone (AMH), and Haptoglobin (Hp) in Pregnant and Non-Pregnant Ewes and Their Association with Fetal Mortality, Maternal Weight, and Twinning Rate. Veterinary Sciences. 2025; 12(5):463. https://doi.org/10.3390/vetsci12050463
Chicago/Turabian StyleOzturan, Halil Gunes, Selim Aslan, Feride Zabitler Tepik, Isfendiyar Darbaz, Serkan Sayiner, and Axel Wehrend. 2025. "Concentrations of Progesterone (P4), Anti-Müllerian Hormone (AMH), and Haptoglobin (Hp) in Pregnant and Non-Pregnant Ewes and Their Association with Fetal Mortality, Maternal Weight, and Twinning Rate" Veterinary Sciences 12, no. 5: 463. https://doi.org/10.3390/vetsci12050463
APA StyleOzturan, H. G., Aslan, S., Zabitler Tepik, F., Darbaz, I., Sayiner, S., & Wehrend, A. (2025). Concentrations of Progesterone (P4), Anti-Müllerian Hormone (AMH), and Haptoglobin (Hp) in Pregnant and Non-Pregnant Ewes and Their Association with Fetal Mortality, Maternal Weight, and Twinning Rate. Veterinary Sciences, 12(5), 463. https://doi.org/10.3390/vetsci12050463








